Associations of Serum Total 25OHD, 25OHD3, and epi-25OHD3 with Insulin Resistance: Cross-Sectional Analysis of the National Health and Nutrition Examination Survey, 2011–2016
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. The Determination of Vitamin D Metabolites
2.3. Determination of Fasting Glucose, Insulin, and HOMA-IR
2.4. Other Covariates
2.5. Statistical Analysis
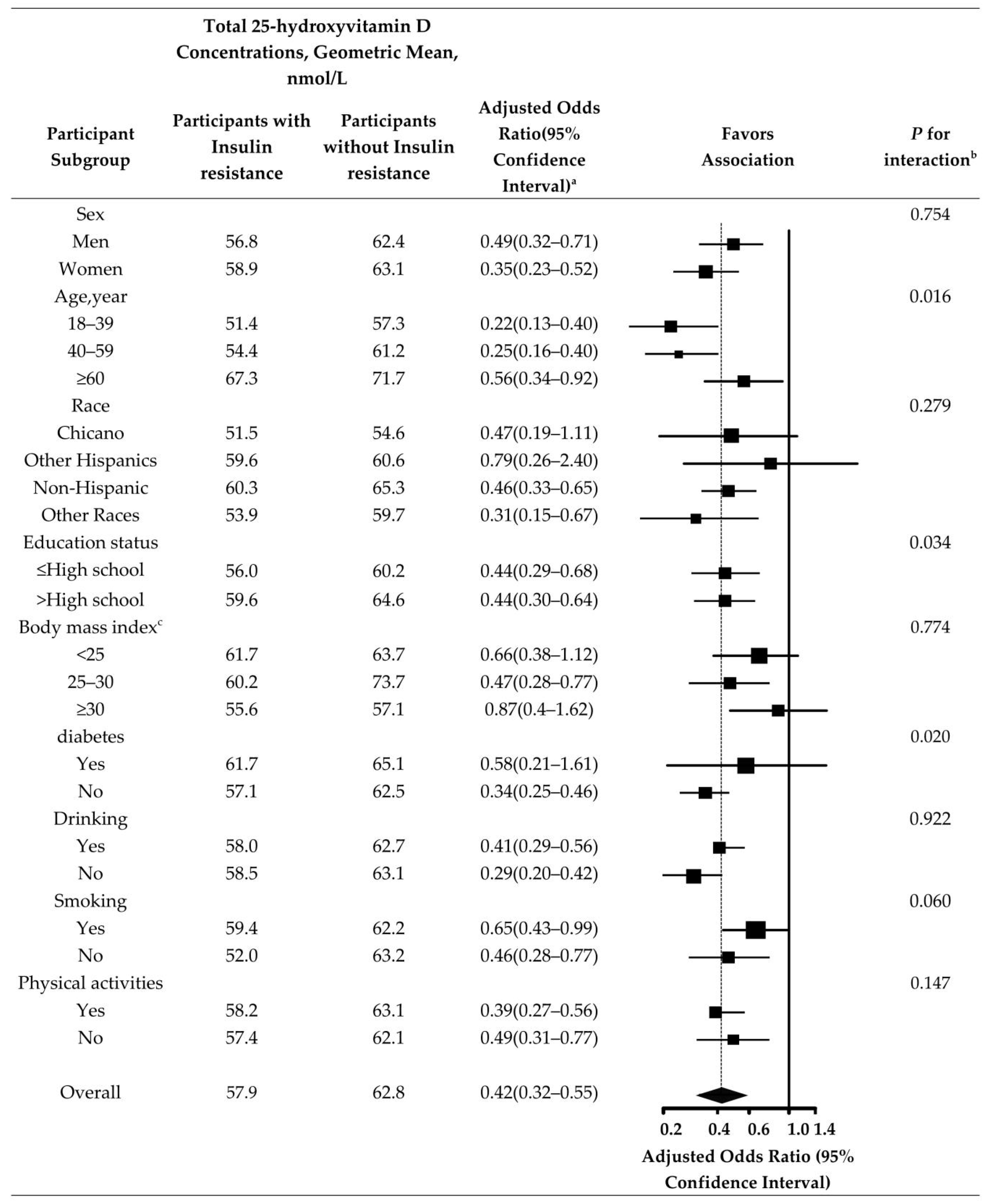
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Binkley, N.; Ramamurthy, R.; Krueger, D. Low vitamin D status: Definition, prevalence, consequences, and correction. Rheum. Dis. Clin. N. Am. 2012, 38, 45–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krist, A.H.; Davidson, K.W.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Epling, J.W., Jr.; Kubik, M.; et al. Screening for Vitamin D Deficiency in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1436–1442. [Google Scholar] [PubMed]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Dawson-Hughes, B.; Eisman, J.A.; Fuleihan, G.E.; Josse, R.G.; Lips, P.; Morales-Torres, J.; IOF Committee of Scientific Advisors (CSA) Nutrition Working Group. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Souberbielle, J.-C.; Body, J.-J.; Lappe, J.M.; Plebani, M.; Shoenfeld, Y.; Wang, T.J.; Bischoff-Ferrari, H.A.; Cavalier, E.; Ebeling, P.R.; Fardellone, P.; et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun. Rev. 2010, 9, 709–715. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Altieri, B.; Annweiler, C.; Balercia, G.; Pal, H.B.; Boucher, B.J.; Cannell, J.J.; Foresta, C.; Grübler, M.R.; Kotsa, K.; et al. Vitamin D and chronic diseases: The current state of the art. Arch. Toxicol. 2017, 91, 97–107. [Google Scholar] [CrossRef]
- Barragan, M.; Good, M.; Kolls, J.K. Regulation of Dendritic Cell Function by Vitamin D. Nutrients 2015, 7, 8127–8151. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Jorde, R.; Kawahara, T.; Dawson-Hughes, B. Vitamin D Supplementation for Prevention of Type 2 Diabetes Mellitus: To D or Not to D? J. Clin. Endocrinol. Metab. 2020, 105, 3721–3733. [Google Scholar] [CrossRef]
- Sacerdote, A.; Dave, P.; Lokshin, V.; Bahtiyar, G. Type 2 Diabetes Mellitus, Insulin Resistance, and Vitamin D. Curr. Diab. Rep. 2019, 19, 101. [Google Scholar] [CrossRef]
- Gulseth, H.L.; Wium, C.; Angel, K.; Eriksen, E.F.; Birkeland, K.I. Effects of Vitamin D Supplementation on Insulin Sensitivity and Insulin Secretion in Subjects with Type 2 Diabetes and Vitamin D Deficiency: A Randomized Controlled Trial. Diabetes Care 2017, 40, 872–878. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, M.; Ren, Z.; Ren, H.; Li, M.; Quan, D.; Chen, L.; Qiu, L. Exploratory study on classification of diabetes mellitus through a combined Random Forest Classifier. BMC Med. Inform. Decis. Mak. 2021, 21, 105. [Google Scholar] [CrossRef]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, L.; Whiting, D.; Weil, C.; Unwin, N. The International Diabetes Federation diabetes atlas methodology for estimating global and national prevalence of diabetes in adults. Diabetes Res. Clin. Pract. 2011, 94, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Onyango, A.N. Cellular Stresses and Stress Responses in the Pathogenesis of Insulin Resistance. Oxid Med. Cell. Longev. 2018, 2018, 4321714. [Google Scholar] [CrossRef] [PubMed]
- Simonson, G.D.; Kendall, D.M. Diagnosis of insulin resistance and associated syndromes: The spectrum from the metabolic syndrome to type 2 diabetes mellitus. Coron. Artery. Dis. 2005, 16, 465–472. [Google Scholar] [CrossRef]
- Barchetta, I.; De Bernardinis, M.; Capoccia, D.; Baroni, M.G.; Fontana, M.; Fraioli, A.; Morini, S.; Leonetti, F.; Cavallo, M.G. Hypovitaminosis D is independently associated with metabolic syndrome in obese patients. PLoS ONE 2013, 8, e68689. [Google Scholar]
- Olson, M.L.; Maalouf, N.M.; Oden, J.D.; White, P.C.; Hutchison, M.R. Vitamin D deficiency in obese children and its relationship to glucose homeostasis. J. Clin. Endocrinol. Metab. 2012, 97, 279–285. [Google Scholar] [CrossRef]
- Kayaniyil, S.; Retnakaran, R.; Harris, S.B.; Vieth, R.; Knight, J.A.; Gerstein, H.C.; Perkins, B.A.; Zinman, B.; Hanley, A.J. Prospective associations of vitamin D with beta-cell function and glycemia: The PROspective Metabolism and ISlet cell Evaluation (PROMISE) cohort study. Diabetes 2011, 60, 2947–2953. [Google Scholar] [CrossRef]
- Dunlop, T.W.; Vaisanen, S.; Frank, C.; Molnár, F.; Sinkkonen, L.; Carlberg, C. The human peroxisome proliferator-activated receptor delta gene is a primary target of 1alpha,25-dihydroxyvitamin D3 and its nuclear receptor. J. Mol. Biol. 2005, 349, 248–260. [Google Scholar] [CrossRef]
- Nazarian, S.; St, P.J.; Boston, R.C.; Jones, S.A.; Mariash, C.N. Vitamin D3 supplementation improves insulin sensitivity in subjects with impaired fasting glucose. Transl. Res. 2011, 158, 276–281. [Google Scholar] [CrossRef]
- Bourlon, P.M.; Billaudel, B.; Faure-Dussert, A. Influence of vitamin D3 deficiency and 1,25 dihydroxyvitamin D3 on de novo insulin biosynthesis in the islets of the rat endocrine pancreas. J. Endocrinol. 1999, 160, 87–95. [Google Scholar] [CrossRef]
- Forno, E.; Han, Y.Y.; Muzumdar, R.H.; Celedón, J.C. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J. Allergy. Clin. Immunol. 2015, 136, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Fulgoni, V.R. Intake of Potatoes Is Associated with Higher Diet Quality, and Improved Nutrient Intake and Adequacy among US Adolescents: NHANES 2001-2018 Analysis. Nutrients 2021, 13, 2614. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Harlow, S.D.; Park, S.K. Urinary arsenic and insulin resistance in US adolescents. Int. J. Hyg. Environ. Health 2015, 218, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Matli, B.; Schulz, A.; Koeck, T.; Falter, T.; Lotz, J.; Rossmann, H.; Pfeiffer, N.; Beutel, M.; Münzel, T.; Strauch, K.; et al. Distribution of HOMA-IR in a population-based cohort and proposal for reference intervals. Clin. Chem. Lab. Med. 2021, 59, 1844–1851. [Google Scholar] [CrossRef]
- Shashaj, B.; Luciano, R.; Contoli, B.; Morino, G.S.; Spreghini, M.R.; Rustico, C.; Sforza, R.W.; Dallapiccola, B.; Manco, M. Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta Diabetol. 2016, 53, 251–260. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.J.; Liu, Z.Y.; Li, Q.; Kong, Y.W.; Chen, Y.W.; Sun, Y.H.; Dong, J.Z. Effect of Insulin Resistance on Recurrence after Radiofrequency Catheter Ablation in Patients with Atrial Fibrillation. Cardiovasc. Drugs Ther. 2022; accpeted. [Google Scholar]
- Qi, T.; Xueqin, L.; Peipei, S.; Lingzhong, X. Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: Developments in research and prospects for the future. Drug Discov. Ther. 2015, 9, 380–385. [Google Scholar]
- Li, D.; Wei, H.; Xue, H.; Zhang, J.; Chen, M.; Gong, Y.; Cheng, G. Higher serum 25(OH)D level is associated with decreased risk of impairment of glucose homeostasis: Data from Southwest China. BMC Endocr. Disord. 2018, 18, 25. [Google Scholar] [CrossRef]
- Gao, Y.; Zheng, T.; Ran, X.; Ren, Y.; Chen, T.; Zhong, L.; Yan, D.; Yan, F.; Wu, Q.; Tia, H. Vitamin D and Incidence of Prediabetes or Type 2 Diabetes: A Four-Year Follow-Up Community-Based Study. Dis. Markers 2018, 2018, 1926308. [Google Scholar] [CrossRef]
- Niroomand, M.; Fotouhi, A.; Irannejad, N.; Hosseinpanah, F. Does high-dose vitamin D supplementation impact insulin resistance and risk of development of diabetes in patients with pre-diabetes? A double-blind randomized clinical trial. Diabetes Res. Clin. Pract. 2018, 148, 1–9. [Google Scholar] [CrossRef]
- Pilz, S.; van den Hurk, K.; Nijpels, G.; Stehouwer, C.D.; Van’t Riet, E.; Kienreich, K.; Tomaschitz, A.; Dekker, J.M. Vitamin D status, incident diabetes and prospective changes in glucose metabolism in older subjects: The Hoorn study. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Heshmat, R.; Tabatabaei-Malazy, O.; Abbaszadeh-Ahranjani, S.; Shahbazi, S.; Khooshehchin, G.; Bandarian, F.; Larijani, B. Effect of vitamin D on insulin resistance and anthropometric parameters in Type 2 diabetes; a randomized double-blind clinical trial. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2012, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.A.; Liu, Y.P.; Chen, Y.C.; Yu, W.; Xiong, X.J.; Huang, H.Y.; Li, W.C.; Chen, J.Y. Gender-specific and age-specific associations of the homoeostasis model assessment for IR (HOMA-IR) with albuminuria and renal function impairment: A retrospective cross-sectional study in Southeast China. BMJ Open 2021, 11, e53649. [Google Scholar] [CrossRef] [PubMed]
- Isokuortti, E.; Zhou, Y.; Peltonen, M.; Bugianesi, E.; Clement, K.; Bonnefont-Rousselot, D.; Lacorte, J.M.; Gastaldelli, A.; Schuppan, D.; Schattenberg, J.M.; et al. Use of HOMA-IR to diagnose non-alcoholic fatty liver disease: A population-based and inter-laboratory study. Diabetologia 2017, 60, 1873–1882. [Google Scholar] [CrossRef]
- Lee, C.H.; Shih, A.Z.; Woo, Y.C.; Fong, C.H.; Leung, O.Y.; Janus, E.; Cheung, B.M.; Lam, K.S. Optimal Cut-Offs of Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) to Identify Dysglycemia and Type 2 Diabetes Mellitus: A 15-Year Prospective Study in Chinese. PLoS ONE 2016, 11, e163424. [Google Scholar] [CrossRef]
- Paolisso, G.; Tagliamonte, M.R.; Rizzo, M.R.; Giugliano, D. Advancing age and insulin resistance: New facts about an ancient history. Eur. J. Clin. Investig. 1999, 29, 758–769. [Google Scholar] [CrossRef]
- Gayoso-Diz, P.; Otero-González, A.; Rodriguez-Alvarez, M.X.; Gude, F.; García, F.; De Francisco, A.; Quintela, A.G. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr. Disord. 2013, 13, 47. [Google Scholar] [CrossRef]
- Gluvic, Z.; Zaric, B.; Resanovic, I.; Obradovic, M.; Mitrovic, A.; Radak, D.; Isenovic, E.R. Link between Metabolic Syndrome and Insulin Resistance. Curr. Vasc. Pharm. 2017, 15, 30–39. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, X.; Zhang, Q.; Zou, R. Diabetes mellitus and risk of falls in older adults: A systematic review and meta-analysis. Age Ageing 2016, 45, 761–767. [Google Scholar] [CrossRef]
- Tseng, M.; Fang, C.Y. Acculturation and Insulin Resistance among US Chinese Immigrant Women. Ethn. Dis. 2015, 25, 443–450. [Google Scholar] [CrossRef]
- Rosenberg, D.E.; Jabbour, S.A.; Goldstein, B.J. Insulin resistance, diabetes and cardiovascular risk: Approaches to treatment. Diabetes Obes. Metab. 2005, 7, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Oikonomou, C.; Nychas, G.; Dimitriadis, G.D. Effects of Diet, Lifestyle, Chrononutrition and Alternative Dietary Interventions on Postprandial Glycemia and Insulin Resistance. Nutrients 2022, 14, 823. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Hlozkova, A.; Fleeman, R.; Fletcher, K.; Holubkov, R.; Barnard, N.D. Fat Quantity and Quality, as Part of a Low-Fat, Vegan Diet, Are Associated with Changes in Body Composition, Insulin Resistance, and Insulin Secretion. A 16-Week Randomized Controlled Trial. Nutrients 2019, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Boucher, B.J.; John, W.G.; Noonan, K. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am. J. Clin. Nutr. 2004, 80, 820–825. [Google Scholar] [CrossRef]
- Kumar, S.; Davies, M.; Zakaria, Y.; Mawer, E.B.; Gordon, C.; Olukoga, A.O.; Boulton, A.J. Improvement in glucose tolerance and beta-cell function in a patient with vitamin D deficiency during treatment with vitamin D. Postgrad. Med. J. 1994, 70, 440–443. [Google Scholar] [CrossRef]
- Pramono, A.; Jocken, J.W.E.; Blaak, E.E.; van Baak, M.A. The Effect of Vitamin D Supplementation on Insulin Sensitivity: A Systematic Review and Meta-analysis. Diabetes Care 2020, 43, 1659–1669. [Google Scholar] [CrossRef]
- Gallagher, J.C.; Yalamanchili, V.; Smith, L.M. The effect of vitamin D supplementation on serum 25OHD in thin and obese women. J. Steroid Biochem. Mol. Biol. 2013, 136, 195–200. [Google Scholar] [CrossRef]
- Pereira-Santos, M.; Costa, P.R.; Assis, A.M.; Santos, C.A.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2015, 16, 341–349. [Google Scholar] [CrossRef]
- Wright, C.S.; Weinheimer-Haus, E.M.; Fleet, J.C.; Peacock, M.; Campbell, W.W. The Apparent Relation between Plasma 25-Hydroxyvitamin D and Insulin Resistance is Largely Attributable to Central Adiposity in Overweight and Obese Adults. J. Nutr. 2015, 145, 2683–2689. [Google Scholar] [CrossRef]
- Nsiah-Kumi, P.A.; Erickson, J.M.; Beals, J.L.; Ogle, E.A.; Whiting, M.; Brushbreaker, C.; Borgeson, C.D.; Qiu, F.; Yu, F.; Larsen, J.L. Vitamin D Insufficiency Is Associated with Diabetes Risk in Native American Children. Clin. Pediatr. 2012, 51, 146–153. [Google Scholar] [CrossRef]
- Tao, S.; Yuan, Q.; Mao, L.; Chen, F.L.; Ji, F.; Cui, Z.H. Vitamin D deficiency causes insulin resistance by provoking oxidative stress in hepatocytes. Oncotarget 2017, 8, 67605–67613. [Google Scholar] [CrossRef] [PubMed]
- Rasic-Milutinovic, Z.; Perunicic-Pekovic, G.; Pljesa, S. Clinical significance and pathogenic mechanisms of insulin resistance in chronic renal insufficiency (part II): Pathogenic factors of insulin resistance in chronic renal insufficiency. Med. Pregl. 2000, 53, 159–163. [Google Scholar] [PubMed]
- Dorothy, T.; Shawn, S.D. Vitamin D: Emerging new roles in insulin sensitivity. Nutr. Res. Rev. 2009, 22, 82–92. [Google Scholar]
- Tai, K.; Need, A.G.; Horowitz, M.; Chapman, I.M. Vitamin D, glucose, insulin, and insulin sensitivity. Nutrition 2007, 24, 279–285. [Google Scholar] [CrossRef]
- Santos, L.R.d.; Lima, A.G.A.; Braz, A.F.; Melo, S.R.D.; Morais, J.B.S.; Severo, J.S.; de Oliveira, A.R.S.; Cruz, K.J.C.; Marreiro, D.D.N. Role of vitamin D in insulin resistance in obese individuals. Nutrire 2017, 42, 17. [Google Scholar] [CrossRef]
- Sung, C.C.; Liao, M.T.; Lu, K.C.; Wu, C.C. Role of Vitamin D in Insulin Resistance. J. Biomed. Biotechnol. 2012, 2012, 614395. [Google Scholar] [CrossRef]

| 25-Hydroxyvitamin D Concentration, Median (IQR), nmol/L | p Value a | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | No. (%) a | Total 25-Hydroxyvitamin D | 25-Hydroxyvitamin D3 | 3-epi-25-Hydroxyvitamin D3 | Total 25-Hydroxyvitamin D | 25-Hydroxyvitamin D3 | 3-epi-25-Hydroxyvitamin D3 |
| Overall | 6026 | 62.5 (45.7–81.1) | 58.8 (41.6–77.2) | 3.3 (2.0–5.0) | |||
| Sex | 0.027 | 0.021 | <0.001 | ||||
| Men | 3252 (54.0) | 63.1 (47.2–80.9) | 59.4 (43.1–77.3) | 3.4 (2.1–5.2) | |||
| Women | 2875 (46.0) | 61.6 (43.8–81.4) | 57.9 (40.2–77.1) | 3.2 (1.9–4.8) | |||
| Age, year | <0.001 | <0.001 | <0.001 | ||||
| 18–39 | 1663 (27.6) | 56.9 (42.0–71.2) | 54.5 (39.4–69.1) | 3.0 (1.9–4.3) | |||
| 40–59 | 2348 (39.0) | 60.2 (43.8–77.5) | 56.4 (40.0–73.5) | 3.1 (1.9–4.7) | |||
| ≥60 | 2015 (33.4) | 73.3 (53.4–92.4) | 67.2 (46.3–87.4) | 3.9 (2.4–6.0) | |||
| Race | <0.001 | <0.001 | <0.001 | ||||
| Chicano | 834 (13.8) | 53.7 (41.0–68.4) | 50.4 (37.9–64.9) | 2.9 (1.8–4.1) | |||
| Other Hispanics | 653 (10.8) | 61.1 (49.3–73.9) | 57.4 (45.5–70.5) | 3.3 (2.2–4.4) | |||
| Non-Hispanic | 3740 (62.1) | 66.2 (47.1–85.4) | 62.2 (42.4–81.5) | 3.5 (2.1–5.4) | |||
| Other Races | 799 (13.3) | 58.3 (43.6–77.7) | 54.0 (39.7–73.3) | 3.0 (1.9–4.4) | |||
| Body mass index | <0.001 | <0.001 | <0.001 | ||||
| <25 | 1791 (29.7) | 65.8 (48.0–85.2) | 61.9 (44.0–82.2) | 3.4 (2.2–5.3) | |||
| 25≤30 | 1956 (32.5) | 64.0 (48.0–82.0) | 60.3 (44.7–78.6) | 3.4 (2.2–5.3) | |||
| ≥30 | 2279 (37.8) | 68.8 (42.5–76.6) | 54.4 (37.4–71.7) | 3.0 (1.8–4.6) | |||
| Drinking | 0.531 | 0.052 | <0.001 | ||||
| Yes | 4290 (71.2) | 62.8 (45.8–80.9) | 59.5 (42.2–77.4) | 3.4 (2.1–5.1) | |||
| No | 1736 (28.8) | 61.7 (45.6–82.1) | 56.8 (40.1–76.8) | 3.1 (1.9–4.7) | |||
| Diabetes | 0.009 | 0.146 | 0.856 | ||||
| Yes | 787 (13.1) | 64.9 (46.7–84.8) | 58.3 (39.9–77.0) | 3.3 (2.0–4.9) | |||
| No | 5239 (86.9) | 62.1 (45.5–80.8) | 58.9 (41.9–77.3) | 3.3 (2.0–5.0) | |||
| Insulin resistance | <0.001 | <0.001 | <0.001 | ||||
| Yes | 3664 (60.8) | 60.5 (44.4–78.5) | 56.1 (40.1–74.7) | 3.2 (2.0–4.9) | |||
| No | 2362 (39.2) | 65.8 (48.6–84.9) | 62.3 (44.6–81.3) | 3.4 (2.2–5.2) | |||
| Smoking | 0.069 | 0.204 | <0.001 | ||||
| Yes | 2587 (42.9) | 63.9 (46.2–82.8) | 60.0 (41.8–78.9) | 3.5 (2.1–5.3) | |||
| No | 3439 (57.1) | 61.4 (45.4–79.8) | 57.7 (41.5–76.3) | 3.1 (2.0–4.8) | |||
| Creatinine (urine) mg/dL | <0.001 | <0.001 | <0.001 | ||||
| <0.81 | 1971 (32.7) | 64.8 (49.3–82.9) | 61.1 (45.8–79.7) | 3.4 (2.2–5.2) | |||
| 0.81–1.44 | 2038 (33.8) | 62.1 (45.8–80.1) | 58.6 (41.7–76.4) | 3.3 (2.0–5.0) | |||
| ≥1.44 | 2017 (33.5) | 60.8 (42.7–80.4) | 56.0 (37.8–75.5) | 3.2 (1.8–4.8) | |||
| Albumin (urine) (mg/L) | <0.001 | <0.001 | <0.001 | ||||
| <5.6 | 1999 (33.2) | 67.0 (50.3–86.8) | 62.7 (46.0–82.3) | 3.5 (2.2–5.3) | |||
| 5.6–13.2 | 2014 (33.4) | 62.6 (45.5–81.2) | 58.9 (41.7–77.8) | 3.3 (2.0–4.9) | |||
| ≥13.2 | 2013 (33.4) | 58.1 (42.1–74.6) | 54.6 (38.2–71.5) | 3.1 (1.9–4.7) | |||
| Cholesterol (mmol/L) | <0.001 | <0.001 | 0.06 | ||||
| <4.4 | 1983 (32.9) | 60.2 (44.2–77.5) | 56.8 (40.7–73.7) | 3.18 (2.0–4.9) | |||
| 4.4–5.25 | 2000 (33.2) | 63.5 (45.9–82.4) | 60.0 (41.9–78.4) | 3.4 (2.1–5.1) | |||
| ≥5.25 | 2043 (33.9) | 63.9 (63.9–83.5) | 59.8 (42.4–79.3) | 3.3 (2.1–4.9) | |||
| Education status | <0.001 | <0.001 | <0.001 | ||||
| <High school | 1335 (22.2) | 58.5 (43.9–75.3) | 54.6 (40.2–71.0) | 3.0 (1.9–4.5) | |||
| High school | 1300 (21.6) | 60.8 (43.9–80.9) | 57.3 (39.7–76.6) | 3.2 (2.0–4.9) | |||
| >High school | 3391 (56.3) | 64.7 (47.5–83.0) | 60.9 (43.079.4) | 3.4 (2.1–5.3) | |||
| Physical activities | 0.093 | <0.001 | <0.001 | ||||
| Yes | 3896 (64.7) | 62.8 (46.7–80.9) | 59.7 (43.2–77.8) | 3.4 (2.1–5.2) | |||
| No | 2130 (35.3) | 61.9 (44.2–82.0) | 56.8 (38.5–76.1) | 3.1 (1.8–4.7) | |||
| Dietary intake | |||||||
| Carbohydrate (g) | 0.001 | 0.117 | 0.359 | ||||
| <177.81 | 2006 (33.3) | 63.0 (45.1–82.8) | 58.1 (40.5–78.4) | 3.3 (2.0–5.0) | |||
| 177.81–263.25 | 2013 (33.4) | 64.2 (46.4–82.8) | 60.0 (42.3–78.6) | 3.3 (2.0–5.2) | |||
| ≥263.25 | 2007 (33.3) | 61.0 (45.5–78.2) | 58.3 (42.5–75.3) | 3.3 (2.1–4.8) | |||
| Protein (g) | 0.753 | 0.112 | 0.096 | ||||
| <58.41 | 2006 (33.3) | 62.5 (44.5–82.4) | 57.9 (40.2–77.4) | 3.2 (1.9–4.9) | |||
| 58.41–85.58 | 1981 (32.9) | 62.5 (46.2–81.5) | 59.2 (42.4–78.4) | 3.3 (2.1–5.1) | |||
| ≥85.58 | 2039 (33.8) | 62.4 (46.3–79.7) | 59.1 (42.8–76.0) | 3.3 (2.1–4.9) | |||
| Fat (g) | 0.149 | 0.178 | 0.287 | ||||
| <54.16 | 2006 (33.3) | 62.4 (44.8–81.4) | 57.4 (40.3–76.7) | 3.1 (2.0–4.9) | |||
| 54.16–83.33 | 2013 (33.4) | 62.9 (46.4–82.9) | 59.3 (42.2–78.8) | 3.3 (2.1–5.0) | |||
| ≥83.33 | 2007 (33.3) | 62.1 (45.8–79.6) | 59.4 (42.7–76.1) | 3.3 (2.1–5.1) | |||
| Dietary fiber (g) | <0.001 | <0.001 | <0.001 | ||||
| <11.15 | 2004 (33.3) | 58.7 (42.1–78.0) | 54.4 (36.9–72.8) | 3.1 (1.8–4.7) | |||
| 11.15–18.20 | 2005 (33.3) | 63.3 (46.4–82.2) | 59.5 (42.6–78.7) | 3.3 (2.1–5.0) | |||
| ≥18.20 | 2017 (33.4) | 64.8 (48.8–83.0) | 61.2 (45.5–79.2) | 3.5 (2.2–5.2) | |||
| Vitamin D (μg) | <0.001 | <0.001 | <0.001 | ||||
| <2.10 | 1976 (32.8) | 57.8 (41.8–77.7) | 53.4 (37.3–72.8) | 3.1 (1.8–4.7) | |||
| 2.10–4.70 | 2030 (33.7) | 63.0 (44.9–81.7) | 58.8 (40.7–77.7) | 3.2 (2.0–5.1) | |||
| ≥4.70 | 2020 (33.5) | 66.0 (50.5–84.1) | 62.3 (47.2–80.1) | 3.5 (2.3–5.2) | |||
| Calcium (mg) | <0.001 | <0.001 | <0.001 | ||||
| <617.0 | 2005 (33.3) | 58.5 (42.0–78.2) | 53.3 (36.9–72.8) | 3.0 (1.8–4.7) | |||
| 617.0–975.84 | 2014 (33.4) | 63.7 (47.0–83.3) | 59.8 (42.6–78.8) | 3.4 (2.1–5.0) | |||
| ≥975.84 | 2007 (33.3) | 64.2 (48.6–81.4) | 61.3 (45.4–78.7) | 3.5 (2.3–5.3) | |||
| With Insulin Resistance/Without Insulin Resistance | 70th vs. 30th Percentile | 80th vs. 20th Percentile | ||
|---|---|---|---|---|
| 25-hydroxyvitamin D (nmol/L) | 60.5/65.8 | 47.5/80.7 | 40.3/88.8 | |
| Model 1 a | 1 [Reference] | 0.32 (0.24–0.43) | 0.34 (0.25–0.46) | 0.36 (0.26–0.49) |
| Model 2 b | 1 [Reference] | 0.56 (0.40–0.78) | 0.58 (0.41–0.82) | 0.59 (0.41–0.86) |
| Model 3 c | 1 [Reference] | 0.57 (0.41–0.80) | 0.58 (0.40–0.83) | 0.61 (0.42–0.90) |
| Model 4 d | 1 [Reference] | 0.61 (0.43–0.86) | 0.62 (0.43–0.90) | 0.67 (0.45–0.99) |
| 25-hydroxyvitamin D3 (nmol/L) | 56.1/62.3 | 43.4/77.2 | 35.8/85.4 | |
| Model 1 a | 1 [Reference] | 0.34 (0.26–0.44) | 0.35 (0.27–0.46) | 0.35 (0.27–0.47) |
| Model 2 b | 1 [Reference] | 0.63 (0.46–0.85) | 0.65 (0.47–0.90) | 0.66 (0.47–0.93) |
| Model 3 c | 1 [Reference] | 0.64 (0.47–0.86) | 0.66 (0.48–0.92) | 0.70 (0.49–0.98) |
| Model 4 d | 1 [Reference] | 0.67 (0.49–0.91) | 0.71 (0.51–0.99) | 0.75 (0.53–1.08) |
| 3-epi-25-hydroxyvitamin D3 (nmol/L) | 3.2/3.9 | 2.2/4.7 | 1.7/5.9 | |
| Model 1 a | 1 [Reference] | 0.64 (0.53–0.77) | 0.51 (0.41–0.63) | 0.60 (0.49–0.73) |
| Model 2 b | 1 [Reference] | 0.99 (0.80–1.24) | 0.81 (0.63–1.04) | 0.92 (0.72–1.18) |
| Model 3 c | 1 [Reference] | 1.00 (0.82–1.26) | 0.82 (0.64–1.05) | 0.96 (0.75–1.23) |
| Model 4 d | 1 [Reference] | 1.05 (0.92–1.18) | 0.85 (0.66–1.10) | 1.00 (0.78–1.28) |
| Tertile 1 | Tertile 2 | Tertile 3 | p Value for Trend a | |
|---|---|---|---|---|
| Total 25-hydroxyvitamin D (nmol/L) | <51.5 | 51.5 to 74.7 | >74.7 | |
| With Insulin resistance/without Insulin resistance, No. | 1324/669 | 1238/807 | 1102/886 | |
| Model 1 b | 1 [Reference] | 0.76 (0.67–0.87) | 0.59 (0.52–0.68) | <0.001 |
| Model 2 c | 1 [Reference] | 0.82 (0.71–0.95) | 0.76 (0.65–0.89) | <0.001 |
| Model 3 d | 1 [Reference] | 0.82 (0.71–0.96) | 0.77 (0.66–0.90) | <0.001 |
| Model 4 e | 1 [Reference] | 0.84 (0.72–0.97) | 0.79 (0.67–0.93) | <0.001 |
| 25-hydroxyvitamin D3 (nmol/L) | <47.4 | 47.4 to 70.0 | >70.0 | |
| With Insulin resistance/without Insulin resistance, No. | 1324/665 | 1391/918 | 949/779 | |
| Model 1 b | 1 [Reference] | 0.77 (0.67–0.88) | 0.58 (0.51–0.67) | <0.001 |
| Model 2 c | 1 [Reference] | 0.84 (0.73–0.98) | 0.77 (0.66–0.90) | <0.001 |
| Model 3 d | 1 [Reference] | 0.85 (0.73–0.99) | 0.78 (0.66–0.91) | <0.001 |
| Model 4 e | 1 [Reference] | 0.86 (0.74–1.00) | 0.79 (0.68–0.93) | <0.001 |
| 3-epi-25-hydroxyvitamin D3 (nmol/L) | <2.2 | 2.2 to 4.0 | >4.0 | |
| With Insulin resistance/without Insulin resistance, No. | 1093/613 | 1253/821 | 1318/928 | |
| Model 1 b | 1 [Reference] | 0.83 (0.73–0.95) | 0.77 (0.68–0.88) | <0.001 |
| Model 2 c | 1 [Reference] | 0.96 (0.83–1.11) | 1.05 (0.90–1.20) | <0.001 |
| Model 3 d | 1 [Reference] | 0.97 (0.83–1.12) | 1.06 (0.91–1.23) | <0.001 |
| Model 4 e | 1 [Reference] | 0.98 (0.84–1.14) | 1.08 (0.92–1.26) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Huang, R. Associations of Serum Total 25OHD, 25OHD3, and epi-25OHD3 with Insulin Resistance: Cross-Sectional Analysis of the National Health and Nutrition Examination Survey, 2011–2016. Nutrients 2022, 14, 3526. https://doi.org/10.3390/nu14173526
Zhou M, Huang R. Associations of Serum Total 25OHD, 25OHD3, and epi-25OHD3 with Insulin Resistance: Cross-Sectional Analysis of the National Health and Nutrition Examination Survey, 2011–2016. Nutrients. 2022; 14(17):3526. https://doi.org/10.3390/nu14173526
Chicago/Turabian StyleZhou, Meiling, and Ruixue Huang. 2022. "Associations of Serum Total 25OHD, 25OHD3, and epi-25OHD3 with Insulin Resistance: Cross-Sectional Analysis of the National Health and Nutrition Examination Survey, 2011–2016" Nutrients 14, no. 17: 3526. https://doi.org/10.3390/nu14173526
APA StyleZhou, M., & Huang, R. (2022). Associations of Serum Total 25OHD, 25OHD3, and epi-25OHD3 with Insulin Resistance: Cross-Sectional Analysis of the National Health and Nutrition Examination Survey, 2011–2016. Nutrients, 14(17), 3526. https://doi.org/10.3390/nu14173526





