A Review of the Effect of Preparations from Vegetables of the Asteraceae Family and Cucurbitaceae Family on the Cardiovascular System and Its Diseases
Abstract
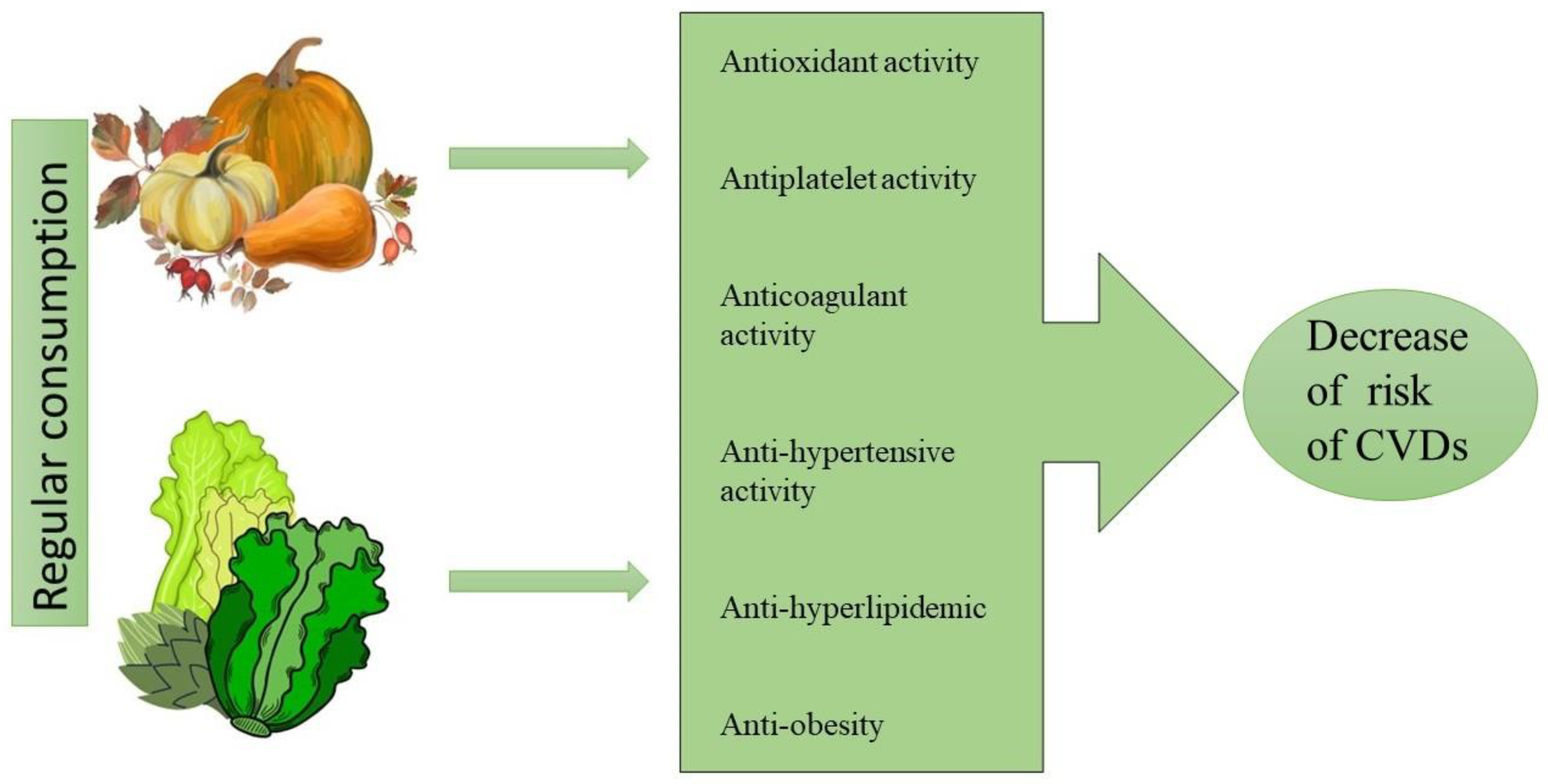
1. Introduction
2. Oxidative Stress and Cardiovascular System; the Role of Antioxidants
3. Pharmacological Characteristics of the Asteraceae Family of Plants
3.1. Vegetables from the Asteraceae Family and Their Effect on Oxidative Stress
3.1.1. In Vitro Studies
3.1.2. In Vivo Studies
3.2. Vegetables from the Asteraceae Family and Their Antiplatelet Activity
3.2.1. In Vitro Studies
3.2.2. In Vivo Studies
3.3. Anticoagulant Activity of Asteraceae Vegetables
4. Characteristics of the Biological Properties of Plants from the Cucurbitaceae Family
4.1. Vegetables from the Cucurbitaceae Family and Their Effect on Oxidative Stress
4.1.1. In Vitro Studies
4.1.2. In Vivo Studies
4.2. Vegetables from the Cucurbitaceae Family and Their Antiplatelet Activity
4.2.1. In Vitro Studies
4.2.2. In Vivo Studies
4.3. Anticoagulant Activity of Cucurbitaceae Vegetables
5. Anti-Hyperlipidemia Effects of Preparations from Vegetables of the Asteraceae Family and Cucurbitaceae
6. Other Cardioprotective Properties (Anti-Hypertension and Anti-Obesity) of Preparations from Plants of the Asteraceae Family and Cucurbitaceae Family
| Vegetables | Cardioprotective Activity | Type of Solvent | Dose | Biological Material | Studies | References |
|---|---|---|---|---|---|---|
| Antioxidant Activity | ||||||
| Cichorium intybus, Lactuca sativa, Helianthus tuberosus (Asteraceae) | Scavenging free radical, inhibition of lipid peroxidation, and carbonylation of plasma protein | methanol | 1–50 µg/mL | Human plasma | in vitro | [30] |
| Cichorium intybus (Asteraceae) | Scavenging free radicals | methanol | 100 mg/mL | Human serum | in vitro | [27] |
| Lactuca sativa, (Asteraceae) | Scavenging free radicals | water | 20 mg/mL | Human serum | in vitro | [20] |
| Launaea taraxacifolia, (Asteraceae) | Decrease in free radical production | ethanol-aqueous | 1–20 μg/μL | PLB985 cells | in vitro | [31] |
| Cucurbita pepo, Cucurbita pepo convar. Giromontina, Cucumis sativus, Cucurbita pepo var. patisoniana (Cucurbitaceae) | Scavenging free radicals, inhibition of lipid peroxidation, and carbonylation of plasma protein | methanol | 1–50 µg/mL | Human plasma | in vitro | [44] |
| Momordica dioica (Cucurbitaceae) | Scavenging free radicals | methanol/water (70%, 50%, 30%) | 1–5000 µg/mL | - | in vitro | [47] |
| Cucumis melo var. agrestis (Cucurbitaceae) | Scavenging free radicals | methanol/water (70%, 50%, 30%) | 1–5000 µg/mL | - | in vitro | [47] |
| Citrullus colocynthus (Cucurbitaceae) | Scavenging free radicals | methanol/water (70%, 50%, 30%) | 1–5000 µg/mL | - | in vitro | [47] |
| Momordica charantia (Cucurbitaceae) | Inhibition of lipid peroxidation, scavenging free radicals | methanol, chloroform | 100 mg/mL | Human plasma | in vitro | [48] |
| Cichorium intybus (Asteraceae) | Decrease in biomarkers of oxidative stress in blood | methanol | 100%, 50%, 25% | Male albino Wistar rats | in vivo | [27] |
| Cutrullus colocynthis (Cucurbitaceae) | Decrease in lipid peroxidation biomarkers in liver tissue | no solvent/dry extract | 300 mg/kg | Diabetic albino rats | in vivo | [49] |
| Cucurbita ficifolia (Cucurbitaceae) | Decrease in lipid peroxidation | water | 300 mg/kg | Male Sprague–Dawley rats | in vivo | [50] |
| Antiplatelet activity | ||||||
| Cichorium intybus, Lactuca sativa, Helianthus tuberosus (Asteraceae) | Inhibition of adhesion of thrombin- and ADP-activated platelets to fibrinogen | methanol | 1–50 µg/mL | Human blood platelets | in vitro | [33] |
| Cucurbita pepo, Cucurbita pepo convar. Giromontina, Cucumis sativus, Cucurbita pepo var. patisoniana (Cucurbitaceae) | Inhibition of adhesion of thrombin- and ADP-activated platelets to collagen and fibrinogen | methanol | 1–50 µg/mL | Human blood platelets | in vitro | [51] |
| Cichorium intybus (Asteraceae) | Inhibition of platelet aggregation | water | 300 mL of 20 g chicory coffee | Human | in vivo | [34] |
| Lagenaria siceriaria (Cucurbitaceae) | Inhibition of ADP-induced platelet aggregation | ethanol | 250, 5000 and 1000 mg/kg | Swiss albino mouse | in vivo | [52] |
| Anticoagulant activity | ||||||
| Cucurbita pepo var. patisoniana (Cucucrbitaceae) | Inhibition of total thrombus formation | methanol | 50 µg/mL | Whole blood | in vitro | [51] |
| Anti-hyperlipidemic activity | ||||||
| Launaea taraxacifolia (Asteraceae) | Inhibition of lipid accumulation | ethanol-aqueous | 20 μg/μL | HepG2 cells | in vitro | [31] |
| Cichorium intybus (Asteraceae) | Improvement of lipid profile | no solvent | 10 g/100 g of diet | male Wistar rats | in vivo | [54] |
| Citrullus colocynthis (Cucurbitaceae) | Decrease in serum triglyceride and cholesterol | methanol | 60 mg/kg | Sprague–Dawley rats | in vivo | [59] |
| Cucurbita moschata (Cucurbitaceae) | Decrease in triglyceride and cholesterol in blood | ethanol-aqueous | 500 mg/kg | Adult mice | in vivo | [62] |
| Antihypertensive activity | ||||||
| Cucurbita pepo (Cucurbitaceae) | Decrease of nitric oxide | no solvent | 40 or 100 mg/kg of body weight | Sprague–Dawley rats (serum) | in vivo | [57] |
| Cynara cardunculus (Asteraceae) | Decrease in blood pressure | water | 18% juice | Patients with mild-hypertension | in vivo | [56] |
| Cucumis sativus (Cucurbitaceae) | modulation of endothelium-derived relaxing factors | ethanol-aqueous | 50–300 mg/kg | Sprague–Dawley rats | in vivo | [59] |
| Anti-obesity and body weight control | ||||||
| Cichorium intybus (Asteraceae) | Decrease in triglycerides content | methanol | 1 pg/mL to 10 μg/mL | 3T3-L1 preadipocytes cell | in vitro | [60] |
| Cucurbita pepo (Cucurbitaceae) | Decrease in LDL and triglycerides | methanol | 100, 200, 400 mg/kg | male Wistar rats | in vivo | [63] |
| Cucurbita pepo (Cucurbitaceae) | Decrease in blood pressure, lower body weight, and LDL level | ethanol/methanol | 100 mg/kg | male Wistar rats | in vivo | [58]. |
7. Toxicity of Vegetable Preparations from Cucurbitaceae and Asteraceae Families
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, H.H.; Heemskerk, J.W.M.; Levi, M.; Reitsma, P.H. New Fundamentals in Hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.-H.; Sim, E.-H.; Goh, R.-Y.; Park, J.-I.; Han, J.-Y. Platelet Activation: The Mechanisms and Potential Biomarkers. BioMed Res. Int. 2016, 2016, 9060143. [Google Scholar] [CrossRef] [PubMed]
- Jr, R.; Llamas, D.M.; Alcantara, S.; Ciocson, K.S.; Escabusa, C.; Rivera, J.R.; Santos, S. Anticoagulant Properties of Medicinal Plants of Asteraceae: A Systematic Review. Asian J. Biol. Life Sci. 2021, 10, 231–237. [Google Scholar]
- Zhong, S.; Li, L.; Shen, X.; Li, Q.; Xu, W.; Wang, X.; Tao, Y.; Yin, H. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic. Biol. Med. 2019, 144, 266–278. [Google Scholar] [CrossRef]
- Wadhera, R.K.; Steen, D.L.; Khan, I.; Giugliano, R.P.; Foody, J.M. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J. Clin. Lipidol. 2015, 10, 472–489. [Google Scholar] [CrossRef]
- Michel, J.; Rani, N.Z.A.; Husain, K. A Review on the Potential Use of Medicinal Plants From Asteraceae and Lamiaceae Plant Family in Cardiovascular Diseases. Front. Pharmacol. 2020, 11, 852. [Google Scholar] [CrossRef]
- Asgary, S.; Rastqar, A.; Keshvari, M. Functional Food and Cardiovascular Disease Prevention and Treatment: A Review. J. Am. Coll. Nutr. 2018, 37, 429–455. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. Vegetables from the Cucurbitaceae family and their products: Positive effect on human health. Nutrition 2020, 78, 110788. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef]
- Tang, G.-Y.; Meng, X.; Li, Y.; Zhao, C.-N.; Liu, Q.; Li, H.-B. Effects of Vegetables on Cardiovascular Diseases and Related Mechanisms. Nutrients 2017, 9, 857. [Google Scholar] [CrossRef]
- Olas, B. Anti-Aggregatory Potential of Selected Vegetables—Promising Dietary Components for the Prevention and Treatment of Cardiovascular Disease. Adv. Nutr. Int. Rev. J. 2019, 10, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-C.; Sheen, J.-M.; Hu, W.L.; Hung, Y.-C. Polyphenols and Oxidative Stress in Atherosclerosis-Related Ischemic Heart Disease and Stroke. Oxidative Med. Cell. Longev. 2017, 2017, 8526438. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Giri, L.; Suyal, R.; Jugran, A.K.; Zucca, P.; Rescigno, A.; Peddio, S.; Bobiş, O.; et al. Antioxidant potential of family Cucurbitaceae with special emphasis on Cucurbita genus: A key to alleviate oxidative stress-mediated disorders. Phytother. Res. 2021, 35, 3533–3557. [Google Scholar] [CrossRef] [PubMed]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, S.C.; Răchișan, A.L.; Negrean, V.; Perné, M.-G.; Donca, V.I.; Alexescu, T.-G.; Para, I.; et al. The effects of favonoids in cardiovascular diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. ChemInform Abstract: The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Jafarinia, M.; Jafarinia, M. A Review of Medicinal Properties of some Asteraceae Family Plants on Immune System. Rep. Health Care 2019, 5, 1–7. [Google Scholar]
- Achika, J.I.; Arthur, D.E.; Gerald, I.; Adedayo, A. A Review on the Phytoconstituents and Related Medicinal Properties of Plants in the Asteraceae Family. IOSR J. Appl. Chem. 2014, 7, 1–8. [Google Scholar] [CrossRef]
- Abdalla, M.; Li, F.; Wenzel-Storjohann, A.; Sulieman, S.; Tasdemir, D.; Mühling, K. Comparative Metabolite Profile, Biological Activity and Overall Quality of Three Lettuce (Lactuca sativa L., Asteraceae) Cultivars in Response to Sulfur Nutrition. Pharmaceutics 2021, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Gostin, A.-I.; Waisundara, V.Y. Edible flowers as functional food: A review on artichoke (Cynara cardunculus L.). Trends Food Sci. Technol. 2019, 86, 381–391. [Google Scholar] [CrossRef]
- Jedrejek, D.; Kontek, B.; Lis, B.; Stochmal, A.; Olas, B. Evaluation of antioxidant activity of phenolic fractions from the leaves and petals of dandelion in human plasma treated with H2O2 and H2O2/Fe. Chem. Biol. Interact. 2017, 262, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lis, B.; Rolnik, A.; Jedrejek, D.; Soluch, A.; Stochmal, A.; Olas, B. Dandelion (Taraxacum officinale L.) root components exhibit anti-oxidative and antiplatelet action in an in vitro study. J. Funct. Foods 2019, 59, 16–24. [Google Scholar] [CrossRef]
- Majewski, M.; Lis, B.; Juśkiewicz, J.; Ognik, K.; Borkowska-Sztachańska, M.; Jedrejek, D.; Stochmal, A.; Olas, B. Phenolic Fractions from Dandelion Leaves and Petals as Modulators of the Antioxidant Status and Lipid Profile in an In Vivo Study. Antioxidants 2020, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Lis, B.; Olas, B. Pro-health activity of dandelion (Taraxacum officinale L.) and its food products—History and present. J. Funct. Foods 2019, 59, 40–48. [Google Scholar] [CrossRef]
- Atawodi, S.E.; Adepoju, O.A.; Nzelibe, H.C. Antihyperglycaemic and hypolipidemic effect of methanol extracts of Ageratum conyzoides L. (Asteraceae) in normal and diabetic rats. Trop. J. Pharm. Res. 2017, 16, 989. [Google Scholar] [CrossRef][Green Version]
- Epure, A.; E E Pârvu, A.; Vlase, L.; Benedec, D.; Hanganu, D.; Gheldiu, A.-M.; Toma, V.A.; Oniga, I. Phytochemical Profile, Antioxidant, Cardioprotective and Nephroprotective Activity of Romanian Chicory Extract. Plants 2021, 10, 64. [Google Scholar] [CrossRef]
- Abbas, Z.K.; Saggu, S.; Sakeran, M.I.; Zidan, N.; Rehman, H.; Ansari, A.A. Phytochemical, antioxidant and mineral composition of hydroalcoholic extract of chicory (Cichorium intybus L.) leaves. Saudi J. Biol. Sci. 2014, 22, 322–326. [Google Scholar] [CrossRef]
- Awwad, A.; Poucheret, P.; Idres, A.Y.; Bidel, L.; Tousch, D. The bitter Asteraceae: An interesting approach to delay the metabolic syndrome progression. NFS J. 2020, 18, 29–38. [Google Scholar] [CrossRef]
- Rolnik, A.; Soluch, A.; Kowalska, I.; Olas, B. Antioxidant and hemostatic properties of preparations from Asteraceae family and their chemical composition—Comparative studies. Biomed. Pharmacother. 2021, 142, 111982. [Google Scholar] [CrossRef]
- Koukoui, O.; Agbangnan, P.; Boucherie, S.; Yovo, M.; Nusse, O.; Combettes, L.; Sohounhloué, D. Phytochemical Study and Evaluation of Cytotoxicity, Antioxidant and Hypolipidemic Properties of Launaea taraxacifolia Leaves Extracts on Cell Lines HepG2 and PLB985. Am. J. Plant Sci. 2015, 6, 1768–1779. [Google Scholar] [CrossRef]
- Nignpense, B.E.; Chinkwo, K.A.; Blanchard, C.L.; Santhakumar, A.B. Polyphenols: Modulators of Platelet Function and Platelet Microparticle Generation? Int. J. Mol. Sci. 2019, 21, 146. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, A.; Stochmal, A.; Olas, B. The in vitro anti-platelet activities of plant extracts from the Asteraceae family. Biomed. Pharmacother. 2022, 149. [Google Scholar] [CrossRef]
- Schumacher, E.; Vigh, E.; Molnár, V.; Kenyeres, P.; Fehér, G.; Késmárky, G.; Tóth, K.; Garai, J. Thrombosis Preventive Potential of Chicory Coffee Consumption: A Clinical Study. Phytother. Res. 2011, 25, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Singha, S.; Kar, A.; Chanda, J.; Banerjee, S.; Dasgupta, B.; Haldar, P.K.; Sharma, N. Therapeutic importance of Cucurbitaceae: A medicinally important family. J. Ethnopharmacol. 2021, 282, 114599. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Mahajan, N.; Sah, S.; Nath, S.K.; Paudyal, B. Oxidative stress and its biomarkers in systemic lupus erythematosus. J. Biomed. Sci. 2014, 21, 23. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A. Edible seeds from Cucurbitaceae family as potential functional foods: Immense promises, few concerns. Biomed. Pharmacother. 2017, 91, 330–337. [Google Scholar] [CrossRef]
- Salehi, B.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jugran, A.K.; Calina, D.; et al. Cucurbits Plants: A Key Emphasis to Its Pharmacological Potential. Molecules 2019, 24, 1854. [Google Scholar] [CrossRef]
- Dhiman, K.; Gupta, A.; Sharma, D.K.; Gill, N.S.; Goyal, A. A review on the medicinally important plants of the family Cucurbitaceae. Asian. J. Clin. Nutr. 2012, 4, 16–26. [Google Scholar] [CrossRef]
- Bortolotti, M.; Mercatelli, D.; Polito, L. Momordica charantia, a Nutraceutical Approach for Inflammatory Related Diseases. Front. Pharmacol. 2019, 10, 486. [Google Scholar] [CrossRef]
- Montesano, D.; Rocchetti, G.; Putnik, P.; Lucini, L. Bioactive profile of pumpkin: An overview on terpenoids and their health-promoting properties. Curr. Opin. Food Sci. 2018, 22, 81–87. [Google Scholar] [CrossRef]
- Chamundeeswari, D.; Vasantha, J.; Iman, R.; Priya, B.; Chithra, R.; Shalini, K.; Sharon, V. In vitroantiplatelet activity-guided fractionation of aerial parts of Melothria maderaspatana. Indian J. Pharm. Sci. 2006, 68, 668. [Google Scholar] [CrossRef]
- Chekroun, E.; Benariba, N.; Adida, H.; Bechiri, A.; Azzi, R.; Djaziri, R. Antioxidant activity and phytochemical screening of two Cucurbitaceae: Citrullus colocynthis fruits and Bryonia dioica roots. Asian Pac. J. Trop. Dis. 2015, 5, 632–637. [Google Scholar] [CrossRef]
- Rolnik, A.; Kowalska, I.; Soluch, A.; Stochmal, A.; Olas, B. Comparative Phytochemical, Antioxidant and Haemostatic Studies of Preparations from Selected Vegetables from Cucurbitaceae Family. Molecules 2020, 25, 4326. [Google Scholar] [CrossRef] [PubMed]
- Veliká, B.; Kron, I. Antioxidant properties of benzoic acid derivatives against Superoxide radical. Free Radic. Antioxid. 2012, 2, 62–67. [Google Scholar] [CrossRef]
- Xanthopoulou, M.N.; Nomikos, T.; Fragopoulou, E.; Antonopoulou, S. Antioxidant and lipoxygenase inhibitory activities of pumpkin seed extracts. Food Res. Int. 2009, 42, 641–646. [Google Scholar] [CrossRef]
- Yasir, M.; Sultana, B.; Nigam, P.S.; Owusu-Apenten, R. Antioxidant and genoprotective activity of selected cucurbitaceae seed extracts and LC–ESIMS/MS identification of phenolic components. Food Chem. 2016, 199, 307–313. [Google Scholar] [CrossRef]
- Rezaeizadeh, A.; Zuki, A.B.; Abdollahi, M.; Goh, Y.M.; Noordin, M.M.; Hamid, M.; Azmi, T. Determination of antioxidant activity in methanolic and chloroformic extracts of Momordica charantia. Afr. J. Biotechnol. 2011, 10, 4932–4940. [Google Scholar]
- Dallak, M. In vivo, hypolipidemic and antioxidant effects of Citrullus colocynthis pulp extract in alloxan-induced diabetic rats. Afr. J. Biotechnol. 2011, 10, 9898–9903. [Google Scholar] [CrossRef]
- Xia, T.; Wang, Q. Hypoglycaemic role of Cucurbita ficifolia (Cucurbitaceae) fruit extract in streptozotocin induced diabetic rats. J. Sci. Food Agric. 2007, 87, 1753–1757. [Google Scholar] [CrossRef]
- Rolnik, A.; Skalski, B.; Stochmal, A.; Olas, B. Preparations from selected cucurbit vegetables as antiplatelet agents. Sci. Rep. 2021, 11, 22694. [Google Scholar] [CrossRef] [PubMed]
- Rajput, M.S.; Balekar, N.; Jain, D.K. Inhibition of ADP-induced platelet aggregation and involvement of non-cellular blood chemical mediators are responsible for the antithrombotic potential of the fruits of Lagenaria siceraria. Chin. J. Nat. Med. 2014, 12, 599–606. [Google Scholar] [CrossRef]
- Gogoi, D.; Jha, S.; Chattopadhyay, P.; Mukherjee, A.K. A simple, cost-effective, and rapid separation process for the isolation of anticoagulant active fraction from the fruit extract of Momordica charantia: Characterization of bioactive components and anticoagulant mechanism of active fraction in a mouse model. J. Sep. Sci. 2020, 43, 3902–3912. [Google Scholar] [CrossRef] [PubMed]
- Keshk, W.A.; Noeman, S.A. Impact of Chicory-Supplemented Diet on Hyperlipidemia. J. Food Biochem. 2015, 39, 164–172. [Google Scholar] [CrossRef]
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef]
- Roghani, F.; Kamkhah, A.-F. Artichoke Leaf Juice Contains Antihypertensive Effect in Patients with Mild Hypertension. J. Diet. Suppl. 2009, 6, 328–341. [Google Scholar] [CrossRef]
- El-Mosallamy, A.E.; Sleem, A.A.; Abdel-Salam, O.M.; Shaffie, N.; Kenawy, S.A. Antihypertensive and Cardioprotective Effects of Pumpkin Seed Oil. J. Med. Food 2012, 15, 180–189. [Google Scholar] [CrossRef]
- Ramadan, B.K.; Mohammad, S.A.; Mahmoud, E.S.; Ouda, E.A. Role of pumpkin seed oil on some cardiovascular and renal aspects in adult male albino rats. Al-Azhar. Med. J. 2016, 45, 931–956. [Google Scholar] [CrossRef]
- Wahid, M.; Saqib, F.; Chicea, L.; Ahmedah, H.T.; Sajer, B.H.; Marc Vlaic, R.A.; Pop, O.L.; Mogą, M.; Gavris, C. Metabolomics analysis delineates the therapeutic effects of hydroethanolic extract of Cucumis sativus L. seeds on hypertension and isoproterenol-induced myocardial infarction. Biomed. Pharmacother. 2022, 148, 112704. [Google Scholar] [CrossRef]
- Dickel, M.L.; Rates, S.; Ritter, M.R. Plants popularly used for loosing weight purposes in Porto Alegre, South Brazil. J. Ethnopharmacol. 2007, 109, 60–71. [Google Scholar] [CrossRef]
- Muthusamy, V.; Anand, S.; Sangeetha, K.; Sujatha, S.; Arun, B.; Lakshmi, B. Tannins present in Cichorium intybus enhance glucose uptake and inhibit adipogenesis in 3T3-L1 adipocytes through PTP1B inhibition. Chem. Interact. 2008, 174, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Eo, H.; Park, K.; Jin, M.; Park, E.-J.; Kim, S.-H.; Park, J.E.; Kim, S. A water-soluble extract from Cucurbita moschata shows anti-obesity effects by controlling lipid metabolism in a high fat diet-induced obesity mouse model. Biochem. Biophys. Res. Commun. 2007, 359, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Ghahremanloo, A.; Hajipour, R.; Hemmati, M.; Moossavi, M.; Mohaqiq, Z. The beneficial effects of pumpkin extract on atherogenic lipid, insulin resistance and oxidative stress status in high-fat diet-induced obese rats. J. Complement. Integr. Med. 2018, 15. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolnik, A.; Olas, B. A Review of the Effect of Preparations from Vegetables of the Asteraceae Family and Cucurbitaceae Family on the Cardiovascular System and Its Diseases. Nutrients 2022, 14, 3601. https://doi.org/10.3390/nu14173601
Rolnik A, Olas B. A Review of the Effect of Preparations from Vegetables of the Asteraceae Family and Cucurbitaceae Family on the Cardiovascular System and Its Diseases. Nutrients. 2022; 14(17):3601. https://doi.org/10.3390/nu14173601
Chicago/Turabian StyleRolnik, Agata, and Beata Olas. 2022. "A Review of the Effect of Preparations from Vegetables of the Asteraceae Family and Cucurbitaceae Family on the Cardiovascular System and Its Diseases" Nutrients 14, no. 17: 3601. https://doi.org/10.3390/nu14173601
APA StyleRolnik, A., & Olas, B. (2022). A Review of the Effect of Preparations from Vegetables of the Asteraceae Family and Cucurbitaceae Family on the Cardiovascular System and Its Diseases. Nutrients, 14(17), 3601. https://doi.org/10.3390/nu14173601






