Targeted Metabolomics Revealed a Sex-Dependent Signature for Metabolic Syndrome in the Mexican Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Health Workers Cohort Study (HWCS)
2.2. Metabolic Syndrome Criteria
2.3. Covariates
2.4. Other Measurements
2.5. Targeted Metabolomics Analysis
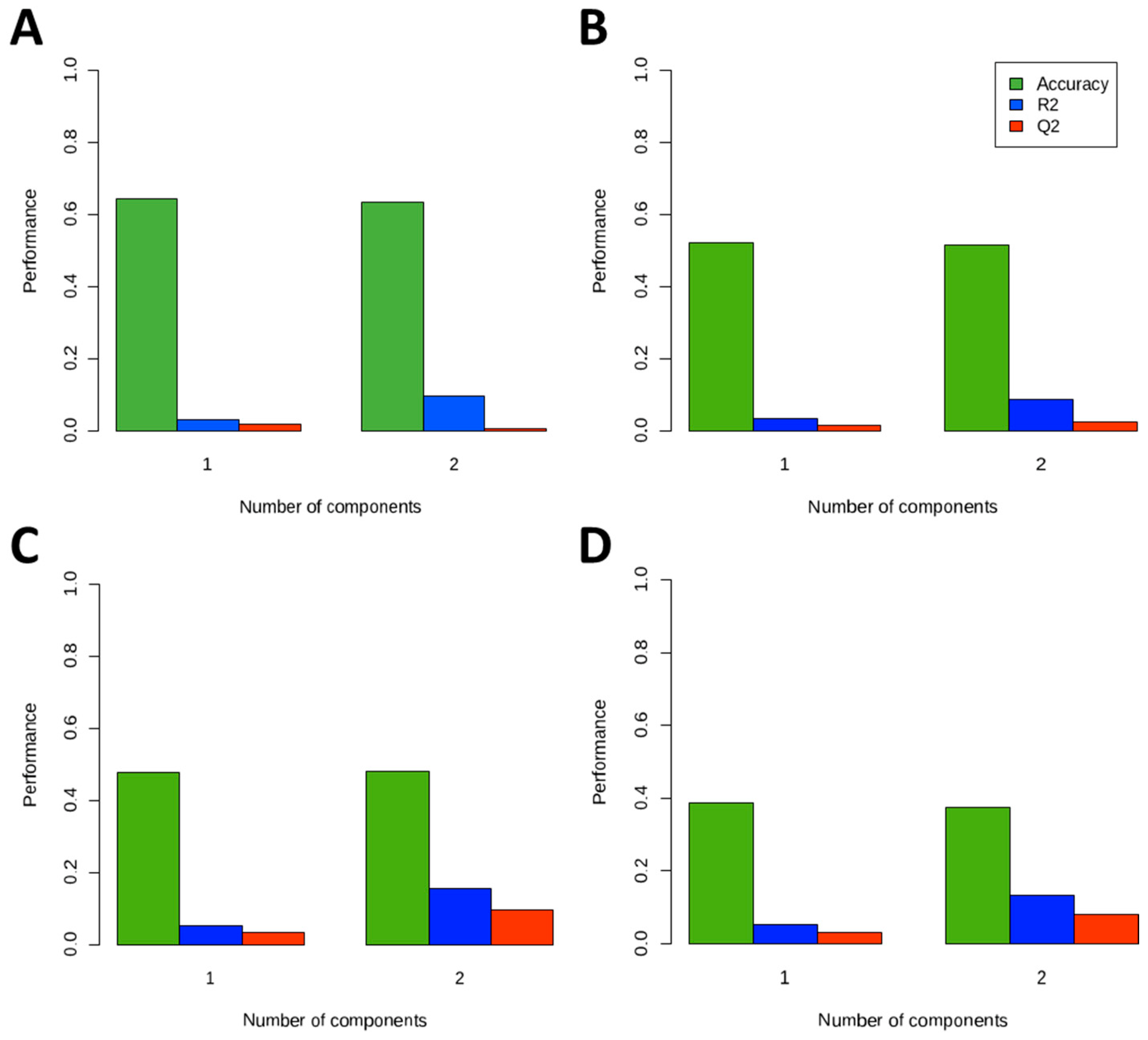
2.6. Statistical Analyses
3. Results
3.1. Demographics and Clinical Characteristics of the Study Population
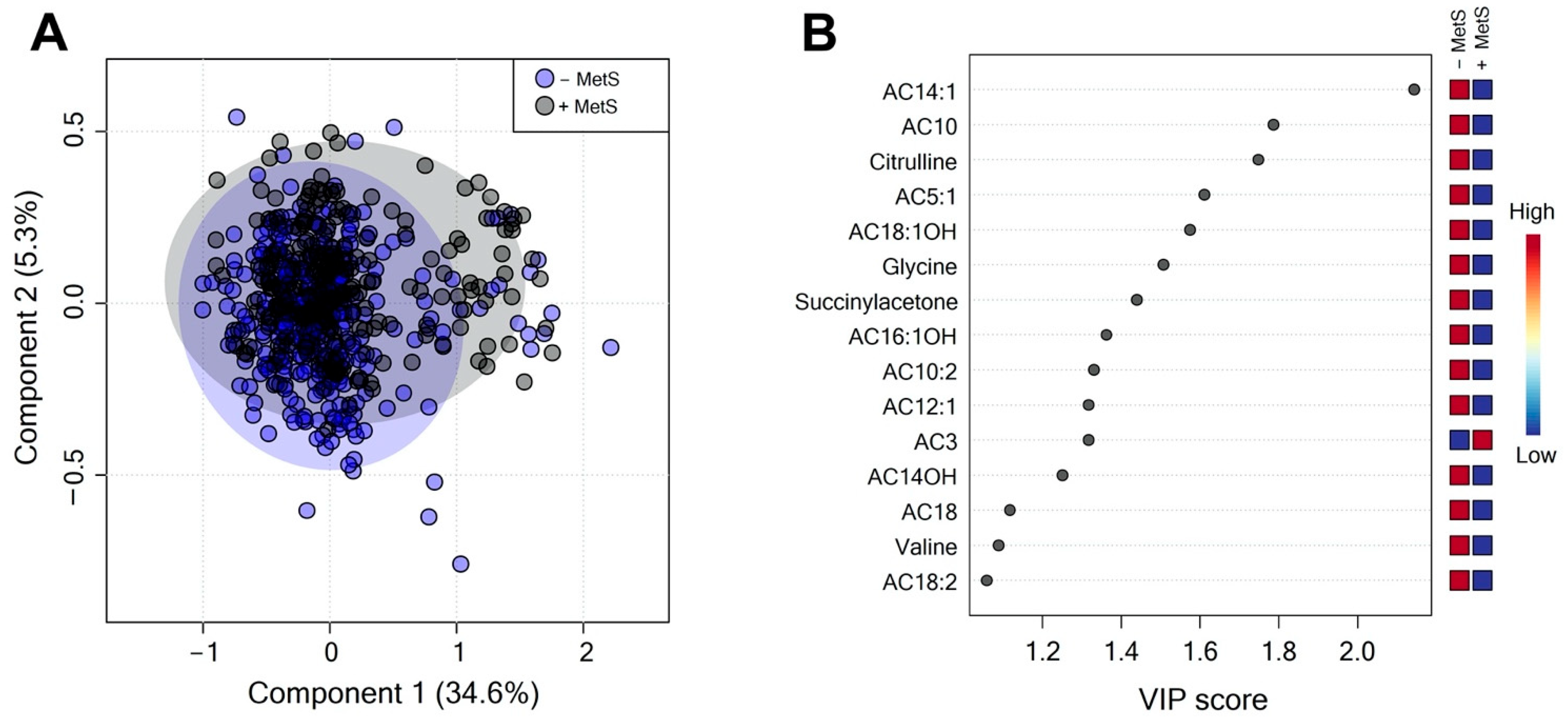
3.2. Serum Metabolite Profile According to Metabolic Syndrome
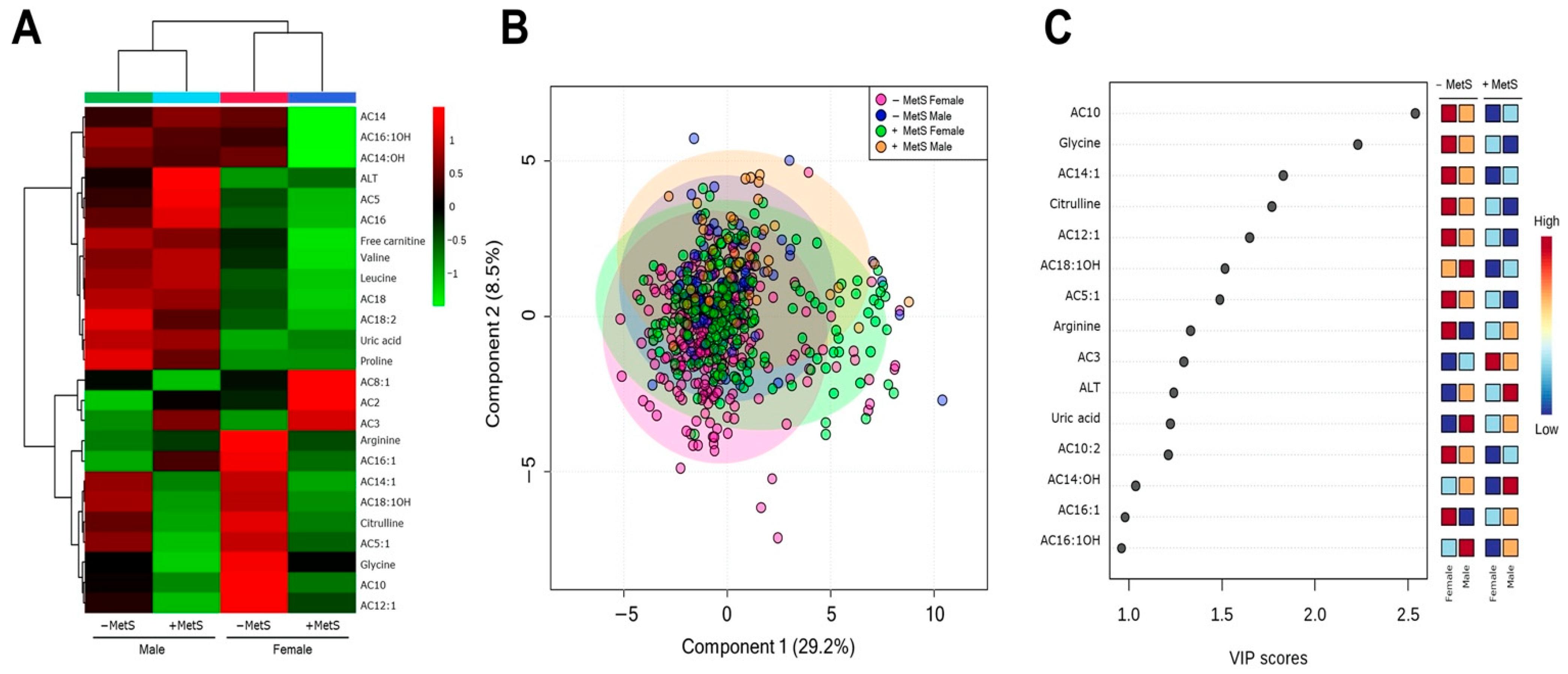
3.3. Metabolic Profile According to Sex-Dependent MetS
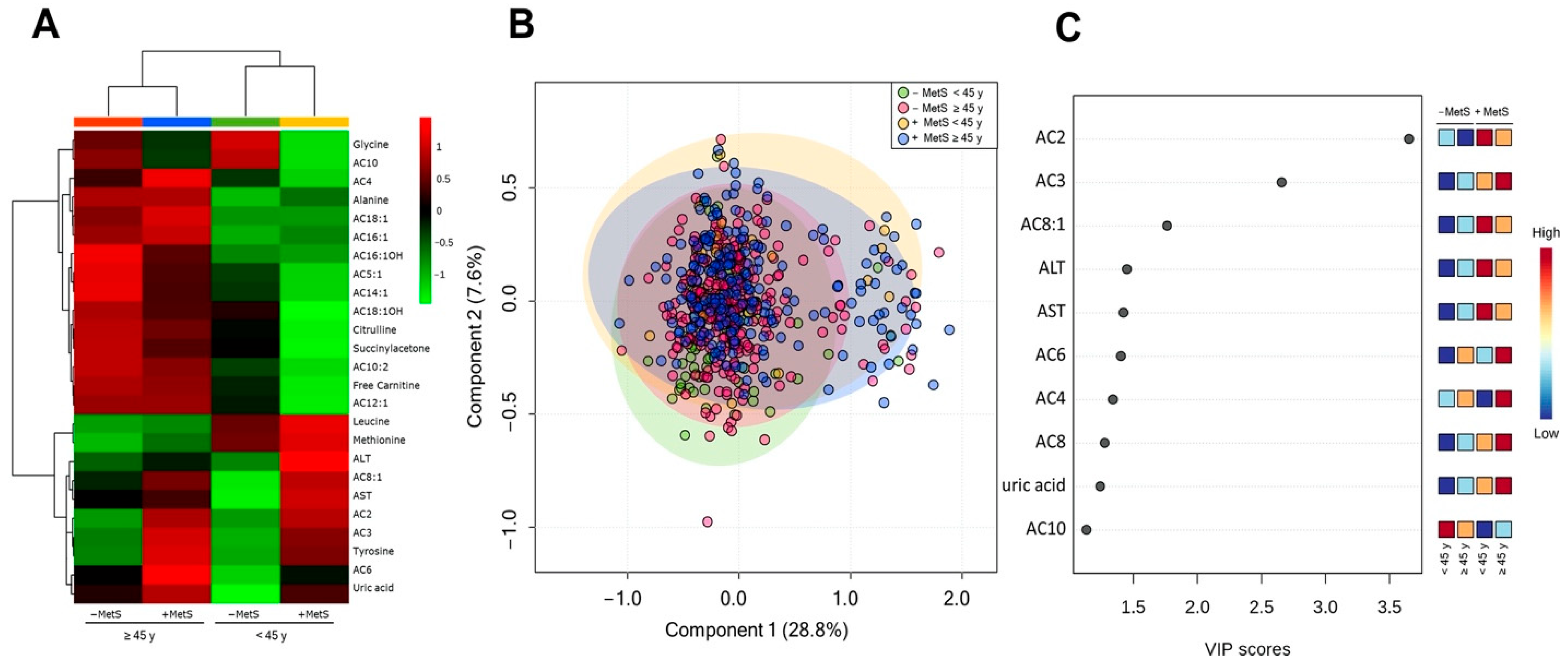
3.4. Metabolic Profile According to Age-Dependent MetS
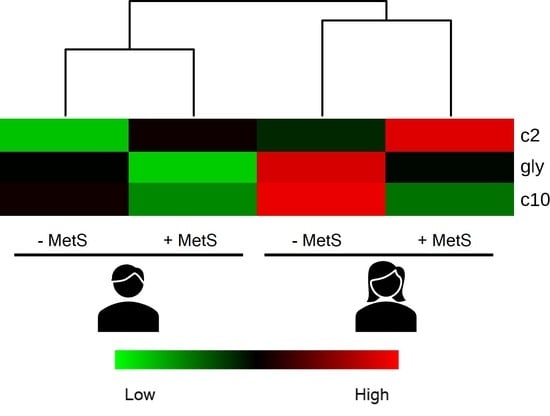
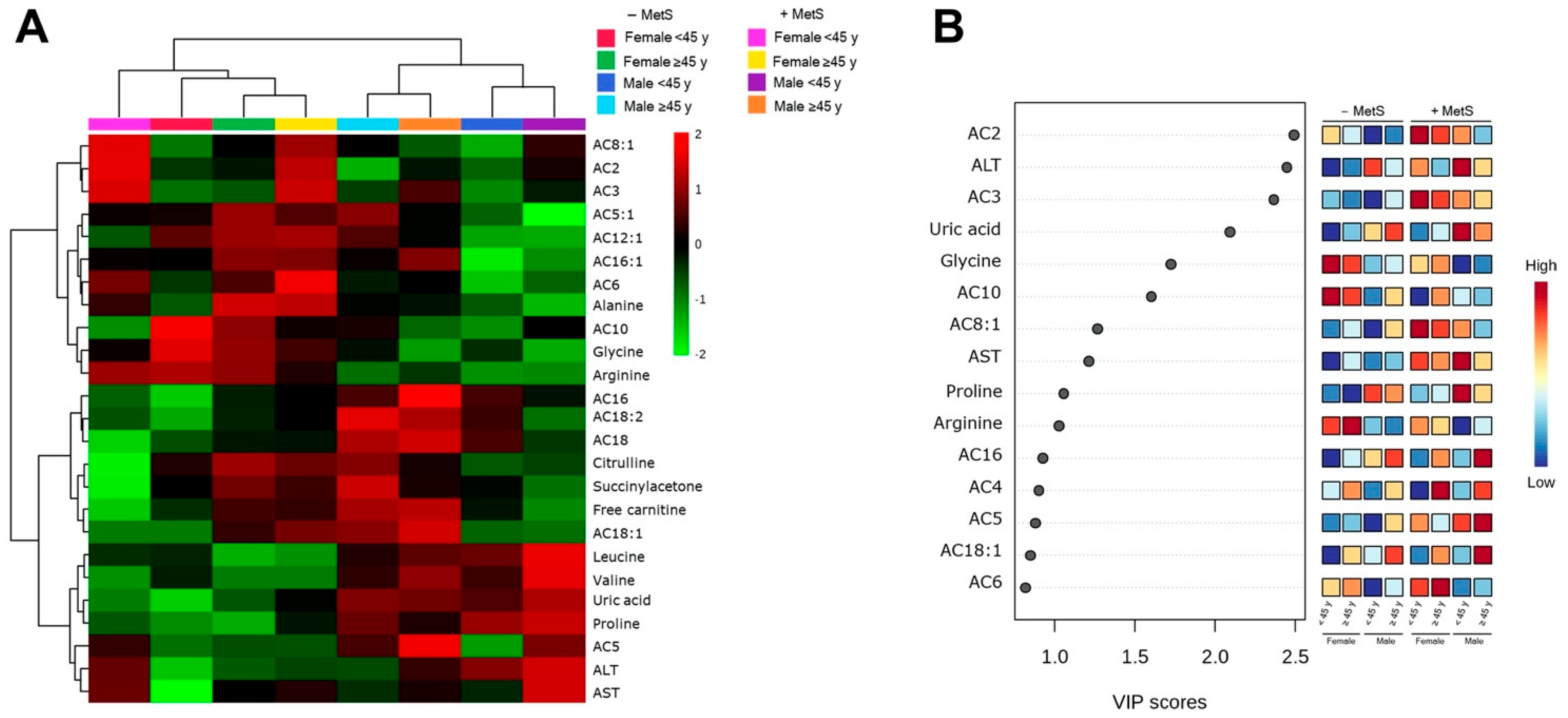
3.5. Metabolic Profile According to Sex-Age Dependent MetS
3.6. Correlation between Body Fat and Metabolites Associated with Sex-Age Dependent MetS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Proline Levels and Glucose Tolerance
| <45 Years | ≥45 Years | ||||
|---|---|---|---|---|---|
| No | Intolerance | No | Intolerance | T2D | |
| Males | 256.4 ± 18.48 | 294.6 ± 27.40 | 210.7 ± 8.60 | 237.7 ± 13.76 | 238.3 ± 11.58 |
| Females | 197.8 ± 60.51 | 239 ± 63.48 * | 200.5 ± 64.21 b | 205.3 ± 61.75 b | 223.2 ± 59.02 a |

References
- Marott, S.C.W.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Benn, M. Components of the Metabolic Syndrome and Risk of Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 3212–3221. [Google Scholar] [CrossRef] [PubMed]
- Pragasam, S.S.J.; Venkatesan, V. Metabolic Syndrome Predisposes to Osteoarthritis: Lessons from Model System. Cartilage 2021, 13, 1598S–1609S. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Jenab, M.; Karimi, M.; Truong, T.; Mahamat-Saleh, Y.; Ferrari, P.; Dashti, S.G.; Kühn, T.; Cross, A.J.; Severi, G.; et al. Metabolic Syndrome and Risk of Gastrointestinal Cancers: An Investigation Using Large-Scale Molecular Data. Clin. Gastroenterol. Hepatol. 2022, 20, e1338–e1352. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Ortiz-Rodríguez, M.A.; Bautista-Ortiz, L.F.; Villa, A.R.; Antúnez-Bautista, P.K.; Aldaz-Rodríguez, M.V.; Estrada-Luna, D.; Denova-Gutiérrez, E.; Camacho-Díaz, B.H.; Martínez-Salazar, M.F. Prevalence of Metabolic Syndrome Among Mexican Adults. Metab. Syndr. Relat. Disord. 2022, 20, 264–272. [Google Scholar] [CrossRef]
- Rojas-Martínez, R.; Aguilar-Salinas, C.A.; Romero-Martínez, M.; Castro-Porras, L.; Gómez-Velasco, D.; Mehta, R. Trends in the Prevalence of Metabolic Syndrome and Its Components in Mexican Adults, 2006–2018. Salud Publica Mex. 2021, 63, 713–724. [Google Scholar] [CrossRef]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the Metabolic Syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef]
- Ziki, M.D.A.; Mani, A. Metabolic Syndrome: Genetic Insights into Disease Pathogenesis. Curr. Opin. Lipidol. 2016, 27, 162–171. [Google Scholar] [CrossRef]
- Monnerie, S.; Comte, B.; Ziegler, D.; Morais, J.A.; Pujos-Guillot, E.; Gaudreau, P. Metabolomic and Lipidomic Signatures of Metabolic Syndrome and Its Physiological Components in Adults: A Systematic Review. Sci. Rep. 2020, 10, 669. [Google Scholar] [CrossRef]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic Syndrome: Definitions and Controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.C.; Chang, H.T.; Chiang, S.C.; Chen, H.S.; Lin, M.H.; Chen, T.J.; Hwang, S.J. Sex Differences in Relationships between Metabolic Syndrome Components and Factors Associated with Health-Related Quality of Life in Middle-Aged Adults Living in the Community: A Cross-Sectional Study in Taiwan. Health Qual. Life Outcomes 2018, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, S.-Y.; Lee, H.; Kim, J.J.; Park, H.E. Sex and Age Differences in the Impact of Metabolic Syndrome and Its Components Including A Body Shape Index on Arterial Stiffness in the General Population. J. Atheroscler. Thromb. 2022, 63371. [Google Scholar] [CrossRef]
- Varlamov, O.; Bethea, C.L.; Roberts, C.T. Sex-Specific Differences in Lipid and Glucose Metabolism. Front. Endocrinol. 2015, 5, 241. [Google Scholar] [CrossRef] [PubMed]
- Trabado, S.; Al-Salameh, A.; Croixmarie, V.; Masson, P.; Corruble, E.; Fève, B.; Colle, R.; Ripoll, L.; Walther, B.; Boursier-Neyret, C.; et al. The Human Plasma-Metabolome: Reference Values in 800 French Healthy Volunteers; Impact of Cholesterol, Gender and Age. PLoS ONE 2017, 12, e0173615. [Google Scholar] [CrossRef] [PubMed]
- Draper, C.F.; Vassallo, I.; di Cara, A.; Milone, C.; Comminetti, O.; Monnard, I.; Godin, J.P.; Scherer, M.; Su, M.M.; Jia, W.; et al. A 48-Hour Vegan Diet Challenge in Healthy Women and Men Induces a BRANCH-Chain Amino Acid Related, Health Associated, Metabolic Signature. Mol. Nutr. Food Res. 2018, 62, 1700703. [Google Scholar] [CrossRef]
- di Cesare, F.; Tenori, L.; Meoni, G.; Gori, A.M.; Marcucci, R.; Giusti, B.; Molino-Lova, R.; Macchi, C.; Pancani, S.; Luchinat, C.; et al. Lipid and Metabolite Correlation Networks Specific to Clinical and Biochemical Covariate Show Differences Associated with Sexual Dimorphism in a Cohort of Nonagenarians. Geroscience 2022, 44, 1109–1128. [Google Scholar] [CrossRef]
- Zhao, X.; Han, Q.; Liu, Y.; Sun, C.; Gang, X.; Wang, G. The Relationship between Branched-Chain Amino Acid Related Metabolomic Signature and Insulin Resistance: A Systematic Review. J. Diabetes Res. 2016, 2016, 2794591. [Google Scholar] [CrossRef]
- Jin, Q.; Ma, R.C.W. Metabolomics in Diabetes and Diabetic Complications: Insights from Epidemiological Studies. Cells 2021, 10, 2832. [Google Scholar] [CrossRef]
- Newgard, C.B. Metabolomics and Metabolic Diseases: Where Do We Stand? Cell Metab. 2017, 25, 43–56. [Google Scholar] [CrossRef]
- Lent-Schochet, D.; McLaughlin, M.; Ramakrishnan, N.; Jialal, I. Exploratory Metabolomics of Metabolic Syndrome: A Status Report. World J. Diabetes 2019, 10, 23–36. [Google Scholar] [CrossRef]
- Siomkajło, M.; Rybka, J.; Mierzchała-Pasierb, M.; Gamian, A.; Stankiewicz-Olczyk, J.; Bolanowski, M.; Daroszewski, J. Specific Plasma Amino Acid Disturbances Associated with Metabolic Syndrome. Endocrine 2017, 58, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Okekunle, A.P.; Li, Y.Y.; Liu, L.; Du, S.; Wu, X.; Chen, Y.; Li, Y.Y.; Qi, J.; Sun, C.; Feng, R. Abnormal Circulating Amino Acid Profiles in Multiple Metabolic Disorders. Diabetes Res. Clin. Pract. 2017, 132, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.N.; Niu, Y.C.; Sun, X.W.; Li, Q.; Zhao, C.; Wang, C.; Guo, F.C.; Sun, C.H.; Li, Y. Histidine Supplementation Improves Insulin Resistance through Suppressed Inflammation in Obese Women with the Metabolic Syndrome: A Randomised Controlled Trial. Diabetologia 2013, 56, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Iida, M.; Harada, S.; Kurihara, A.; Fukai, K.; Kuwabara, K.; Sugiyama, D.; Takeuchi, A.; Okamura, T.; Akiyama, M.; Nishiwaki, Y.; et al. Profiling of Plasma Metabolites in Postmenopausal Women with Metabolic Syndrome. Menopause 2016, 23, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.R.; Ning, Y.; Yu, H.; Tang, N.J. A HPLC-Q-TOF-MS-Based Urinary Metabolomic Approach to Identification of Potential Biomarkers of Metabolic Syndrome. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.L.; Yang, S.; Zhang, W.; Han, F.F.; Xuan, L.L.; Lv, Y.L.; Liu, H.; Liu, L.H.; Tu, W.J. Targeted Metabolomics for Plasma Amino Acids and Carnitines in Patients with Metabolic Syndrome Using HPLC-MS/MS. Dis. Markers 2020, 2020, 8842320. [Google Scholar] [CrossRef]
- Panchal, S.K.; Poudyal, H.; Ward, L.C.; Waanders, J.; Brown, L. Modulation of Tissue Fatty Acids by L-Carnitine Attenuates Metabolic Syndrome in Diet-Induced Obese Rats. Food Funct. 2015, 6, 2496–2506. [Google Scholar] [CrossRef]
- Lee, B.J.; Lin, J.S.; Lin, Y.C.; Lin, P.T. Antiinflammatory Effects of L-Carnitine Supplementation (1000 Mg/d) in Coronary Artery Disease Patients. Nutrition 2015, 31, 475–479. [Google Scholar] [CrossRef]
- Auinger, A.; Rubin, D.; Sabandal, M.; Helwig, U.; Rüther, A.; Schreiber, S.; Foelsch, U.R.; Döring, F.; Schrezenmeir, J. A Common Haplotype of Carnitine Palmitoyltransferase 1b Is Associated with the Metabolic Syndrome. Br. J. Nutr. 2013, 109, 810–815. [Google Scholar] [CrossRef]
- Denova-Gutiérrez, E.; Flores, Y.N.; Gallegos-Carrillo, K.; Ramírez-Palacios, P.; Rivera-Paredez, B.; Muñoz-Aguirre, P.; Velázquez-Cruz, R.; Torres-Ibarra, L.; Meneses-León, J.; Méndez-Hernández, P.; et al. Health Workers Cohort Study: Methods and Study Design. Salud Publica Mex. 2016, 58, 708–716. [Google Scholar] [CrossRef]
- Davis, S.R.; Lambrinoudaki, I.; Lumsden, M.; Mishra, G.D.; Pal, L.; Rees, M.; Santoro, N.; Simoncini, T. Menopause. Nat. Rev. Dis. Primers 2015, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Emaus, N.; Berntsen, G.K.R.; Joakimsen, R.; Fonnebø, V. Longitudinal Changes in Forearm Bone Mineral Density in Women and Men Aged 45-84 Years: The Tromso Study, a Population-Based Study. Am. J. Epidemiol. 2006, 163, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Kolovou, G.; Bilianou, H.; Marvaki, A.; Mikhailidis, D.P. Aging Men and Lipids. Am. J. Mens. Health 2011, 5, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Tate, J.R.; Berg, K.; Couderc, R.; Dati, F.; Kostner, G.M.; Marcovina, S.M.; Rifai, N.; Sakurabayashi, I.; Steinmetz, A. International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Standardization Project for the Measurement of Lipoprotein(a). Phase 2: Selection and Properties of a Proposed Secondary Reference Material for Lipoprotein(a). Clin. Chem. Lab. Med. 1999, 37, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Palacios-González, B.; León-Reyes, G.; Rivera-Paredez, B.; Ibarra-González, I.; Vela-Amieva, M.; Flores, Y.N.; Canizales-Quinteros, S.; Salmerón, J.; Velázquez-Cruz, R. Serum Metabolite Profile Associated with Sex-Dependent Visceral Adiposity Index and Low Bone Mineral Density in a Mexican Population. Metabolites 2021, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Web-Based Inference of Biological Patterns, Functions and Pathways from Metabolomic Data Using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef]
- Chen, Y.L.; Li, H.; Li, S.; Xu, Z.; Tian, S.; Wu, J.; Liang, X.Y.; Li, X.; Liu, Z.L.; Xiao, J.; et al. Prevalence of and Risk Factors for Metabolic Associated Fatty Liver Disease in an Urban Population in China: A Cross-Sectional Comparative Study. BMC Gastroenterol. 2021, 21, 212. [Google Scholar] [CrossRef]
- Pujos-Guillot, E.; Brandolini, M.; Pétéra, M.; Grissa, D.; Joly, C.; Lyan, B.; Herquelot, É.; Czernichow, S.; Zins, M.; Goldberg, M.; et al. Systems Metabolomics for Prediction of Metabolic Syndrome. J. Proteome Res. 2017, 16, 2262–2272. [Google Scholar] [CrossRef]
- Oguoma, V.M.; Abu-Farha, M.; Coffee, N.T.; Alsharrah, S.; Al-Refaei, F.H.; Abubaker, J.; Daniel, M.; Al-Mulla, F. Metabolically Healthy and Unhealthy Obese Phenotypes among Arabs and South Asians: Prevalence and Relationship with Cardiometabolic Indicators. Nutrients 2022, 14, 915. [Google Scholar] [CrossRef]
- McGarry, J.D.; Brown, N.F. The Mitochondrial Carnitine Palmitoyltransferase System. From Concept to Molecular Analysis. Eur. J. Biochem. 1997, 244, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Indiveri, C.; Iacobazzi, V.; Tonazzi, A.; Giangregorio, N.; Infantino, V.; Convertini, P.; Console, L.; Palmieri, F. The Mitochondrial Carnitine/Acylcarnitine Carrier: Function, Structure and Physiopathology. Mol. Asp. Med. 2011, 32, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Koves, T.R.; Li, P.; An, J.; Akimoto, T.; Slentz, D.; Ilkayeva, O.; Dohm, G.L.; Yan, Z.; Newgard, C.B.; Muoio, D.M. Peroxisome Proliferator-Activated Receptor-Gamma Co-Activator 1alpha-Mediated Metabolic Remodeling of Skeletal Myocytes Mimics Exercise Training and Reverses Lipid-Induced Mitochondrial Inefficiency. J. Biol. Chem. 2005, 280, 33588–33598. [Google Scholar] [CrossRef]
- van Hove, J.L.K.; Zhang, W.; Kahler, S.G.; Roe, C.R.; Chen, Y.T.; Terada, N.; Chace, D.H.; Iafolla, A.K.; Ding, J.H.; Millington, D.S. Medium-Chain Acyl-CoA Dehydrogenase (MCAD) Deficiency: Diagnosis by Acylcarnitine Analysis in Blood. Am. J. Hum. Genet. 1993, 52, 958–966. [Google Scholar]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.B.; Newgard, C.B.; et al. Mitochondrial Overload and Incomplete Fatty Acid Oxidation Contribute to Skeletal Muscle Insulin Resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Schooneman, M.G.; Vaz, F.M.; Houten, S.M.; Soeters, M.R. Acylcarnitines: Reflecting or Inflicting Insulin Resistance? Diabetes 2013, 62, 1–8. [Google Scholar] [CrossRef]
- Surowiec, I.; Noordam, R.; Bennett, K.; Beekman, M.; Slagboom, P.E.; Lundstedt, T.; van Heemst, D. Metabolomic and Lipidomic Assessment of the Metabolic Syndrome in Dutch Middle-Aged Individuals Reveals Novel Biological Signatures Separating Health and Disease. Metabolomics 2019, 15, 23. [Google Scholar] [CrossRef]
- Muoio, D.M.; Noland, R.C.; Kovalik, J.P.; Seiler, S.E.; Davies, M.N.; Debalsi, K.L.; Ilkayeva, O.R.; Stevens, R.D.; Kheterpal, I.; Zhang, J.; et al. Muscle-Specific Deletion of Carnitine Acetyltransferase Compromises Glucose Tolerance and Metabolic Flexibility. Cell Metab. 2012, 15, 764–777. [Google Scholar] [CrossRef]
- Mai, M.; Tönjes, A.; Kovacs, P.; Stumvoll, M.; Fiedler, G.M.; Leichtle, A.B. Serum Levels of Acylcarnitines Are Altered in Prediabetic Conditions. PLoS ONE 2013, 8, e82459. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, X.F.; Huang, T.; Luo, H.H.; Chen, J.X.; Zeng, J.; Gu, M.; Li, J.; Sun, X.Y.; Sun, D.; et al. The Association Between Acylcarnitine Metabolites and Cardiovascular Disease in Chinese Patients With Type 2 Diabetes Mellitus. Front. Endocrinol. 2020, 11, 212. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, Z.; Li, Q.; Liu, J.; Tao, L.; Han, Y.; Zhang, J.; Guo, X.; Yang, X. Dose-Response Relationship between Distinct Serum Uric Acid Trajectories and Metabolic Syndrome Risk: A 5-Year Prospective Cohort Study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1189–1199. [Google Scholar] [CrossRef]
- Violante, S.; Achetib, N.; van Roermund, C.W.T.; Hagen, J.; Dodatko, T.; Vaz, F.M.; Waterham, H.R.; Chen, H.; Baes, M.; Yu, C.; et al. Peroxisomes Can Oxidize Medium- and Long-Chain Fatty Acids through a Pathway Involving ABCD3 and HSD17B4. FASEB J. 2019, 33, 4355–4364. [Google Scholar] [CrossRef]
- Ferdinandusse, S.; Mulders, J.; Ijlst, L.; Denis, S.; Dacremont, G.; Waterham, H.R.; Wanders, R.J.A. Molecular Cloning and Expression of Human Carnitine Octanoyltransferase: Evidence for Its Role in the Peroxisomal Beta-Oxidation of Branched-Chain Fatty Acids. Biochem. Biophys. Res. Commun. 1999, 263, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Moon, J.S.; Kang, J.H.; Jang, H.B.; Lee, H.J.; Park, S.I.; Yu, K.S.; Cho, J.Y. Combined Untargeted and Targeted Metabolomic Profiling Reveals Urinary Biomarkers for Discriminating Obese from Normal-Weight Adolescents. Pediatr. Obes. 2017, 12, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Shulman, G.I. Free Fatty Acids in Obesity and Type 2 Diabetes: Defining Their Role in the Development of Insulin Resistance and Beta-Cell Dysfunction. Eur. J. Clin. Investig. 2002, 32 (Suppl. 3), 14–23. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, S.J.; Michaliszyn, S.F.; de Las Heras, J.; Bacha, F.; Lee, S.J.; Chace, D.H.; DeJesus, V.R.; Vockley, J.; Arslanian, S.A. Metabolomic Profiling of Fatty Acid and Amino Acid Metabolism in Youth with Obesity and Type 2 Diabetes: Evidence for Enhanced Mitochondrial Oxidation. Diabetes Care 2012, 35, 605–611. [Google Scholar] [CrossRef]
- LaBarre, J.L.; Peterson, K.E.; Kachman, M.T.; Perng, W.; Tang, L.; Hao, W.; Zhou, L.; Karnovsky, A.; Cantoral, A.; Téllez-Rojo, M.M.; et al. Mitochondrial Nutrient Utilization Underlying the Association Between Metabolites and Insulin Resistance in Adolescents. J. Clin. Endocrinol. Metab. 2020, 105, 2442–2455. [Google Scholar] [CrossRef]
- Sunny, N.E.; Kalavalapalli, S.; Bril, F.; Garrett, T.J.; Nautiyal, M.; Mathew, J.T.; Williams, C.M.; Cusi, K. Cross-Talk between Branched-Chain Amino Acids and Hepatic Mitochondria Is Compromised in Nonalcoholic Fatty Liver Disease. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E311–E319. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between Lipids and Branched-Chain Amino Acids in Development of Insulin Resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef]
- Yao, M.; Xiao, Y.; Yang, Z.; Ge, W.; Liang, F.; Teng, H.; Gu, Y.; Yin, J. Identification of Biomarkers for Preeclampsia Based on Metabolomics. Clin. Epidemiol. 2022, 14, 337–360. [Google Scholar] [CrossRef]
- Connelly, M.A.; Wolak-Dinsmore, J.; Dullaart, R.P.F. Branched Chain Amino Acids Are Associated with Insulin Resistance Independent of Leptin and Adiponectin in Subjects with Varying Degrees of Glucose Tolerance. Metab. Syndr. Relat. Disord. 2017, 15, 183–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddik, M.A.B.; Shin, A.C. Recent Progress on Branched-Chain Amino Acids in Obesity, Diabetes, and Beyond. Endocrinol. Metab. 2019, 34, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and Ectopic Fat, Atherosclerosis, and Cardiometabolic Disease: A Position Statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Patel, M.J.; Batch, B.C.; Svetkey, L.P.; Bain, J.R.; Turer, C.B.; Haynes, C.; Muehlbauer, M.J.; Stevens, R.D.; Newgard, C.B.; Shah, S.H. Race and Sex Differences in Small-Molecule Metabolites and Metabolic Hormones in Overweight and Obese Adults. OMICS 2013, 17, 627–635. [Google Scholar] [CrossRef]
- Liu, Z.; Jeppesen, P.B.; Gregersen, S.; Larsen, L.B.; Hermansen, K. Chronic Exposure to Proline Causes Aminoacidotoxicity and Impaired Beta-Cell Function: Studies In Vitro. Rev. Diabet. Stud. 2016, 13, 66–78. [Google Scholar] [CrossRef]
- Lejawa, M.; Osadnik, K.; Osadnik, T.; Pawlas, N. Association of Metabolically Healthy and Unhealthy Obesity Phenotypes with Oxidative Stress Parameters and Telomere Length in Healthy Young Adult Men. Analysis of the MAGNETIC Study. Antioxidants 2021, 10, 93. [Google Scholar] [CrossRef]
- De Magalhães, E.L.G.; Juvanhol, L.L.; da Silva, D.C.G.; Ferreira, F.G.; Roberto, D.M.T.; Hinnig, P.d.F.; Longo, G.Z. Uric Acid: A New Marker for Metabolic Syndrome? Results of a Population-Based Study with Adults. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2077–2080. [Google Scholar] [CrossRef]
- Tian, S.; Liu, Y.; Feng, A.; Zhang, S. Sex-Specific Differences in the Association of Metabolically Healthy Obesity With Hyperuricemia and a Network Perspective in Analyzing Factors Related to Hyperuricemia. Front. Endocrinol. 2020, 11, 573452. [Google Scholar] [CrossRef]
- Méndez-Salazar, E.O.; Vázquez-Mellado, J.; Casimiro-Soriguer, C.S.; Dopazo, J.; Çubuk, C.; Zamudio-Cuevas, Y.; Francisco-Balderas, A.; Martínez-Flores, K.; Fernández-Torres, J.; Lozada-Pérez, C.; et al. Taxonomic Variations in the Gut Microbiome of Gout Patients with and without Tophi Might Have a Functional Impact on Urate Metabolism. Mol. Med. 2021, 27, 50. [Google Scholar] [CrossRef]
- Alves, A.; Bassot, A.; Bulteau, A.L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hossenzadeh, H. Effects of Glycine on Metabolic Syndrome Components: A Review. J. Endocrinol. Investig. 2022, 45, 927–939. [Google Scholar] [CrossRef]
- Gaggini, M.; Carli, F.; Rosso, C.; Buzzigoli, E.; Marietti, M.; della Latta, V.; Ciociaro, D.; Abate, M.L.; Gambino, R.; Cassader, M.; et al. Altered Amino Acid Concentrations in NAFLD: Impact of Obesity and Insulin Resistance. Hepatology 2018, 67, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Yamakado, M.; Nagao, K.; Imaizumi, A.; Tani, M.; Toda, A.; Tanaka, T.; Jinzu, H.; Miyano, H.; Yamamoto, H.; Daimon, T.; et al. Plasma Free Amino Acid Profiles Predict Four-Year Risk of Developing Diabetes, Metabolic Syndrome, Dyslipidemia, and Hypertension in Japanese Population. Sci. Rep. 2015, 5, 11918. [Google Scholar] [CrossRef] [PubMed]
- Vogelzangs, N.; van der Kallen, C.J.H.; van Greevenbroek, M.M.J.; van der Kolk, B.W.; Jocken, J.W.E.; Goossens, G.H.; Schaper, N.C.; Henry, R.M.A.; Eussen, S.J.P.M.; Valsesia, A.; et al. Metabolic Profiling of Tissue-Specific Insulin Resistance in Human Obesity: Results from the Diogenes Study and the Maastricht Study. Int. J. Obes. 2020, 44, 1376–1386. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; Zhang, W.; Li, H.; Wang, S.; Mu, H.; Zhou, Q.; Zhang, Y.; Tang, Y.; Wang, Y.; et al. Association of Serum Glycine Levels with Metabolic Syndrome in an Elderly Chinese Population. Nutr. Metab. 2018, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S. Amino Acid Metabolism. Cold Spring Harb. Perspect. Biol. 2021, 13, a040584. [Google Scholar] [CrossRef]
- Szlas, A.; Kurek, J.M.; Krejpcio, Z. The Potential of L-Arginine in Prevention and Treatment of Disturbed Carbohydrate and Lipid Metabolism-A Review. Nutrients 2022, 14, 961. [Google Scholar] [CrossRef]
- Jobgen, W.S.; Fried, S.K.; Fu, W.J.; Meininger, C.J.; Wu, G. Regulatory Role for the Arginine-Nitric Oxide Pathway in Metabolism of Energy Substrates. J. Nutr. Biochem. 2006, 17, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Poehlman, E.T. Menopause, Energy Expenditure, and Body Composition. Acta Obstet. Gynecol. Scand. 2005, 84, 1131. [Google Scholar] [CrossRef]
- Franklin, R.M.; Ploutz-Snyder, L.; Kanaley, J.A. Longitudinal Changes in Abdominal Fat Distribution with Menopause. Metabolism 2009, 58, 311–315. [Google Scholar] [CrossRef]
- Harraqui, K.; Oudghiri, D.E.; Hannoun, Z.; Naceiri Mrabti, H.; Aboulghras, S.; Assaggaf, H.M.; S. Rajab, B.; A. Attar, A.; Bouyahya, A.; Bour, A. Frequency of Metabolic Syndrome and Study of Anthropometric, Clinical and Biological Characteristics in Peri- and Postmenopausal Women in the City of Ksar El Kebir (Northern Morocco). Int. J. Environ. Res. Public Health 2022, 19, 6109. [Google Scholar] [CrossRef] [PubMed]

| Overall | Female | Male | |||||
|---|---|---|---|---|---|---|---|
| Characteristics | −MetS | +MetS | −MetS | +MetS | |||
| n = 602 | n = 273 | n = 184 | p value | n = 102 | n = 43 | p value | |
| Age, (years) a | 60 (50–68) | 58 (49–65) | 63 (57–71) | <0.001 | 55 (46–63) | 59 (49–67) | 0.365 |
| BMI, (kg/m2) a | 26.0 (24.1–30.5) | 25.3 (22.9–28.3) | 29.5 (26.8–33.4) | <0.001 | 25.4 (23.3–27.6) | 30.1 (27.2–32.4) | <0.001 |
| Overweight, % | 39 | 37.7 | 39.1 | 0.762 | 45.1 | 32.6 | 0.162 |
| Obesity, % | 27.2 | 16.1 | 46.2 | <0.001 | 11.8 | 53.5 | <0.001 |
| WC, (cm) a | 93 (86–100) | 86 (81–94) | 98 (92–104) | <0.001 | 94 (89–99) | 106 (99–109) | <0.001 |
| Body fat (%) | 42.8 (36.1–47.8) | 43.4 (38.9–47.7) | 47.2 (43.0–50.8) | <0.001 | 30.9 (28.1–33.8) | 35.1 (33.1–38.3) | 0.0001 |
| DBP, (mmHg) a | 75 (69–82) | 74 (67–79) | 76 (69–83) | 0.0009 | 77 (71–82) | 85 (79–91) | <0.001 |
| SBP, (mmHg) a | 120 (109–134) | 114 (105–123) | 127 (116–142) | <0.001 | 120 (111–132) | 132 (122–145) | <0.001 |
| Fasting plasma glucose, (mg/dL) a | 99 (92–109) | 96 (89–102) | 105 (95–120) | <0.001 | 98 (93–107) | 108 (95–119) | 0.015 |
| Total cholesterol, (mg/dL) a | 198 (169–224) | 198 (172–225) | 199 (172–235) | 0.666 | 194 (162–222) | 199 (162–220) | 0.843 |
| HDL-c, (mg/dL) a | 50.7 (42.3–59.8) | 55.7 (49.7–65.4) | 47.7 (41.1–56.2) | <0.001 | 46.7 (40.8–54) | 39.5 (34.7–48) | 0.001 |
| LDL-c, (mg/dL) a | 113.1 (90.9–135.8) | 115.1 (93.3–134.9) | 110.1 (88.1–139) | 0.416 | 118.3 (90.3–138.9) | 102.9 (87.3–131.7) | 0.101 |
| Triglycerides, (mg/dL) a | 141 (105–197) | 123 (94–150) | 181 (139–226) | <0.001 | 129 (99–175) | 196 (166–282) | <0.001 |
| Uric acid, (mg/dL) a | 5.2 (4.4–6.2) | 4.7 (4.1–5.5) | 5.4 (4.6–6.4) | <0.001 | 5.8 (5.2–6.8) | 6.7 (5.7–7.8) | 0.010 |
| AST, (U/I) a | 25 (21–32) | 24 (21–30) | 27 (23–35) | 0.002 | 26 (22–32) | 30 (23–43) | 0.057 |
| ALT, (U/I) a | 26 (19–37) | 29 (24–37) | 35 (24–65) | 0.004 | 29 (24–37) | 35 (24–65) | 0.016 |
| LogFC | Abundance | t | p Value | Adj. p Value | β | |
|---|---|---|---|---|---|---|
| Glycine | −0.027 | −8.04 × 10−18 | −4.19 | 3.19 × 10−5 | 0.0004 | 1.128 |
| AC10 | −0.019 | −3.84 × 10−18 | −3.57 | 0.0003 | 0.0024 | −1.191 |
| Arginine | −0.018 | −3.53 × 10−18 | −2.92 | 0.0036 | 0.0156 | −3.283 |
| AC2 | 0.031 | −6.29 × 10−18 | 2.44 | 0.0146 | 0.0477 | −4.541 |
| AC16:1 | −0.008 | 2.99 × 10−18 | −2.03 | 0.0421 | 0.1084 | −5.453 |
| Females | Males | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| −MetS | +MetS | −MetS | +MetS | ||||||
| <45 years | ≥45 years | <45 years | ≥45 years | <45 years | ≥45 years | <45 years | ≥45 years | ||
| Arginine | r | −0.50 | −0.26 | 0.38 | −0.31 | 0.32 | 0.10 | −0.68 | 0.27 |
| 95% CI | −0.67 to −0.27 | −0.38 to −0.13 | −0.24 to 0.78 | −0.44 to −0.16 | −0.16 to 0.67 | −0.12 to 0.31 | −0.96 to 0.28 | −0.11 to 0.57 | |
| p value | 0.0001 | 0.0001 | 0.219 | <0.0001 | 0.186 | 0.386 | 0.134 | 0.163 | |
| Glycine | r | −0.66 | −0.16 | 0.45 | −0.17 | 0.30 | 0.05 | −0.42 | 0.25 |
| 95% CI | −0.79 to −0.49 | −0.29 to −0.03 | −0.16 to 0.81 | −0.31 to −0.01 | −0.17 to 0.66 | −0.16 to 0.27 | −0.92 to 0.58 | −0.12 to 0.56 | |
| p value | <0.0001 | 0.014 | 0.136 | 0.031 | 0.200 | 0.614 | 0.396 | 0.186 | |
| AC10 | r | −0.36 | −0.06 | 0.35 | −0.06 | 0.16 | 0.06 | −0.72 | 0.40 |
| 95% CI | −0.57 to −0.11 | −0.20 to 0.06 | −0.27 to 0.77 | −0.22 to 0.09 | −0.31 to 0.57 | −0.15 to 0.28 | −0.96 to 0.21 | 0.05 to 0.67 | |
| p value | 0.005 | 0.331 | 0.255 | 0.407 | 0.506 | 0.565 | 0.106 | 0.027 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios-González, B.; León-Reyes, G.; Rivera-Paredez, B.; Ibarra-González, I.; Vela-Amieva, M.; Flores, Y.N.; Canizales-Quinteros, S.; Salmerón, J.; Velázquez-Cruz, R. Targeted Metabolomics Revealed a Sex-Dependent Signature for Metabolic Syndrome in the Mexican Population. Nutrients 2022, 14, 3678. https://doi.org/10.3390/nu14183678
Palacios-González B, León-Reyes G, Rivera-Paredez B, Ibarra-González I, Vela-Amieva M, Flores YN, Canizales-Quinteros S, Salmerón J, Velázquez-Cruz R. Targeted Metabolomics Revealed a Sex-Dependent Signature for Metabolic Syndrome in the Mexican Population. Nutrients. 2022; 14(18):3678. https://doi.org/10.3390/nu14183678
Chicago/Turabian StylePalacios-González, Berenice, Guadalupe León-Reyes, Berenice Rivera-Paredez, Isabel Ibarra-González, Marcela Vela-Amieva, Yvonne N. Flores, Samuel Canizales-Quinteros, Jorge Salmerón, and Rafael Velázquez-Cruz. 2022. "Targeted Metabolomics Revealed a Sex-Dependent Signature for Metabolic Syndrome in the Mexican Population" Nutrients 14, no. 18: 3678. https://doi.org/10.3390/nu14183678
APA StylePalacios-González, B., León-Reyes, G., Rivera-Paredez, B., Ibarra-González, I., Vela-Amieva, M., Flores, Y. N., Canizales-Quinteros, S., Salmerón, J., & Velázquez-Cruz, R. (2022). Targeted Metabolomics Revealed a Sex-Dependent Signature for Metabolic Syndrome in the Mexican Population. Nutrients, 14(18), 3678. https://doi.org/10.3390/nu14183678