Reconfiguration of Gut Microbiota and Reprogramming of Liver Metabolism with Phycobiliproteins Bioactive Peptides to Rehabilitate Obese Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. PPE
2.2. Rats and Diets
2.3. Glucose Tolerance Test
2.4. Serum Biochemical Analysis
2.5. Gene Expression
2.6. Metagenomics
2.7. Metabolomic Study of Rat Serum by UHPLC-MS/MS
2.8. Short-Chain Fatty Acids (SCFAs) Analysis in Feces
2.9. Statistical Analyses
3. Results
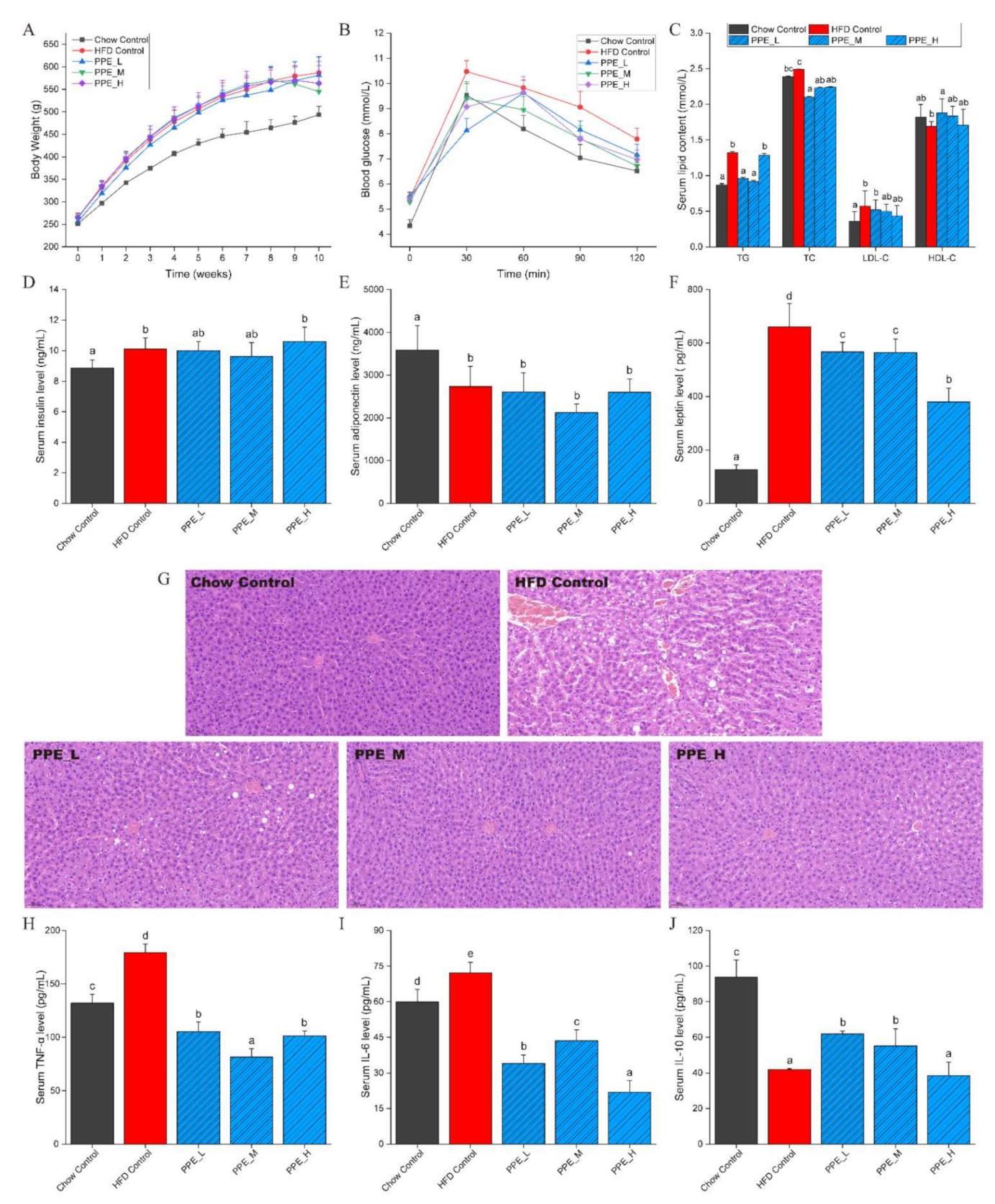
3.1. PPE Reduced Body Weight and Ameliorated Serum Glucose and Lipid Indexes
3.2. PPE Inhibited Liver Lesions and Inflammation in Obese Rats
3.3. PPE Attenuated Liver Steatosis and Insulin Resistance by Regulated Genes in Obese Rats’ Liver
3.4. PPE Attenuated Gut Microbiota Dysbiosis in Obese Rats and Maintained Glucose and Lipid Metabolism Homeostasis
3.5. PPE Promotes SCFAs Production Especially Butyric Acid
3.6. Impact of PPE on the Metabolic Profile in Blood Serum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, J.; Wang, D.; Meng, Z.; Yan, S.; Teng, M.; Jia, M.; Li, R.; Tian, S.; Weiss, C.; Zhou, Z.; et al. Effects of incremental endosulfan sulfate exposure and high fat diet on lipid metabolism, glucose homeostasis and gut microbiota in mice. Environ. Pollut. 2021, 268, 115697. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zeng, T.; Lee, K.; Nobis, M.; Loh, K.; Gou, L.; Xia, Z.; Gao, Z.; Bensellam, M.; Hughes, W.; et al. Peripheral-specific Y1 receptor antagonism increases thermogenesis and protects against diet-induced obesity. Nat. Commun. 2021, 12, 2622. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Backhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, N.; Sun, Y.; Wu, S.; Tian, W.; Lai, Y.; Li, P.; Du, B. Supplementation of Bacillus sp. DU-106 reduces hypercholesterolemia and ameliorates gut dysbiosis in high-fat diet rats. Appl. Microbiol. Biotechnol. 2021, 105, 287–299. [Google Scholar] [CrossRef]
- Koutoukidis, D.A.; Jebb, S.A.; Zimmerman, M.; Otunla, A.; Henry, J.A.; Ferrey, A.; Schofield, E.; Kinton, J.; Aveyard, P.; Marchesi, J.R. The association of weight loss with changes in the gut microbiota diversity, composition, and intestinal permeability: A systematic review and meta-analysis. Gut Microbes 2022, 14, 2020068. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, L.; Zhang, Q.; Zhang, J.; Liu, S.; Li, C.; Wang, L. Glycolipid Metabolism and Metagenomic Analysis of the Therapeutic Effect of a Phenolics-Rich Extract from Noni Fruit on Type 2 Diabetic Mice. J. Agric. Food Chem. 2022, 70, 2876–2888. [Google Scholar] [CrossRef]
- Zhuang, P.; Li, H.; Jia, W.; Shou, Q.; Zhu, Y.; Mao, L.; Wang, W.; Wu, F.; Chen, X.; Wan, X.; et al. Eicosapentaenoic and docosahexaenoic acids attenuate hyperglycemia through the microbiome-gut-organs axis in db/db mice. Microbiome 2021, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wei, W.; Guan, X.; Liu, Y.; Bian, G.; He, D.; Fan, Q.; Cai, X.; Zhang, Y.; Wang, G.; et al. A diet-microbial metabolism feedforward loop modulates intestinal stem cell renewal in the stressed gut. Nat. Commun. 2021, 12, 271. [Google Scholar] [CrossRef]
- Zhubi-Bakija, F.; Bajraktari, G.; Bytyci, I.; Mikhailidis, D.P.; Henein, M.Y.; Latkovskis, G.; Rexhaj, Z.; Zhubi, E.; Banach, M.; The International Lipid Expert Panel. The impact of type of dietary protein, animal versus vegetable, in modifying cardiometabolic risk factors: A position paper from the International Lipid Expert Panel (ILEP). Clin. Nutr. 2021, 40, 255–276. [Google Scholar] [CrossRef]
- Ren, H.; Liu, T.C.; Lu, Y.; Zhang, K.; Xu, Y.; Zhou, P.; Tang, X. A comparison study of the influence of milk protein versus whey protein in high-protein diets on adiposity in rats. Food Funct. 2021, 12, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Hua, P.; Yu, Z.; Xiong, Y.; Liu, B.; Zhao, L. Regulatory Efficacy of Spirulina platensis Protease Hydrolyzate on Lipid Metabolism and Gut Microbiota in High-Fat Diet-Fed Rats. Int. J. Mol. Sci. 2018, 19, 4023. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Tong, Y.; Yan, D.; Jia, S.; Ostenson, C.G.; Chen, Z. The Soybean Peptide Vglycin Preserves the Diabetic beta-cells through Improvement of Proliferation and Inhibition of Apoptosis. Sci. Rep. 2015, 5, 15599. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhou, F.; Zhao, M.; Su, G.; Sun, B. Chicken breast muscle hydrolysates ameliorate acute alcohol-induced liver injury in mice through alcohol dehydrogenase (ADH) activation and oxidative stress reduction. Food Funct. 2018, 9, 774–784. [Google Scholar] [CrossRef]
- Azzurra, S.; Sengul, U.; Ramazan, C.; Gokhan, Z. Natural Occurring β-Peptides: A Fascinating World of Bioactive Molecules. Curr. Bioact. Compd. 2018, 14, 3–8. [Google Scholar]
- Stefanucci, A.; Dimmito, M.P.; Tenore, G.; Pieretti, S.; Minosi, P.; Zengin, G.; Sturaro, C.; Calò, G.; Novellino, E.; Cichelli, A.; et al. Plant-derived peptides rubiscolin-6, soymorphin-6 and their c-terminal amide derivatives: Pharmacokinetic properties and biological activity. J. Funct. Foods 2020, 73, 104154. [Google Scholar] [CrossRef]
- Quintal-Bojórquez, N.; Segura-Campos, M.R. Bioactive Peptides as Therapeutic Adjuvants for Cancer. Nutr. Cancer 2021, 73, 1309–1321. [Google Scholar] [CrossRef]
- Youssef, F.S.; Ashour, M.L.; Singab, A.N.B.; Wink, M. A Comprehensive Review of Bioactive Peptides from Marine Fungi and Their Biological Significance. Mar. Drugs 2019, 17, 559. [Google Scholar] [CrossRef]
- Anekthanakul, K.; Senachak, J.; Hongsthong, A.; Charoonratana, T.; Ruengjitchatchawalya, M. Natural ACE inhibitory peptides discovery from Spirulina (Arthrospira platensis) strain C1. Peptides 2019, 118, 170107. [Google Scholar] [CrossRef]
- Li, Y.; Aiello, G.; Bollati, C.; Bartolomei, M.; Arnoldi, A.; Lammi, C. Phycobiliproteins from Arthrospira platensis (Spirulina): A New Source of Peptides with Dipeptidyl Peptidase-IV Inhibitory Activity. Nutrients 2020, 12, 794. [Google Scholar] [CrossRef]
- Nagaoka, S.; Shimizu, K.; Kaneko, H.; Shibayama, F.; Morikawa, K.; Kanamaru, Y.; Otsuka, A.; Hirahashi, T.; Kato, T. A novel protein C-phycocyanin plays a crucial role in the hypocholesterolemic action of Spirulina platensis concentrate in rats. J. Nutr. 2005, 135, 2425–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, Y.; Lin, L.; Yang, X.; Pan, Q.; Cheng, X. Antidiabetic potential of phycocyanin: Effects on KKAy mice. Pharm. Biol. 2013, 51, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Carrizzo, A.; Conte, G.M.; Sommella, E.; Damato, A.; Ambrosio, M.; Sala, M.; Scala, M.C.; Aquino, R.P.; De Lucia, M.; Madonna, M.; et al. Novel Potent Decameric Peptide of Spirulina platensis Reduces Blood Pressure Levels Through a PI3K/AKT/eNOS-Dependent Mechanism. Hypertension 2019, 73, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Cherng, S.-C.; Cheng, S.-N.; Tarn, A.; Chou, T.-C. Anti-inflammatory activity of c-phycocyanin in lipopolysaccharide-stimulated RAW 264.7 macrophages. Life Sci. 2007, 81, 1431–1435. [Google Scholar] [CrossRef]
- Estrada, J.E.P.; Bescos, P.B.; del Fresno, A.M.V. Antioxidant activity of different fractions of Spirulina platensis protean extract. Farmaco 2001, 56, 497–500. [Google Scholar] [CrossRef]
- Li, Y.; Lammi, C.; Boschin, G.; Arnoldi, A.; Aiello, G. Recent Advances in Microalgae Peptides: Cardiovascular Health Benefits and Analysis. J. Agric. Food Chem. 2019, 67, 11825–11838. [Google Scholar] [CrossRef]
- Ou, Y.; Ren, Z.; Wang, J.; Yang, X. Phycocyanin ameliorates alloxan-induced diabetes mellitus in mice: Involved in insulin signaling pathway and GK expression. Chem. Biol. Interact. 2016, 247, 49–54. [Google Scholar] [CrossRef]
- Liu, J.; Bai, X.; Fu, P. In silico and in vitro assessment of bioactive peptides from Arthrospira platensis phycobiliproteins for DPP-IV inhibitory activity, ACE inhibitory activity, and antioxidant activity. J. Appl. Phycol. 2022, 34, 1497–1511. [Google Scholar] [CrossRef]
- Gargouri, M.; Magne, C.; El Feki, A. Hyperglycemia, oxidative stress, liver damage and dysfunction in alloxan-induced diabetic rat are prevented by Spirulina supplementation. Nutr. Res. 2016, 36, 1255–1268. [Google Scholar] [CrossRef]
- Heo, M.G.; Choung, S.Y. Anti-obesity effects of Spirulina maxima in high fat diet induced obese rats via the activation of AMPK pathway and SIRT1. Food Funct. 2018, 9, 4906–4915. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, H.; Wang, J.; Shan, D.; Xu, Q. Serum metabolomics analysis of the intervention effect of whole grain oats on insulin resistance induced by high-fat diet in rats. Food Res. Int. 2020, 135, 109297. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Noguchi, H.; Park, J.; Takagi, T. MetaGene: Prokaryotic gene finding from environmental genome shotgun sequences. Nucleic Acids Res. 2006, 34, 5623–5630. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Zhao, G.; Nyman, M.; Jonsson, J.A. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed. Chromatogr. 2006, 20, 674–682. [Google Scholar] [CrossRef]
- Bar, N.; Korem, T.; Weissbrod, O.; Zeevi, D.; Rothschild, D.; Leviatan, S.; Kosower, N.; Lotan-Pompan, M.; Weinberger, A.; Le Roy, C.I.; et al. A reference map of potential determinants for the human serum metabolome. Nature 2020, 588, 135–140. [Google Scholar] [CrossRef]
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Censin, J.C.; Peters, S.A.E.; Bovijn, J.; Ferreira, T.; Pulit, S.L.; Magi, R.; Mahajan, A.; Holmes, M.V.; Lindgren, C.M. Causal relationships between obesity and the leading causes of death in women and men. PLoS Genet. 2019, 15, e1008405. [Google Scholar] [CrossRef] [PubMed]
- Sheu, M.J.; Hsieh, Y.Y.; Lai, C.H.; Chang, C.C.; Wu, C.H. Antihyperlipidemic and Antioxidant Effects of C-phycocyanin in Golden Syrian Hamsters Fed with a Hypercholesterolemic Diet. J. Tradit. Complement Med. 2013, 3, 41–47. [Google Scholar] [CrossRef]
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; Gonzalez-Gay, M.A.; Gómez-Reino, J.J.; Mera, A.; Lago, F.; Gómez, R.; Gualillo, O. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109. [Google Scholar] [CrossRef]
- Naeini, F.; Namkhah, Z.; Ostadrahimi, A.; Tutunchi, H.; Hosseinzadeh-Attar, M.J. A Comprehensive Systematic Review of the Effects of Naringenin, a Citrus-Derived Flavonoid, on Risk Factors for Nonalcoholic Fatty Liver Disease. Adv. Nutr. 2021, 12, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Jay, A.G.; Hamilton, J.A. The enigmatic membrane fatty acid transporter CD36: New insights into fatty acid binding and their effects on uptake of oxidized LDL. Prostaglandins Leukot. Essent. Fat. Acids 2018, 138, 64–70. [Google Scholar] [CrossRef]
- Ruiz, R.; Jideonwo, V.; Ahn, M.; Surendran, S.; Tagliabracci, V.S.; Hou, Y.; Gamble, A.; Kerner, J.; Irimia-Dominguez, J.M.; Puchowicz, M.A.; et al. Sterol Regulatory Element-binding Protein-1 (SREBP-1) Is Required to Regulate Glycogen Synthesis and Gluconeogenic Gene Expression in Mouse Liver. J. Biol. Chem. 2014, 289, 5510–5517. [Google Scholar] [CrossRef]
- Yang, J.Y.; Zhang, T.T.; Dong, Z.; Shi, H.H.; Xu, J.; Mao, X.Z.; Wang, Y.M.; Xue, C.H. Dietary Supplementation with Exogenous Sea-Cucumber-Derived Ceramides and Glucosylceramides Alleviates Insulin Resistance in High-Fructose-Diet-Fed Rats by Upregulating the IRS/PI3K/Akt Signaling Pathway. J. Agric. Food Chem. 2021, 69, 9178–9187. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.C.; Strassburger, K.; Nowotny, B.; Kolb, H.; Nowotny, P.; Burkart, V.; Zivehe, F.; Hwang, J.H.; Stehle, P.; Pacini, G.; et al. Intake of Lactobacillus reuteri improves incretin and insulin secretion in glucose-tolerant humans: A proof of concept. Diabetes Care 2015, 38, 1827–1834. [Google Scholar] [CrossRef]
- Qin, R.; Wang, J.; Chao, C.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. RS5 Produced More Butyric Acid through Regulating the Microbial Community of Human Gut Microbiota. J. Agric. Food Chem. 2021, 69, 3209–3218. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Gueimonde, M.; Duncan, S.H.; Flint, H.J.; de los Reyes-Gavilan, C.G. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol. Lett. 2015, 362, fnv176. [Google Scholar] [CrossRef]
- Benevides, L.; Burman, S.; Martin, R.; Robert, V.; Thomas, M.; Miquel, S.; Chain, F.; Sokol, H.; Bermudez-Humaran, L.G.; Morrison, M.; et al. New Insights into the Diversity of the Genus Faecalibacterium. Front. Microbiol. 2017, 8, 1790. [Google Scholar] [CrossRef] [PubMed]
- Roth-Schulze, A.J.; Penno, M.A.S.; Ngui, K.M.; Oakey, H.; Bandala-Sanchez, E.; Smith, A.D.; Allnutt, T.R.; Thomson, R.L.; Vuillermin, P.J.; Craig, M.E.; et al. Type 1 diabetes in pregnancy is associated with distinct changes in the composition and function of the gut microbiome. Microbiome 2021, 9, 167. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Kang, Y.; Li, Y.; Du, Y.; Guo, L.; Chen, M.; Huang, X.; Yang, F.; Hong, J.; Kong, X. Konjaku flour reduces obesity in mice by modulating the composition of the gut microbiota. Int. J. Obes. 2019, 43, 1631–1643. [Google Scholar] [CrossRef]
- Huang, J.; Huang, J.; Yin, T.; Lv, H.; Zhang, P.; Li, H. Enterococcus faecium R0026 Combined with Bacillus subtilis R0179 Prevent Obesity-Associated Hyperlipidemia and Modulate Gut Microbiota in C57BL/6 Mice. J. Microbiol. Biotechnol. 2021, 31, 181–188. [Google Scholar] [CrossRef]
- Marciňáková, M.; Simonová, M.; Strompfová, V.; Lauková, A. Oral application of Enterococcus faecium strain EE3 in healthy dogs. Folia Microbiol. 2006, 51, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Hong, E.; Oh, S.; Kim, E. Non-Viable Lactobacillus johnsonii JNU3402 Protects against Diet-Induced Obesity. Foods 2020, 9, 1494. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.B.; Yassour, M.; Sauk, J.; Garner, A.; Jiang, X.; Arthur, T.; Lagoudas, G.K.; Vatanen, T.; Fornelos, N.; Wilson, R.; et al. A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med. 2017, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-L.; Chu, H.-F.; Dai, F.-J.; Yu, T.-Y.; Chau, C.-F. Intestinal Health Benefits of the Water-Soluble Carbohydrate Concentrate of Wild Grape (Vitis thunbergii) in Hamsters. J. Agric. Food Chem. 2012, 60, 4854–4858. [Google Scholar] [CrossRef]
- Yuan, Y.Q.; Liu, Q.B.; Zhao, F.Q.; Cao, J.; Shen, X.R.; Li, C. Holothuria leucospilota Polysaccharides Ameliorate Hyperlipidemia in High-Fat Diet-Induced Rats via Short-Chain Fatty Acids Production and Lipid Metabolism Regulation. Int. J. Mol. Sci. 2019, 20, 4738. [Google Scholar] [CrossRef]
- Xia, Y.G.; Wang, T.L.; Yu, S.M.; Liang, J.; Kuang, H.X. Structural characteristics and hepatoprotective potential of Aralia elata root bark polysaccharides and their effects on SCFAs produced by intestinal flora metabolism. Carbohydr. Polym. 2019, 207, 256–265. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, P.; Li, Y.F.; Wu, J.; Tan, X.M.; Zhou, J.J.; Sun, Z.L.; Chen, X.; Zhang, G.F.; Zhang, H.P.; et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci. Adv. 2020, 6, eaba8555. [Google Scholar] [CrossRef]
- Lee, J.; Park, A.; Kim, M.J.; Lim, H.J.; Rha, Y.A.; Kang, H.G. Spirulina Extract Enhanced a Protective Effect in Type 1 Diabetes by Anti-Apoptosis and Anti-ROS Production. Nutrients 2017, 9, 1363. [Google Scholar] [CrossRef]
- Ren, Z.; Xie, Z.; Cao, D.; Gong, M.; Yang, L.; Zhou, Z.; Ou, Y. C-Phycocyanin inhibits hepatic gluconeogenesis and increases glycogen synthesis via activating Akt and AMPK in insulin resistance hepatocytes. Food Funct. 2018, 9, 2829–2839. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhen, D.; Hu, C.; Liu, Y.; Shen, X.; Fu, P.; He, Y. Reconfiguration of Gut Microbiota and Reprogramming of Liver Metabolism with Phycobiliproteins Bioactive Peptides to Rehabilitate Obese Rats. Nutrients 2022, 14, 3635. https://doi.org/10.3390/nu14173635
Liu J, Zhen D, Hu C, Liu Y, Shen X, Fu P, He Y. Reconfiguration of Gut Microbiota and Reprogramming of Liver Metabolism with Phycobiliproteins Bioactive Peptides to Rehabilitate Obese Rats. Nutrients. 2022; 14(17):3635. https://doi.org/10.3390/nu14173635
Chicago/Turabian StyleLiu, Jing, Dongyu Zhen, Changbao Hu, Yawen Liu, Xuanri Shen, Pengcheng Fu, and Yanfu He. 2022. "Reconfiguration of Gut Microbiota and Reprogramming of Liver Metabolism with Phycobiliproteins Bioactive Peptides to Rehabilitate Obese Rats" Nutrients 14, no. 17: 3635. https://doi.org/10.3390/nu14173635
APA StyleLiu, J., Zhen, D., Hu, C., Liu, Y., Shen, X., Fu, P., & He, Y. (2022). Reconfiguration of Gut Microbiota and Reprogramming of Liver Metabolism with Phycobiliproteins Bioactive Peptides to Rehabilitate Obese Rats. Nutrients, 14(17), 3635. https://doi.org/10.3390/nu14173635








