Prevalence of Food Allergy in France up to 5.5 Years of Age: Results from the ELFE Cohort
Abstract
:1. Introduction
2. Materials and Methods
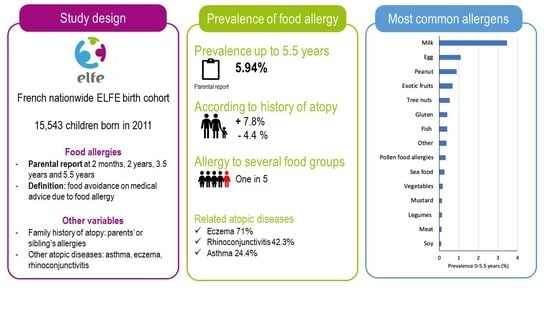
2.1. Study Design
2.2. Food Allergies
2.3. Other Data
2.4. Sample Selection
2.5. Statistical Analysis
3. Results
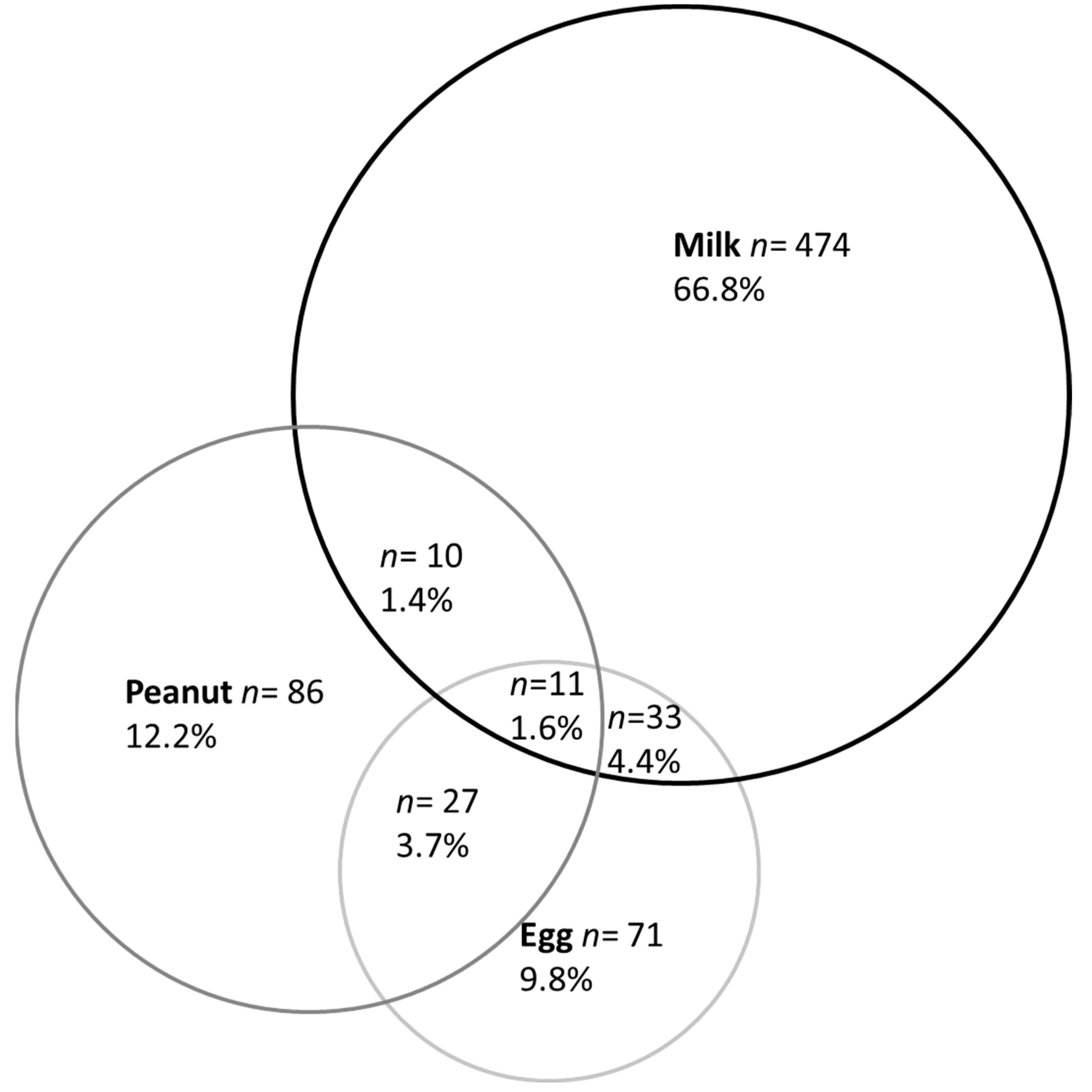
3.1. Prevalence of Parent-Reported Food Allergy
3.2. Prevalence of Physician-Reported Food Allergy
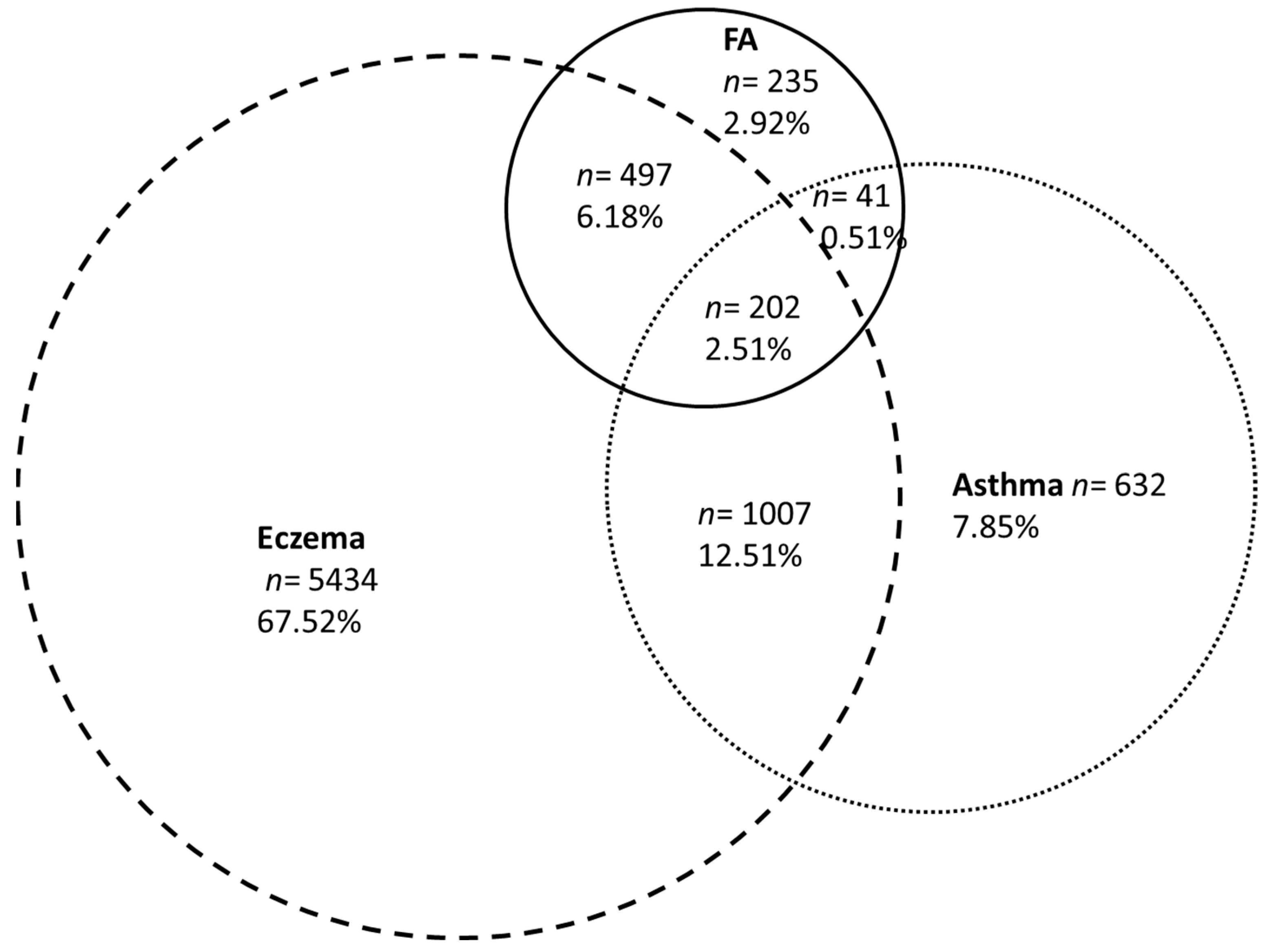
3.3. Family History of Atopy and Allergic Diseases
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prescott, S.; Allen, K.J. Food allergy: Riding the second wave of the allergy epidemic. Pediatr. Allergy Immunol. 2011, 22, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A.; The EAACI Food Allergy and Anaphylaxis Guidelines Group. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 2014, 69, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Panesar, S.S.; Javad, S.; de Silva, D.; Nwaru, B.I.; Hickstein, L.; Muraro, A.; Roberts, G.; Worm, M.; Bilò, M.B.; Cardona, V.; et al. The epidemiology of anaphylaxis in Europe: A systematic review. Allergy 2013, 68, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Campbell, D.E.; Motosue, M.S.; Campbell, R.L. Global Trends in Anaphylaxis Epidemiology and Clinical Implications. J. Allergy Clin. Immunol. Pract. 2020, 8, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Grabenhenrich, L.; Trendelenburg, V.; Bellach, J.; Yürek, S.; Reich, A.; Fiandor, A.; Rivero, D.; Sigurdardottir, S.; Clausen, M.; Papadopoulos, N.G.; et al. Frequency of food allergy in school-aged children in eight European countries—The EuroPrevall-iFAAM birth cohort. Allergy 2020, 75, 2294–2308. [Google Scholar] [CrossRef]
- Pénard-Morand, C.; Raherison, C.; Kopferschmitt, C.; Caillaud, D.; Lavaud, F.; Charpin, D.; Bousquet, J.; Annesi-Maesano, I. Prevalence of food allergy and its relationship to asthma and allergic rhinitis in schoolchildren. Allergy 2005, 60, 1165–1171. [Google Scholar] [CrossRef]
- Rancé, F.; Grandmottet, X.; Grandjean, H. Prevalence and main characteristics of schoolchildren diagnosed with food allergies in France. Clin. Exp. Allergy 2005, 35, 167–172. [Google Scholar] [CrossRef]
- ANSES. INCA 3: Evolution des habitudes et modes de consommation, de nouveaux enjeux en matière de sécurité sanitaire et de nutrition; Anses—Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail: MaisonAlfort, France, 2017.
- Roduit, C.; Frei, R.; Depner, M.; Schaub, B.; Loss, G.; Genuneit, J.; Pfefferle, P.; Hyvärinen, A.; Karvonen, A.M.; Riedler, J.; et al. Increased food diversity in the first year of life is inversely associated with allergic diseases. J. Allergy Clin. Immunol. 2014, 133, 1056–1064. [Google Scholar] [CrossRef]
- Perkin, M.R.; Logan, K.; Marrs, T.; Radulovic, S.; Craven, J.; Flohr, C.; Lack, G.; Young, L.; Offord, V.; DeSousa, M.; et al. Enquiring About Tolerance (EAT) study: Feasibility of an early allergenic food introduction regimen. J. Allergy Clin. Immunol. 2016, 137, 1477–1486.e8. [Google Scholar] [CrossRef]
- Charles, M.A.; Thierry, X.; Lanoe, J.-L.; Bois, C.; Dufourg, M.-N.; Popa, R.; Cheminat, M.; Zaros, C.; Geay, B. Cohort Profile: The French national cohort of children (ELFE): Birth to 5 years. Int. J. Epidemiol. 2020, 49, 368–369j. [Google Scholar] [CrossRef]
- Anto, J.M.; Bousquet, J.; Akdis, M.; Auffray, C.; Keil, T.; Momas, I.; Postma, D.S.; Valenta, R.; Wickman, M.; Cambon-Thomsen, A.; et al. Mechanisms of the Development of Allergy (MeDALL): Introducing novel concepts in allergy phenotypes. J. Allergy Clin. Immunol. 2017, 139, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Juillard, H. Weighting of Elfe Survey Data at Time 0. Available online: Https://Pandora.vjf.inserm.fr/public/2015 (accessed on 1 April 2015).
- Blondel, B.; Lelong, N.; Kermarrec, M.; Goffinet, F. Coordination nationale des Enquêtes Nationales Périnatales. Trends in perinatal health in France between 1995 and 2010: Results from the National Perinatal Surveys. J. Gynecol. Obstet. Biol. Reprod. 2012, 41, 151–166. [Google Scholar] [CrossRef] [PubMed]
- McGowan, E.C.; Keet, C.A. Prevalence of self-reported food allergy in the National Health and Nutrition Examination Survey (NHANES) 2007–2010. J. Allergy Clin. Immunol. 2013, 132, 1216–1219.e5. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Springston, E.E.; Warrier, M.R.; Smith, B.; Kumar, R.; Pongracic, J.; Holl, J.L. The Prevalence, Severity, and Distribution of Childhood Food Allergy in the United States. Pediatrics 2011, 128, e9–e17. [Google Scholar] [CrossRef] [PubMed]
- Saleh-Langenberg, J.; Bootsma, G.M.; Van Ginkel, C.D.; Kollen, B.J.; Blok, B.M.J.F.-D.; Dubois, A.E.J. The prevalence of food allergy and epinephrine auto-injectors in Dutch food-allergic adolescents. Pediatr. Allergy Immunol. 2016, 27, 755–759. [Google Scholar] [CrossRef]
- Peters, R.L.; Koplin, J.J.; Gurrin, L.C.; Dharmage, S.C.; Wake, M.; Ponsonby, A.-L.; Tang, M.L.; Lowe, A.J.; Matheson, M.; Dwyer, T.; et al. The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: HealthNuts age 4-year follow-up. J. Allergy Clin. Immunol. 2017, 140, 145–153.e8. [Google Scholar] [CrossRef]
- Venkataraman, D.; Erlewyn-Lajeunesse, M.; Kurukulaaratchy, R.J.; Potter, S.; Roberts, G.; Matthews, S.; Arshad, S.H. Prevalence and longitudinal trends of food allergy during childhood and adolescence: Results of the Isle of Wight Birth Cohort study. Clin. Exp. Allergy 2018, 48, 394–402. [Google Scholar] [CrossRef]
- Venter, C.; Patil, V.; Grundy, J.; Glasbey, G.; Twiselton, R.; Arshad, S.H.; Dean, T. Prevalence and cumulative incidence of food hyper-sensitivity in the first 10 years of life. Pediatr. Allergy Immunol. 2016, 27, 452–458. [Google Scholar] [CrossRef]
- Flom, J.D.; Sicherer, S.H. Epidemiology of Cow’s Milk Allergy. Nutrients 2019, 11, 1051. [Google Scholar] [CrossRef]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children—EuroPrevall birth cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef]
- Xepapadaki, P.; Fiocchi, A.; Grabenhenrich, L.; Roberts, G.; Grimshaw, K.E.C.; Fiandor, A.; Larco, J.I.; Sigurdardottir, S.L.; Clausen, M.; Papadopoulos, L.; et al. Incidence and natural history of hen’s egg allergy in the first 2 years of life—the EuroPrevall birth cohort study. Allergy 2016, 71, 350–357. [Google Scholar] [CrossRef]
- Sasaki, M.; Koplin, J.; Dharmage, S.; Field, M.J.; Sawyer, S.M.; McWilliam, V.; Peters, R.; Gurrin, L.; Vuillermin, P.J.; Douglass, J.; et al. Prevalence of clinic-defined food allergy in early adolescence: The SchoolNuts study. J. Allergy Clin. Immunol. 2018, 141, 391–398.e4. [Google Scholar] [CrossRef]
- Brough, H.A.; Caubet, J.-C.; Mazon, A.; Haddad, D.; Bergmann, M.M.; Wassenberg, J.; Panetta, V.; Gourgey, R.; Radulovic, S.; Nieto, M.; et al. Defining challenge-proven coexistent nut and sesame seed allergy: A prospective multicenter European study. J. Allergy Clin. Immunol. 2020, 145, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.H.; Wong, W.H.; Heine, R.G.; Hosking, C.S.; Hill, D.J.; Allen, K.J. Early clinical predictors of remission of peanut allergy in children. J. Allergy Clin. Immunol. 2008, 121, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Skripak, J.M.; Matsui, E.C.; Mudd, K.; Wood, R.A. The natural history of IgE-mediated cow’s milk allergy. J. Allergy Clin. Immunol. 2007, 120, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, V.; Peters, R.; Tang, M.L.K.; Dharmage, S.; Ponsonby, A.-L.; Gurrin, L.; Perrett, K.; Koplin, J.; Allen, K.J.; Dwyer, T.; et al. Patterns of tree nut sensitization and allergy in the first 6 years of life in a population-based cohort. J. Allergy Clin. Immunol. 2019, 143, 644–650.e5. [Google Scholar] [CrossRef] [PubMed]
- Cousin, M.; Verdun, S.; Seynave, M.; Vilain, A.-C.; Lansiaux, A.; DeCoster, A.; Sauvage, C. Phenotypical characterization of peanut allergic children with differences in cross-allergy to tree nuts and other legumes. Pediatr. Allergy Immunol. 2017, 28, 245–250. [Google Scholar] [CrossRef]
- Deschildre, A.; Elegbédé, C.F.; Just, J.; Bruyère, O.; Van Der Brempt, X.; Papadopoulos, A.; Beaudouin, E.; Renaudin, J.-M.; Crepet, A.; Moneret-Vautrin, D.-A. Peanut-allergic patients in the MIRABEL survey: Characteristics, allergists’ dietary advice and lessons from real life. Clin. Exp. Allergy 2016, 46, 610–620. [Google Scholar] [CrossRef]
- Bergmann, M.M.; Caubet, J.-C.; Boguniewicz, M.; Eigenmann, P. Evaluation of food allergy in patients with atopic dermatitis. J. Allergy Clin. Immunol. Pract. 2013, 1, 22–28. [Google Scholar] [CrossRef]
- Mastrorilli, C.; Caffarelli, C.; Hoffmann-Sommergruber, K. Food allergy and atopic dermatitis: Prediction, progression, and prevention. Pediatr. Allergy Immunol. 2017, 28, 831–840. [Google Scholar] [CrossRef]

| Allergens Families | Food Avoidance Reported by the Parents in the Open-Ended Item | |
|---|---|---|
| Milk | Milk *, butter, cream, cheese, beef and veal allergies | |
| Egg | ||
| Peanut | ||
| Exotic fruits | Kiwi, pineapple, banana | |
| Fish | ||
| Gluten | ||
| Soy | ||
| Tree nuts | Almond, cashew nut, pistachio, hazelnut, walnut, nut, chestnut, sesame, tree nut, coconut, macadamia nut | |
| Legumes | Peas, lentils, legumes, fava bean, chickpeas, flat bean, split bean | |
| Mustard | ||
| Sea food | Sea food, shellfish, crab, shrimp | |
| Meat | Meat, chicken, turkey, lamb, pork, poultry, frog | |
| Vegetables | Eggplant, pepper, onion, yam, corn, zucchini, cabbage, artichoke, leek, mushroom, spinach, endive, vegetables, potato | |
| Pollen food allergies | Fruit (fig, mandarin, citrus, blueberry, stone fruit, blackcurrant, orange), apiaceae (parsley, celery, carrot), rosaceae (peach, apple, pear, mirabelle plum) | |
| Other | Food additives (coloring, aroma, preservatives, etc.), foods with several ingredients (sweetened beverages, fruits juice, spice, etc.), other foods (honey, vinegar, pickle, etc.) | |
| Weighted % (n) | ||
|---|---|---|
| Males | 51.35% (8410) | |
| Gestational age | ||
| Preterm (<37 weeks) | 5.17% (866) | |
| Term (37 weeks or more) | 94.83% (15,269) | |
| C-section delivery | 18.80% (3001) | |
| Never breastfed | 27.11% (4305) | |
| Maternal age at delivery, years | ||
| <25 | 12.12% (1567) | |
| 25–29 | 30.65% (4890) | |
| 30–34 | 34.14% (6041) | |
| 35 years or more | 23.10% (3891) | |
| Maternal education level | ||
| Up to lower secondary | 5.33% (592) | |
| Upper secondary | 40.52% (5330) | |
| Intermediate | 20.40% (3553) | |
| 3-year university degree | 16.33% (2811) | |
| At least 5-year university degree | 17.43% (3160) | |
| Family history of atopy | 48.09% (7878) | |
| Weighted Prevalence % (95%CI *) | |
|---|---|
| At least one allergen | 5.94 (5.54–6.34) |
| Milk | 3.40 (3.09–3.71) |
| Egg | 0.99 (0.80–1.18) |
| Peanut | 0.93 (0.75–1.12) |
| Exotic fruits | 0.65 (0.51–0.80) |
| Tree nuts | 0.54 (0.40–0.68) |
| Gluten | 0.41 (0.28–0.54) |
| Fish | 0.37 (0.26–0.48) |
| Pollen-food allergies | 0.28 (0.17–0.38) |
| Vegetables | 0.19 (0.12–0.26) |
| Sea food/shellfish | 0.25 (0.14–0.36) |
| Legumes | 0.15 (0.08–0.22) |
| Mustard | 0.12 (0.05–0.19) |
| Meat | 0.08 (0.03–0.14) |
| Soy | 0.08 (0.03–0.13) |
| Other | 0.33 (0.22–0.45) |
| Single-Group Food Allergy | Multiple-Group Food Allergies | ||
|---|---|---|---|
| Allergens | |||
| Milk (n = 578) | 83.2% | 16.8% | |
| Egg (n = 142) | 35.9% | 64.1% | |
| Peanut (n = 134) | 41.0% | 59.0% | |
| Exotic fruit (n = 101) | 56.4% | 43.6% | |
| Tree nuts (n = 80) | 38.8% | 61.3% | |
| Gluten (n = 56) | 32.1% | 67.9% | |
| Fish (n = 56) | 41.1% | 58.9% | |
| Vegetables (n = 34) | 50.0% | 50.0% | |
| Pollen food allergy syndrome (n = 35) | 60.0% | 40.0% | |
| Seafood (n = 26) | 38.5% | 61.5% | |
| Legumes (n = 23) | 43.5% | 56.5% | |
| Mustard (n = 15) | 13.3% | 86.7% | |
| Soy (n = 14) | 7.1% | 92.9% | |
| Meat (n = 13) | 30.8% | 69.2% | |
| Others (n = 45) | 64.4% | 35.6% | |
| Comorbidities | |||
| Eczema | 68.5% | 78.5% | |
| Asthma | 22.1% | 35.4% | |
| Allergic rhinitis or conjunctivitis | 39.4% | 52.7% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamazouzt, S.; Adel-Patient, K.; Deschildre, A.; Roduit, C.; Charles, M.A.; de Lauzon-Guillain, B.; Divaret-Chauveau, A. Prevalence of Food Allergy in France up to 5.5 Years of Age: Results from the ELFE Cohort. Nutrients 2022, 14, 3624. https://doi.org/10.3390/nu14173624
Tamazouzt S, Adel-Patient K, Deschildre A, Roduit C, Charles MA, de Lauzon-Guillain B, Divaret-Chauveau A. Prevalence of Food Allergy in France up to 5.5 Years of Age: Results from the ELFE Cohort. Nutrients. 2022; 14(17):3624. https://doi.org/10.3390/nu14173624
Chicago/Turabian StyleTamazouzt, Sarah, Karine Adel-Patient, Antoine Deschildre, Caroline Roduit, Marie Aline Charles, Blandine de Lauzon-Guillain, and Amandine Divaret-Chauveau. 2022. "Prevalence of Food Allergy in France up to 5.5 Years of Age: Results from the ELFE Cohort" Nutrients 14, no. 17: 3624. https://doi.org/10.3390/nu14173624