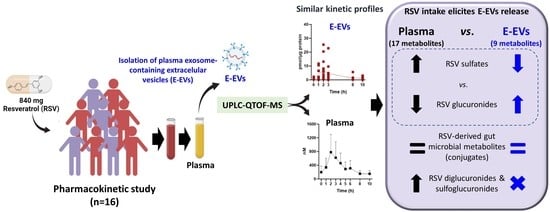
Exosome-Containing Extracellular Vesicles Contribute to the Transport of Resveratrol Metabolites in the Bloodstream: A Human Pharmacokinetic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and RSV Capsules
2.2. Study Design
2.3. Blood Sampling
2.4. EVs Isolation and Characterization
2.5. Protein Determination and Western Blot Analysis
2.6. Analysis of Resveratrol (RSV) and Derived Metabolites
2.7. Statistical Analysis
3. Results
3.1. Volunteers’ Characteristics
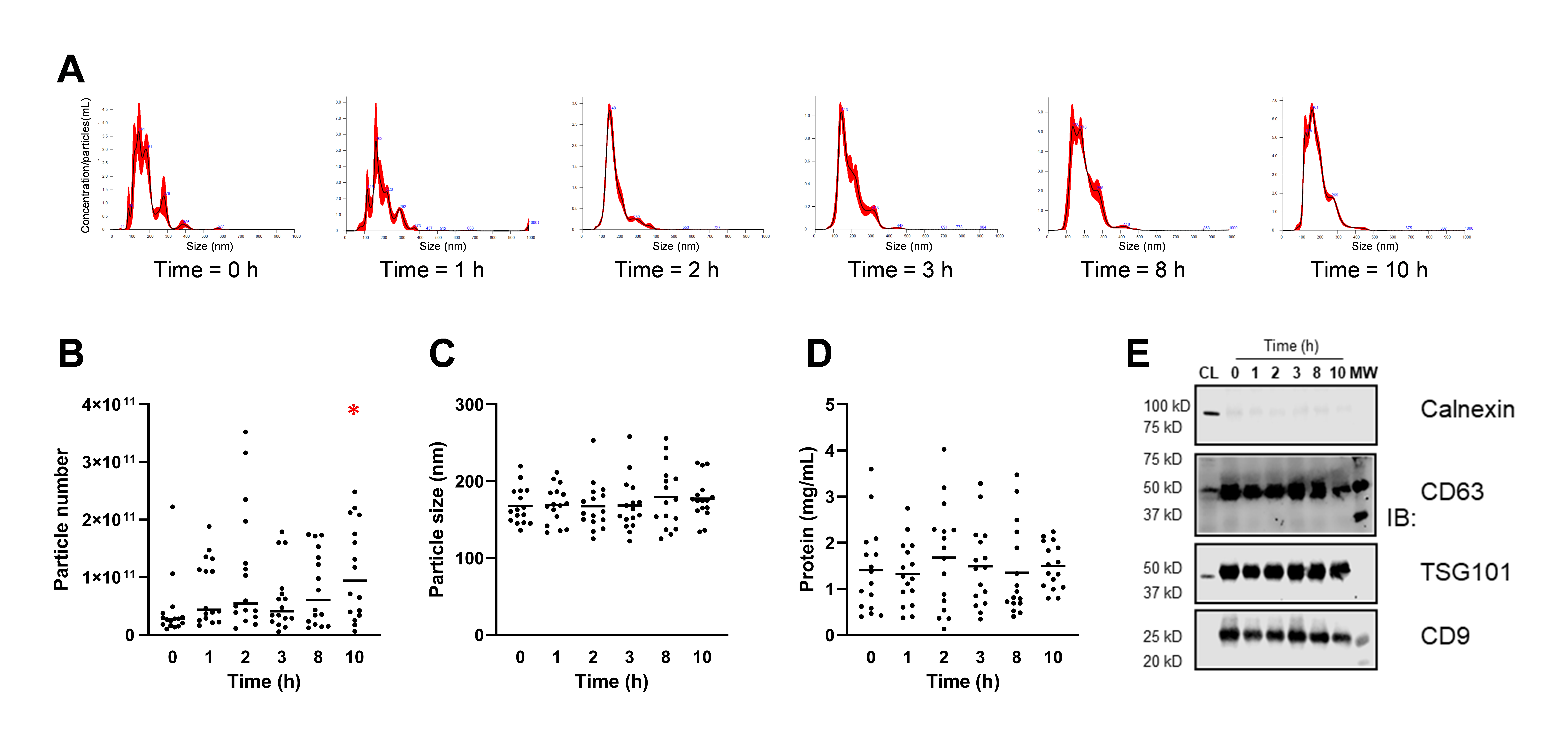
3.2. EVs Isolation and Characterization
3.3. Identification of RSV and Derived Metabolites
3.4. Pharmacokinetics of RSV Metabolites in Plasma and E-EVs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Théry, C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell. Vesicles 2019, 8, 1648167. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Sanderson, M.P.; Stoeck, A.; Altevogt, P. Exosomes: From biogenesis and secretion to biological function. Immunol. Lett. 2006, 107, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science. 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- O’Grady, T.; Njock, M.-S.; Lion, M.; Bruyr, J.; Mariavelle, E.; Galvan, B.; Boeckx, A.; Struman, I.; Dequiedt, F. Sorting and packaging of RNA into extracellular vesicles shape intracellular transcript levels. BMC Biol. 2022, 20, 72. [Google Scholar] [CrossRef]
- Santiago-Dieppa, D.R.; Steinberg, J.; Gonda, D.; Cheung, V.J.; Carter, B.S.; Chen, C.C. Extracellular vesicles as a platform for ‘liquid biopsy’ in glioblastoma patients. Expert Rev. Mol. Diagn. 2014, 14, 819–825. [Google Scholar] [CrossRef]
- Vallejo, F.; Yuste, J.E.; Teruel-Montoya, R.; Luengo-Gil, G.; Bohdan, N.; Espín, S.; García-Barberá, N.; Martínez, C.; Vicente, V.; Espín, J.C.; et al. First exploratory study on the metabolome from plasma exosomes in patients with paroxysmal nocturnal hemoglobinuria. Thromb. Res. 2019, 183, 80–85. [Google Scholar] [CrossRef]
- Núñez-Sánchez, M.A.; González-Sarrías, A.; Romo-Vaquero, M.; García-Villalba, R.; Selma, M.V.; Tomás-Barberán, F.A.; García-Conesa, M.-T.; Espín, J.C. Dietary phenolics against colorectal cancer-From promising preclinical results to poor translation into clinical trials: Pitfalls and future needs. Mol. Nutr. Food Res. 2015, 59, 1274–1291. [Google Scholar] [CrossRef]
- Iglesias-Aguirre, C.E.; Cortés-Martín, A.; Ávila-Gálvez, M.Á.; Giménez-Bastida, J.A.; Selma, M.V.; González-Sarrías, A.; Espín, J.C. Main drivers of (poly)phenol effects on human health: Metabolite production and/or gut microbiota-associated metabotypes? Food Funct. 2021, 12, 10324–10355. [Google Scholar] [CrossRef]
- Rajha, H.N.; Paule, A.; Aragonès, G.; Barbosa, M.; Caddeo, C.; Debs, E.; Dinkova, R.; Eckert, G.P.; Fontana, A.; Gebrayel, P.; et al. Recent Advances in Research on Polyphenols: Effects on Microbiota, Metabolism, and Health. Mol. Nutr. Food Res. 2022, 66, 2100670. [Google Scholar] [CrossRef]
- Canfrán-Duque, A.; Pastor, Ó.; Quintana-Portillo, R.; Lerma, M.; de la Peña, G.; Martín-Hidalgo, A.; Fernández-Hernando, C.; Lasunción, M.A.; Busto, R. Curcumin promotes exosomes/microvesicles secretion that attenuates lysosomal cholesterol traffic impairment. Mol. Nutr. Food Res. 2014, 58, 687–697. [Google Scholar] [CrossRef]
- Canfrán-Duque, A.; Pastor, O.; Reina, M.; Lerma, M.; Cruz-Jentoft, A.J.; Lasunción, M.A.; Busto, R. Curcumin Mitigates the Intracellular Lipid Deposit Induced by Antipsychotics In Vitro. PLoS ONE 2015, 10, e0141829. [Google Scholar] [CrossRef]
- García-Seisdedos, D.; Babiy, B.; Lerma, M.; Casado, M.E.; Martínez-Botas, J.; Lasunción, M.A.; Pastor, Ó.; Busto, R. Curcumin stimulates exosome/microvesicle release in an in vitro model of intracellular lipid accumulation by increasing ceramide synthesis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158638. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Y.; Huang, S.; Xu, H.; Li, H.; Liu, B. Resveratrol-primed exosomes strongly promote the recovery of motor function in SCI rats by activating autophagy and inhibiting apoptosis via the PI3K signaling pathway. Neurosci. Lett. 2020, 736, 135262. [Google Scholar] [CrossRef]
- Figueira, I.; Bastos, P.; González-Sarrías, A.; Espín, J.C.; Costa-Silva, B.; Nunes dos Santos, C. Can exosomes transfer the preconditioning effects triggered by (poly)phenol compounds between cells? Food Funct. 2022, accepted. [Google Scholar]
- Song, H.; Liu, B.; Dong, B.; Xu, J.; Zhou, H.; Na, S.; Liu, Y.; Pan, Y.; Chen, F.; Li, L.; et al. Exosome-Based Delivery of Natural Products in Cancer Therapy. Front. Cell Dev. Biol. 2021, 9, 650426. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Iglesias-Aguirre, C.E.; Cortés-Martín, A.; Vallejo, F.; Cattivelli, A.; del Pozo-Acebo, L.; Del Saz, A.; López de las Hazas, M.C.; Dávalos, A.; Espín, J.C. Milk-Derived Exosomes as Nanocarriers to Deliver Curcumin and Resveratrol in Breast Tissue and Enhance Their Anticancer Activity. Int. J. Mol. Sci. 2022, 23, 2860. [Google Scholar] [CrossRef]
- Arola-Arnal, A.; López de las Hazas, M.-C.; Iglesias-Carres, L.; Mantilla-Escalante, D.C.; Suárez, M.; Busto, R.; Visioli, F.; Bladé, C.; Dávalos, A. Exosomes transport trace amounts of (poly)phenols. Food Funct. 2020, 11, 7784–7792. [Google Scholar] [CrossRef]
- Ivanov, V.; Carr, A.C.; Frei, B. Red Wine Antioxidants Bind to Human Lipoproteins and Protect Them from Metal Ion-Dependent and -Independent Oxidation. J. Agric. Food Chem. 2001, 49, 4442–4449. [Google Scholar] [CrossRef]
- Delmas, D.; Lin, H.-Y. Role of membrane dynamics processes and exogenous molecules in cellular resveratrol uptake: Consequences in bioavailability and activities. Mol. Nutr. Food Res. 2011, 55, 1142–1153. [Google Scholar] [CrossRef]
- Harbi, S.M.; Hussien, R.A.; Hawasawi, I.; Alshdoukhi, I.; Chopra, V.; Alanazi, A.N.; Butler, W.; Koroma, R.; Peters, C.; Garver, D.D.; et al. Red Blood Cells and Lipoproteins: Important Reservoirs and Transporters of Polyphenols and Their Metabolites. J. Agric. Food Chem. 2020, 68, 7005–7013. [Google Scholar] [CrossRef]
- Tung, W.-C.; Rizzo, B.; Dabbagh, Y.; Saraswat, S.; Romanczyk, M.; Codorniu-Hernández, E.; Rebollido-Rios, R.; Needs, P.W.; Kroon, P.A.; Rakotomanomana, N.; et al. Polyphenols bind to low density lipoprotein at biologically relevant concentrations that are protective for heart disease. Arch. Biochem. Biophys. 2020, 694, 108589. [Google Scholar] [CrossRef]
- Iglesias-Aguirre, C.E.; Vallejo, F.; Beltrán, D.; Berná, J.; Puigcerver, J.; Alajarín, M.; Selma, M.V.; Espín, J.C. 4-Hydroxydibenzyl: A novel metabolite from the human gut microbiota after consuming resveratrol. Food Funct. 2022, 13, 7487–7493. [Google Scholar] [CrossRef]
- Azorín-Ortuño, M.; Yáñez-Gascón, M.J.; Vallejo, F.; Pallarés, F.J.; Larrosa, M.; Lucas, R.; Morales, J.C.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Metabolites and tissue distribution of resveratrol in the pig. Mol. Nutr. Food Res. 2011, 55, 1154–1168. [Google Scholar] [CrossRef]
- Vallejo, F.; Larrosa, M.; Escudero, E.; Zafrilla, M.P.; Cerdá, B.; Boza, J.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Concentration and Solubility of Flavanones in Orange Beverages Affect Their Bioavailability in Humans. J. Agric. Food Chem. 2010, 58, 6516–6524. [Google Scholar] [CrossRef]
- González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.Á.; Tomé-Carneiro, J.; Zafrilla, P.; Mulero, J.; Tomás-Barberán, F.A.; Espín, J.C. Identifying the limits for ellagic acid bioavailability: A crossover pharmacokinetic study in healthy volunteers after consumption of pomegranate extracts. J. Funct. Foods 2015, 19, 225–235. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; Giménez-Bastida, J.A.; González-Sarrías, A.; Espín, J.C. New Insights into the Metabolism of the Flavanones Eriocitrin and Hesperidin: A Comparative Human Pharmacokinetic Study. Antioxidants 2021, 10, 435. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Askenase, P.W. Exosomes provide unappreciated carrier effects that assist transfers of their miRNAs to targeted cells; I. They are ‘The Elephant in the Room’. RNA Biol. 2021, 18, 2038–2053. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Gálvez, M.Á.; García-Villalba, R.; Martínez-Díaz, F.; Ocaña-Castillo, B.; Monedero-Saiz, T.; Torrecillas-Sánchez, A.; Abellán, B.; González-Sarrías, A.; Espín, J.C. Metabolic Profiling of Dietary Polyphenols and Methylxanthines in Normal and Malignant Mammary Tissues from Breast Cancer Patients. Mol. Nutr. Food Res. 2019, 63, 1801239. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; González-Sarrías, A.; Martínez-Díaz, F.; Abellán, B.; Martínez-Torrano, A.J.; Fernández-López, A.J.; Giménez-Bastida, J.A.; Espín, J.C. Disposition of Dietary Polyphenols in Breast Cancer Patients’ Tumors, and Their Associated Anticancer Activity: The Particular Case of Curcumin. Mol. Nutr. Food Res. 2021, 65, e2100163. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes. Mol. Nutr. Food Res. 2020, 64, 1900952. [Google Scholar] [CrossRef]
- López de Las Hazas, M.C.; Del Pozo-Acebo, L.; Hansen, M.S.; Gil-Zamorano, J.; Mantilla-Escalante, D.C.; Gómez-Coronado, D.; Marín, F.; Garcia-Ruiz, A.; Rasmussen, J.T.; Dávalos, A. Dietary bovine milk miRNAs transported in extracellular vesicles are partially stable during GI digestion, are bioavailable and reach target tissues but need a minimum dose to impact on gene expression. Eur. J. Nutr. 2022, 61, 1043–1056. [Google Scholar] [CrossRef]
- Qi, Y.; Guo, L.; Jiang, Y.; Shi, Y.; Sui, H.; Zhao, L. Brain delivery of quercetin-loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles. Drug Deliv. 2020, 27, 745–755. [Google Scholar] [CrossRef]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef]
- Flanagan, E.; Müller, M.; Hornberger, M.; Vauzour, D. Impact of Flavonoids on Cellular and Molecular Mechanisms Underlying Age-Related Cognitive Decline and Neurodegeneration. Curr. Nutr. Rep. 2018, 7, 49–57. [Google Scholar] [CrossRef]
- García-Villalba, R.; Tomás-Barberán, F.A.; Iglesias-Aguirre, C.E.; Giménez-Bastida, J.A.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagitannins, urolithins, and neuroprotection: Human evidence and the possible link to the gut microbiota. Mol. Asp. Med. 2022, 101109. [Google Scholar] [CrossRef]
- Chevillet, J.R.; Kang, Q.; Ruf, I.K.; Briggs, H.A.; Vojtech, L.N.; Hughes, S.M.; Cheng, H.H.; Arroyo, J.D.; Meredith, E.K.; Gallichotte, E.N.; et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl. Acad. Sci. USA 2014, 111, 14888–14893. [Google Scholar] [CrossRef]
- Iglesias-Aguirre, C.E.; Vallejo, F.; Beltrán, D.; Aguilar-Aguilar, E.; Puigcerver, J.; Alajarín, M.; Berná, J.; Selma, M.V.; Espín, J.C. Lunularin Producers versus Non-producers: Novel Human Metabotypes Associated with the Metabolism of Resveratrol by the Gut Microbiota. J. Agric. Food Chem. 2022, 70, 10521–10531. [Google Scholar] [CrossRef]
- Buschmann, D.; Kirchner, B.; Hermann, S.; Märte, M.; Wurmser, C.; Brandes, F.; Kotschote, S.; Bonin, M.; Steinlein, O.K.; Pfaffl, M.W.; et al. Evaluation of serum extracellular vesicle isolation methods for profiling miRNAs by next-generation sequencing. J. Extracell. Vesicles 2018, 7, 1481321. [Google Scholar] [CrossRef]
- López Andrés, N.; Tesse, A.; Regnault, V.; Louis, H.; Cattan, V.; Thornton, S.N.; Labat, C.; Kakou, A.; Tual-Chalot, S.; Faure, S.; et al. Increased Microparticle Production and Impaired Microvascular Endothelial Function in Aldosterone-Salt-Treated Rats: Protective Effects of Polyphenols. PLoS ONE 2012, 7, e39235. [Google Scholar] [CrossRef]
- Horn, P.; Amabile, N.; Angeli, F.S.; Sansone, R.; Stegemann, B.; Kelm, M.; Springer, M.L.; Yeghiazarians, Y.; Schroeter, H.; Heiss, C. Dietary flavanol intervention lowers the levels of endothelial microparticles in coronary artery disease patients. Br. J. Nutr. 2014, 111, 1245–1252. [Google Scholar] [CrossRef]


| Compound | RT (min) | Mass Accuracy (m/z−) | Molecular Formula | Error (ppm) | Score | Occurrence |
|---|---|---|---|---|---|---|
| RSV-diG (isomer-1) | 4.42 | 579.1355 | C26H28O15 | −1.95 | 97.31 | P |
| RSV-diG (isomer-2) | 4.89 | 579.1355 | C26H28O15 | −1.85 | 97.46 | P |
| RSV-SG (isomer-1) | 5.21 | 483.0603 | C20H20O12S | −1.15 | 98.68 | P |
| RSV-SG (isomer-2) | 5.76 | 483.0603 | C20H20O12S | 0.10 | 99.15 | P |
| RSV-4′G | 6.27 | 403.1035 | C20H20O9 | 0.01 | 99.57 | P, E-EVs |
| RSV-4′S * | 7.57 | 307.0282 | C14H12O6S | −1.96 | 98.01 | P |
| DHRSV-4′G | 7.66 | 405.1191 | C20H22O9 | 0.45 | 99.24 | P, E-EVs |
| RSV-3G * | 7.71 | 403.1035 | C20H20O9 | −0.03 | 97.50 | P, E-EVs |
| DHRSV-S (isomer-1) | 8.12 | 309.0438 | C14H14O6S | −2.63 | 92.35 | P |
| DHRSV-3G * | 8.40 | 405.1191 | C20H22O9 | −2.41 | 97.45 | P, E-EVs |
| RSV-3S * | 8.72 | 307.0282 | C14H12O6S | −2.85 | 94.23 | P, E-EVs |
| DHRSV-S (isomer-2) | 8.81 | 309.0438 | C14H14O6S | 1.51 | 97.51 | P, E-EVs |
| RSV * | 10.94 | 227.0714 | C14H12O3 | 0.07 | 90.87 | P |
| LUNU-G (isomer-1) | 11.54 | 389.1242 | C20H22O8 | −3.45 | 94.95 | P, E-EVs |
| LUNU-G (isomer-2) | 11.62 | 389.1242 | C20H22O8 | 0.73 | 99.36 | P, E-EVs |
| LUNU-S (isomer-1) | 11.64 | 293.0489 | C14H14O5S | −1.55 | 91.21 | P, E-EVs |
| LUNU-S (isomer-2) | 12.18 | 293.0489 | C14H14O5S | −3.53 | 90.93 | P |
| Metabolites | T1/2 (h) | Tmax (h) | Cmax | Clast/Cmax | AUC0–24 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Plasma | E-EVs | Plasma | E-EVs | Plasma (nM) | E-EVs (pmol/µg protein) | Plasma | E-EVs | Plasma (nM·h) | E-EVs (pmol/µg protein·h) | |
| DHRSV-3G | 13.0 ± 11.9 | 11.7 ± 12.2 | 6.0 ± 2.8 | 5.9 ± 3.9 | 902 ± 895 | 28.1 ± 22.0 | 0.67 ± 0.31 | 0.55 ± 0.32 | 6196 ± 7702 | 144 ± 132 |
| RSV-3S | 4.8 ± 2.4 | # | 2.7 ± 1.6 | 1.8 ± 1.0 | 6481 ± 5387 | 10.5 ± 11.5 | 0.36 ± 0.20 | 0.66 ± 0.37 | 30,208 ± 24391 | 19.2 ± 19.4 |
| RSV-4′S | 6.9 ± 2.2 | – | 2.6 ± 1.2 | – | 271 ± 688 | – | 0.47 ± 0.11 | – | 1881 ± 5011 | – |
| RSV-3G | 4.1 ± 2.2 | 3.1 ± 1.8 | 2.3 ± 0.7 | 2.6 ± 2.2 | 843 ± 530 | 9.0 ± 8.5 | 0.23 ± 0.13 | 0.29 ± 0.34 | 3510 ± 2050 | 26.2 ± 23.6 |
| T1/2 (h) | Tmax (h) | Cmax | Clast/Cmax | AUC0–24 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean Diff. | p | Mean Diff. | p | Mean Diff. | p | Mean Diff. | p | Mean Diff. | p | |
| Metabolite pairs in plasma | ||||||||||
| RSV-3S vs. RSV-4′S | −2.11 | ns | 0.17 | ns | 6209 | <0.001 | 28327 | <0.001 | −0.12 | ns |
| RSV-3S vs. RSV-3G | 0.71 | ns | 0.42 | ns | 5638 | <0.001 | 26698 | <0.001 | 0.13 | ns |
| RSV-3S vs. DHRSV-3G | −8.21 | 0.003 | −3.26 | <0.001 | 5579 | <0.001 | 24012 | <0.001 | −0.32 | <0.001 |
| RSV-4′S vs. RSV-3G | 2.83 | ns | 0.25 | ns | −571.1 | ns | −1629 | ns | 0.24 | 0.005 |
| RSV-4′S vs. DHRSV-3G | −6.09 | 0.03 | −3.44 | <0.001 | −630.8 | ns | −4315 | ns | −0.19 | 0.03 |
| RSV-3G vs. DHRSV-3G | −8.92 | <0.001 | −3.69 | <0.001 | −59.69 | ns | −2686 | ns | −0.44 | <0.001 |
| Metabolite pairs in E-EVs | ||||||||||
| RSV-3S vs. RSV-3G | – | – | −0.86 | ns | 4.29 | ns | 0.33 | ns | −5.178 | ns |
| RSV-3S vs. DHRSV-3G | – | – | −4.08 | 0.005 | −14.82 | ns | 0.07 | ns | −123.0 | 0.004 |
| RSV-3G vs. DHRSV-3G | – | 0.03 a | −3.214 | 0.01 | −19.11 | 0.008 | −0.2647 | ns | −117.8 | 0.002 |
| Time Points | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolites | 0 h | 1 h | 2 h | 3 h | 8 h | 10 h | |||||||
| Plasma | E-EVs | Plasma | E-EVs | Plasma | E-EVs | Plasma | E-EVs | Plasma | E-EVs | Plasma | E-EVs | Mean Plasma/E-EVs (-Fold) | |
| RSV-3G | 0.2 ± 0.2 | 0.04 ± 0.03 | 0.3 ± 0.3 | 0.1 ± 0.2 | 0.8 ± 0.5 | 0.2 ± 0.3 | 0.6 ± 0.4 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.04 ± 0.04 | 0.15 ± 0.09 | 0.03 ± 0.03 | |
| Plasma/E-EVs (-fold) | 5.2 | 3 | 4 | 6 | 2.5 | 5 | 4.3 ± 1.3 | ||||||
| RSV-3S | 1.6 ± 1.6 | 0.1 ± 0.03 | 2.9 ± 2.9 | 0.2 ± 0.3 | 4.2 ± 4.8 | 0.4 ± 0.6 | 2.6 ± 2.7 | 0.2 ± 0.1 | 1.9 ± 1.6 | 0.1 ± 0.04 | 1.9 ± 1.8 | 0.3 ± 0.3 | |
| Plasma/E-EVs (-fold) | 16 | 14.5 | 10.5 | 13 | 19 | 6.3 | 13.2 ± 4.4 | ||||||
| DHRSV-3G | 0.3 ± 0.6 | 0.3 ± 0.6 | 0.3 ± 0.5 | 0.4 ± 0.4 | 0.5 ± 0.7 | 0.4 ± 0.3 | 0.6 ± 0.8 | 0.4 ± 0.4 | 0.7 ± 0.8 | 0.5 ± 0.3 | 0.7 ± 0.8 | 0.6 ± 0.4 | |
| Plasma/E-EVs (-fold) | 1 | 0.7 | 1.2 | 1.5 | 1.4 | 1.2 | 1.1 ± 0.3 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias-Aguirre, C.E.; Ávila-Gálvez, M.Á.; López de las Hazas, M.-C.; Dávalos, A.; Espín, J.C. Exosome-Containing Extracellular Vesicles Contribute to the Transport of Resveratrol Metabolites in the Bloodstream: A Human Pharmacokinetic Study. Nutrients 2022, 14, 3632. https://doi.org/10.3390/nu14173632
Iglesias-Aguirre CE, Ávila-Gálvez MÁ, López de las Hazas M-C, Dávalos A, Espín JC. Exosome-Containing Extracellular Vesicles Contribute to the Transport of Resveratrol Metabolites in the Bloodstream: A Human Pharmacokinetic Study. Nutrients. 2022; 14(17):3632. https://doi.org/10.3390/nu14173632
Chicago/Turabian StyleIglesias-Aguirre, Carlos Eduardo, María Ángeles Ávila-Gálvez, María-Carmen López de las Hazas, Alberto Dávalos, and Juan Carlos Espín. 2022. "Exosome-Containing Extracellular Vesicles Contribute to the Transport of Resveratrol Metabolites in the Bloodstream: A Human Pharmacokinetic Study" Nutrients 14, no. 17: 3632. https://doi.org/10.3390/nu14173632
APA StyleIglesias-Aguirre, C. E., Ávila-Gálvez, M. Á., López de las Hazas, M.-C., Dávalos, A., & Espín, J. C. (2022). Exosome-Containing Extracellular Vesicles Contribute to the Transport of Resveratrol Metabolites in the Bloodstream: A Human Pharmacokinetic Study. Nutrients, 14(17), 3632. https://doi.org/10.3390/nu14173632