Associations between Maternal Dietary Patterns, Biomarkers and Delivery Outcomes in Healthy Singleton Pregnancies: Multicenter Italian GIFt Study
Abstract
:1. Introduction
- Collect energy and nutrient intakes by 7-Day weighted dietary Record (7-DR), and compare with Italian nutritional recommendations;
- Define prevalent dietary patterns in a population of healthy normal-weight Italian pregnancies as extracted from Food Frequency Questionnaires (FFQ);
- Characterize maternal oxidative and inflammatory status by quantifying blood biomarkers;
- Investigate associations between maternal nutritional intakes and dietary patterns, blood biomarkers of inflammation and oxidative stress, and pregnancy outcomes, also stratifying for geographical area.
2. Materials and Methods
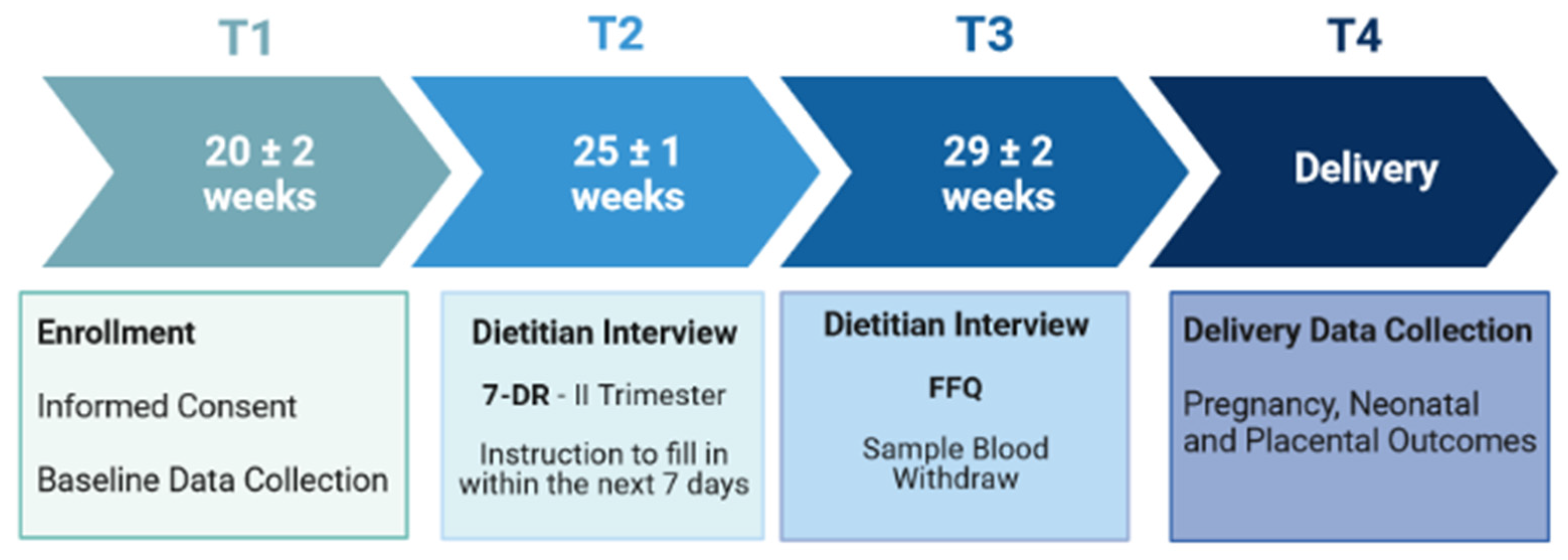
2.1. Study Protocol
2.2. Study Participants
2.3. Study Design and Data Collection
2.3.1. Nutritional Data Collection
2.3.2. Blood Testing: Nutritional, Inflammatory and Oxidative Biomarkers
2.4. Statistical Analysis
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GIFt study | Gestational Intake of Food towards healthy outcomes |
| AGA | Appropriate for Gestational Age |
| BMI | Body Mass Index |
| EIP | Energy Intake in Pregnancy |
| FFQ | Food Frequency Questionnaire |
| GWG | Gestational Weight Gain |
| GA | Gestational Age |
| IOM | Institute of Medicine |
| LARN | Livelli di Assunzione di Riferimento di Nutrienti ed energia |
| LGA | Large for Gestational Age |
| NW | Normal Weight |
| NPI | neonatal ponderal index |
| PAL | Physical Activity Level |
| PIP | Protein Intake in Pregnancy |
| RBCs | Red Blood Cells |
| SGA | Small for Gestational Age |
References
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Pandolfini, C.; Ricci, C.; Siziba, L.P.; Huhn, S.; Genuneit, J.; Bonati, M. Intrauterine Exposures and Maternal Health Status during Pregnancy in Relation to Later Child Health: A Review of Pregnancy Cohort Studies in Europe. Int. J. Environ. Res. Public Health 2021, 18, 7702. [Google Scholar] [CrossRef] [PubMed]
- Cetin, I.; Mandò, C.; Calabrese, S. Maternal predictors of intrauterine growth restriction. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Parisi, F.; Laoreti, A.; Cetin, I. Multiple Micronutrient Needs in Pregnancy in Industrialized Countries. Ann. Nutr. Metab. 2014, 65, 13–21. [Google Scholar] [CrossRef]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalán, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and maternal obesity: Epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef]
- Cetin, I.; Berti, C.; Calabrese, S. Role of micronutrients in the periconceptional period. Hum. Reprod. Update 2010, 16, 80–95. [Google Scholar] [CrossRef]
- Baker, B.C.; Hayes, D.; Jones, R.L. Effects of micronutrients on placental function: Evidence from clinical studies to animal models. Reproduction 2018, 156, R69–R82. [Google Scholar] [CrossRef]
- Wu, G.; Imhoff-Kunsch, B.; Girard, A.W. Biological Mechanisms for Nutritional Regulation of Maternal Health and Fetal Development. Paediatr. Périnat. Epidemiology 2012, 26, 4–26. [Google Scholar] [CrossRef]
- Biagi, C.; Di Nunzio, M.; Bordoni, A.; Gori, D.; Lanari, M. Effect of Adherence to Mediterranean Diet during Pregnancy on Children’s Health: A Systematic Review. Nutrients 2019, 11, 997. [Google Scholar] [CrossRef]
- Concina, F.; Pani, P.; Carletti, C.; Rosolen, V.; Knowles, A.; Parpinel, M.; Ronfani, L.; Mariuz, M.; Brumatti, L.V.; Valent, F.; et al. Nutrient Intake during Pregnancy and Adherence to Dietary Recommendations: The Mediterranean PHIME Cohort. Nutrients 2021, 13, 1434. [Google Scholar] [CrossRef]
- Parisi, F.; Rousian, M.; Steegers-Theunissen, R.P.M.; Koning, A.H.J.; Willemsen, S.P.; de Vries, J.H.M.; Cetin, I.; Steegers, E.A.P. Early first trimester maternal ‘high fish and olive oil and low meat’ dietary pattern is associated with accelerated human embryonic development. Eur. J. Clin. Nutr. 2018, 72, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Blumfield, M.L.; Hure, A.J.; MacDonald-Wicks, L.; Smith, R.; Collins, C.E. A systematic review and meta-analysis of micronutrient intakes during pregnancy in developed countries. Nutr. Rev. 2013, 71, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Blumfield, M.L.; Hure, A.J.; Macdonald-Wicks, L.; Smith, R.; Collins, C.E. Systematic review and meta-analysis of energy and macronutrient intakes during pregnancy in developed countries. Nutr. Rev. 2012, 70, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Northstone, K.; Emmett, P.M.; Rogers, I. Dietary patterns in pregnancy and associations with nutrient intakes. Br. J. Nutr. 2008, 99, 406–415. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, D.; Mao, X.; Xia, Y.; Baker, P.N.; Zhang, H. Maternal Dietary Patterns and Pregnancy Outcome. Nutrients 2016, 8, 351. [Google Scholar] [CrossRef]
- Tsakoumaki, F.; Kyrkou, C.; Fotiou, M.; Dimitropoulou, A.; Biliaderis, C.G.; Athanasiadis, A.P.; Menexes, G.; Michaelidou, A.-M. Framework of Methodology to Assess the Link between a Posteriori Dietary Patterns and Nutritional Adequacy: Application to Pregnancy. Metabolites 2022, 12, 395. [Google Scholar] [CrossRef]
- Yeh, K.-L.; Kautz, A.; Lohse, B.; Groth, S. Associations between Dietary Patterns and Inflammatory Markers during Pregnancy: A Systematic Review. Nutrients 2021, 13, 834. [Google Scholar] [CrossRef]
- Kusinski, L.C.; Murphy, H.R.; Rolfe, E.D.L.; Rennie, K.L.; Griep, L.M.O.; Hughes, D.; Taylor, R.; Meek, C.L. Dietary Intervention in Pregnant Women with Gestational Diabetes; Protocol for the DiGest Randomised Controlled Trial. Nutrients 2020, 12, 1165. [Google Scholar] [CrossRef]
- Hofmeyr, G.J.; Manyame, S.; Medley, N.; Williams, M.J. Calcium supplementation commencing before or early in pregnancy, for preventing hypertensive disorders of pregnancy. Cochrane Database Syst. Rev. 2019, 2019, CD011192. [Google Scholar] [CrossRef]
- Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain during Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; The National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Lisso, F.; Massari, M.; Gentilucci, M.; Novielli, C.; Corti, S.; Stellio, L.N.; Milazzo, R.; Troiano, E.; Schaefer, E.; Cetin, I.; et al. Longitudinal Nutritional Intakes in Italian Pregnant Women in Comparison with National Nutritional Guidelines. Nutrients 2022, 14, 1944. [Google Scholar] [CrossRef]
- BDA Working Group. Food Composition Database for Epidemiological Studies in Italy. Available online: www.bda-ieo.it (accessed on 29 March 2022).
- SINU (Società Italiana di Nutrizione Umana—Italian Society of Human Nutrition). LARN. Nutrients and Energy Reference Intake Levels for Italian Population. Available online: https://eng.sinu.it/larn/ (accessed on 29 March 2022).
- Porrini, M.; Gentile, M.G.; Fidanza, F. Biochemical validation of a self-administered semi-quantitative food-frequency questionnaire. Br. J. Nutr. 1995, 74, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Pirkle, J.L. CDC Laboratory Procedure Manual Folate Microbiologic Assay NHANES 2011–2012. 2012. Available online: https://citeseer.ist.psu.edu/viewdoc/download?doi=10.1.1.645.9088&rep=rep1&type=pdf (accessed on 1 September 2022).
- Molloy, A.M.; Scott, J.M. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997, 281, 43–53. [Google Scholar] [CrossRef] [PubMed]
- O’Broin, S.; Kelleher, B. Microbiological assay on microtitre plates of folate in serum and red cells. J. Clin. Pathol. 1992, 45, 344–347. [Google Scholar] [CrossRef]
- Piyathilake, C.J.; Robinson, C.B.; Cornwell, P. A Practical Approach to Red Blood Cell Folate Analysis. Anal. Chem. Insights 2007, 2, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Massari, M.; Novielli, C.; Mandò, C.; Di Francesco, S.; Della Porta, M.; Cazzola, R.; Panteghini, M.; Savasi, V.; Maggini, S.; Schaefer, E.; et al. Multiple Micronutrients and Docosahexaenoic Acid Supplementation during Pregnancy: A Randomized Controlled Study. Nutrients 2020, 12, 2432. [Google Scholar] [CrossRef] [PubMed]
- Anelli, G.M.; Cardellicchio, M.; Novielli, C.; Antonazzo, P.; Mazzocco, M.I.; Cetin, I.; Mandò, C. Mitochondrial content and hepcidin are increased in obese pregnant mothers. J. Matern. Neonatal Med. 2018, 31, 2388–2395. [Google Scholar] [CrossRef]
- Zambon, M.; Mandò, C.; Lissoni, A.; Anelli, G.M.; Novielli, C.; Cardellicchio, M.; Leone, R.; Monari, M.N.; Massari, M.; Cetin, I.; et al. Inflammatory and Oxidative Responses in Pregnancies With Obesity and Periodontal Disease. Reprod. Sci. 2018, 25, 1474–1484. [Google Scholar] [CrossRef]
- Hoffmann, K.; Schulze, M.B.; Schienkiewitz, A.; Nöthlings, U.; Boeing, H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am. J. Epidemiol. 2004, 159, 935–944. [Google Scholar] [CrossRef]
- Loaiza, S.; Atalah, E. Birth weight and obesity risk at first grade of high school in a non-concurrent cohort of Chilean children. Public Health Nutr. 2013, 16, 228–232. [Google Scholar] [CrossRef]
- Cantone, D.; Pelullo, C.P.; Cancellieri, M.; Attena, F. Can antenatal classes reduce the rate of cesarean section in southern Italy? Women Birth 2017, 30, e83–e88. [Google Scholar] [CrossRef]
- Zerfu, T.A.; Umeta, M.; Baye, K. Dietary habits, food taboos, and perceptions towards weight gain during pregnancy in Arsi, rural central Ethiopia: A qualitative cross-sectional study. J. Health Popul. Nutr. 2016, 35, 22. [Google Scholar] [CrossRef] [PubMed]
- Torjusen, H.; Lieblein, G.; Næs, T.; Haugen, M.; Meltzer, H.M.; Brantsæter, A.L. Food patterns and dietary quality associated with organic food consumption during pregnancy; data from a large cohort of pregnant women in Norway. BMC Public Health 2012, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P., Jr.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef]
- Maugeri, A.; Magnano San Lio, R.; La Rosa, M.C.; Giunta, G.; Panella, M.; Cianci, A.; Caruso, M.A.T.; Agodi, A.; Barchitta, M. The Relationship between Telomere Length and Gestational Weight Gain: Findings from the Mamma & Bambino Cohort. Biomedicines 2021, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.; Nemeth, E. Iron homeostasis during pregnancy. Am. J. Clin. Nutr. 2017, 106 (Suppl. 6), 1567S–1574S. [Google Scholar] [CrossRef] [PubMed]
- Kocyłowski, R.; Lewicka, I.; Grzesiak, M.; Gaj, Z.; Sobańska, A.; Poznaniak, J.; von Kaisenberg, C.; Suliburska, J. Assessment of dietary intake and mineral status in pregnant women. Arch. Gynecol. Obstet. 2018, 297, 1433–1440. [Google Scholar] [CrossRef]
- von Websky, K.; Hasan, A.A.; Reichetzeder, C.; Tsuprykov, O.; Hocher, B. Impact of vitamin D on pregnancy-related disorders and on offspring outcome. J. Steroid Biochem. Mol. Biol. 2018, 180, 51–64. [Google Scholar] [CrossRef]
- Fiscaletti, M.; Stewart, P.; Munns, C.F. The importance of vitamin D in maternal and child health: A global perspective. Public Health Rev. 2017, 38, 19. [Google Scholar] [CrossRef]
- Karras, S.N.; Dursun, E.; Alaylıoğlu, M.; Gezen-Ak, D.; Annweiler, C.; Al Anouti, F.; Fakhoury, H.M.A.; Bais, A.; Kiortsis, D. Investigating the Role of Functional Polymorphism of Maternal and Neonatal Vitamin D Binding Protein in the Context of 25-Hydroxyvitamin D Cutoffs as Determinants of Maternal-Neonatal Vitamin D Status Profiles in a Sunny Mediterranean Region. Nutrients 2021, 13, 3082. [Google Scholar] [CrossRef]
- CaraDonna, F.; Consiglio, O.; Luparello, C.; Gentile, C. Science and Healthy Meals in the World: Nutritional Epigenomics and Nutrigenetics of the Mediterranean Diet. Nutrients 2020, 12, 1748. [Google Scholar] [CrossRef]
- Parisi, F.; Di Bartolo, I.; Savasi, V.M.; Cetin, I. Micronutrient supplementation in pregnancy: Who, what and how much? Obstet. Med. 2019, 12, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Gemici, A.; Sinen, O.; Bülbül, M. Sexual dimorphism in rats exposed to maternal high fat diet: Alterations in medullary sympathetic network. Metab. Brain Dis. 2021, 36, 1305–1314. [Google Scholar] [CrossRef]
- Savva, C.; Helguero, L.A.; González-Granillo, M.; Melo, T.; Couto, D.; Buyandelger, B.; Gustafsson, S.; Liu, J.; Domingues, M.R.; Li, X.; et al. Maternal high-fat diet programs white and brown adipose tissue lipidome and transcriptome in offspring in a sex- and tissue-dependent manner in mice. Int. J. Obes. 2022, 46, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Zavatta, A.; Parisi, F.; Mandò, C.; Scaccabarozzi, C.; Savasi, V.M.; Cetin, I. Role of Inflammaging on the Reproductive Function and Pregnancy. Clin. Rev. Allergy Immunol. 2022, 2022, 1–16. [Google Scholar] [CrossRef]
- Mandò, C.; Calabrese, S.; Mazzocco, M.I.; Novielli, C.; Anelli, G.M.; Antonazzo, P.; Cetin, I. Sex specific adaptations in placental biometry of overweight and obese women. Placenta 2016, 38, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Reichetzeder, C.; Putra, S.E.D.; Li, J.; Hocher, B. Developmental Origins of Disease—Crisis Precipitates Change. Cell. Physiol. Biochem. 2016, 39, 919–938. [Google Scholar] [CrossRef]
- Parisi, F.; Rousian, M.; Koning, A.H.; Willemsen, S.P.; Steegers, E.A.; Steegers-Theunissen, R.P. Effect of human embryonic morphological development on fetal growth parameters: The Rotterdam Periconceptional Cohort (Predict Study). Reprod. Biomed. Online 2019, 38, 613–620. [Google Scholar] [CrossRef]
- Gabory, A.; Roseboom, T.J.; Moore, T.; Moore, L.G.; Junien, C. Placental contribution to the origins of sexual dimorphism in health and diseases: Sex chromosomes and epigenetics. Biol. Sex Differ. 2013, 4, 5. [Google Scholar] [CrossRef] [Green Version]

| Maternal Characteristics | TOTAL STUDY POPULATION n = 179 | MILAN n = 85 | NAPLES n = 94 | p-Value | |
| Maternal Age, years | 31.8 ± 4.3 | 31.7 ± 4.5 | 31.9 ± 4.1 | ns | |
| Maternal pregestational BMI, kg/m2 | 21.9 ± 2.7 | 21.6 ± 2.8 | 22.3 ± 2.7 | ns | |
| Educational Level, % | low | 18 (9.9%) | 6 (7.0%) | 12 (12.6%) | ns |
| intermediate | 55 (30.4%) | 32 (37.2%) | 23 (24.2%) | ||
| high | 108 (59.7%) | 48 (55.8%) | 60 (63.2%) | ||
| Working Status, % | unemployed | 31 (17.4%) | 5 (6.0%) | 26 (27.4%) | <0.001 |
| worker | 147 (82.6%) | 78 (94.0%) | 69 (72.6%) | ||
| Marital Status, % | not married | 64 (35.8%) | 43 (50.6%) | 21 (22.3%) | <0.001 |
| married | 115 (64.2%) | 42 (49.4%) | 73 (77.7%) | ||
| Supplement Use, % | none | 19 (10.9%) | 12 (14.6%) | 7 (7.6%) | <0.001 |
| iron/folic acid | 37 (21.3%) | 5 (6.1%) | 32 (34.8%) | ||
| multivitamin | 118 (67.8%) | 65 (79.3%) | 53 (57.6%) | ||
| Nutritional, Inflammatory and Oxidative Biomarkers at T3 | TOTAL STUDY POPULATION (n = 166) | MILAN (n = 81) | NAPLES (n = 85) | p-Value | |
| RBCs Folate, ng/mL | 595.2 ± 134.2 | 521.3 ± 123.5 | 633.0 ± 104.9 | <0.001 | |
| Serum Vitamin D, ug/L | 22.1 ± 7.6 | 21.2 ± 7.4 | 22.9 ± 7.7 | ns | |
| Plasma Hpn-25, ng/mL | 4.4 ± 2.9 | 4.9 ± 3.0 | 4.0 ± 2.7 | 0.03 | |
| Plasma TAC, mM | 2.8 ± 1.4 | 2.9 ± 1.5 | 2.7 ± 1.3 | ns | |
| Delivery Outcome at T4 | TOTAL STUDY POPULATION n = 173 | MILAN n = 83 | NAPLES n = 90 | p-Value |
|---|---|---|---|---|
| Maternal GWG, kg | 12.6 ± 4.2 | 13.4 ± 4.3 | 12.0 ± 4.0 | 0.05 |
| GA at Delivery, weeks | 39.6 ± 1.2 | 39.8 ± 1.2 | 39.5 ± 1.2 | 0.02 |
| Placental Weight, gr | 541.4 ± 104.2 | 541.5 ± 110.5 | 542.4 ± 99.8 | ns |
| N/P weight ratio | 6.1 ± 1.0 | 6.1 ± 1.1 | 6.1 ± 0.9 | ns |
| Neonatal Weight, gr | 3231.7 ± 454.23 | 3232.7 ± 503.9 | 3225.2 ± 406.2 | ns |
| NPI, gr/cm3 | 2.7 ± 0.3 | 2.7 ± 0.3 | 2.7 ± 0.3 | ns |
| Neonatal Length, cm | 49.2 ± 2.1 | 49.4 ± 2.3 | 49.1 ± 1.9 | ns |
| Neonatal HC, cm | 34.3 ± 1.3 | 34.1 ± 1.3 | 34.5 ± 1.3 | 0.02 |
| ‘HIGH MEAT, ANIMAL FATS, GRAIN’ DP | ‘HIGH FISH, FRUIT, NUTS’ DP | ‘HIGH EGG and SWEETS, LOW LEGUMES’ DP | |
|---|---|---|---|
| EXPLAINED VARIANCE, % | 11.7 | 11.4 | 10.3 |
| DAIRY | 0.057 | 0.508 | −0.050 |
| CEREALS | 0.548 | −0.210 | −0.324 |
| GRAINS | 0.625 | −0.027 | −0.207 |
| VEGETABLES | 0.107 | 0.448 | −0.280 |
| LEGUMES | 0.286 | 0.022 | −0.414 |
| POTATOES | 0.563 | −0.113 | 0.114 |
| MEAT | 0.575 | −0.023 | −0.255 |
| FISH | 0.177 | 0.718 | −0.210 |
| EGG | 0.164 | 0.356 | 0.515 |
| FRUIT | 0.151 | 0.520 | 0.112 |
| NUTS | 0.053 | 0.531 | 0.261 |
| VEGETABLE FATS | 0.038 | 0.362 | −0.098 |
| ANIMAL FATS | 0.566 | −0.170 | 0.399 |
| SWEETS | 0.334 | −0.013 | 0.489 |
| NON-ALCOHOL BEVERAGE | 0.470 | −0.169 | 0.164 |
| β (95% CI) | |||
|---|---|---|---|
| ‘HIGH MEAT, ANIMAL FATS, GRAINS’ DP | Vitamin D, ug/L | −3.9 (−6.9; −0.9) * | |
| Hpn-25, ng/mL | 0.3 (0.0; 0.5) * | ||
| GA, weeks | F | −0.5 (−0.9; −0.0) * | |
| M | −0.3 (−0.7; −0.2) | ||
| Hpn-25 | Placental Weight, gr | −8.3 (−15.7; −0.8) * | |
| Vitamin D | Neonatal Weight, gr | 31.1 (9.2; 53.10) ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anelli, G.M.; Parisi, F.; Sarno, L.; Fornaciari, O.; Carlea, A.; Coco, C.; Porta, M.D.; Mollo, N.; Villa, P.M.; Guida, M.; et al. Associations between Maternal Dietary Patterns, Biomarkers and Delivery Outcomes in Healthy Singleton Pregnancies: Multicenter Italian GIFt Study. Nutrients 2022, 14, 3631. https://doi.org/10.3390/nu14173631
Anelli GM, Parisi F, Sarno L, Fornaciari O, Carlea A, Coco C, Porta MD, Mollo N, Villa PM, Guida M, et al. Associations between Maternal Dietary Patterns, Biomarkers and Delivery Outcomes in Healthy Singleton Pregnancies: Multicenter Italian GIFt Study. Nutrients. 2022; 14(17):3631. https://doi.org/10.3390/nu14173631
Chicago/Turabian StyleAnelli, Gaia Maria, Francesca Parisi, Laura Sarno, Ottavia Fornaciari, Annunziata Carlea, Chiara Coco, Matteo Della Porta, Nunzia Mollo, Paola Maria Villa, Maurizio Guida, and et al. 2022. "Associations between Maternal Dietary Patterns, Biomarkers and Delivery Outcomes in Healthy Singleton Pregnancies: Multicenter Italian GIFt Study" Nutrients 14, no. 17: 3631. https://doi.org/10.3390/nu14173631
APA StyleAnelli, G. M., Parisi, F., Sarno, L., Fornaciari, O., Carlea, A., Coco, C., Porta, M. D., Mollo, N., Villa, P. M., Guida, M., Cazzola, R., Troiano, E., Pasotti, M., Volpi, G., Vetrani, L., Maione, M., & Cetin, I. (2022). Associations between Maternal Dietary Patterns, Biomarkers and Delivery Outcomes in Healthy Singleton Pregnancies: Multicenter Italian GIFt Study. Nutrients, 14(17), 3631. https://doi.org/10.3390/nu14173631









