The Effect of Fava Bean (Vicia faba L.) Protein Ingestion on Myofibrillar Protein Synthesis at Rest and after Resistance Exercise in Healthy, Young Men and Women: A Randomised Control Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
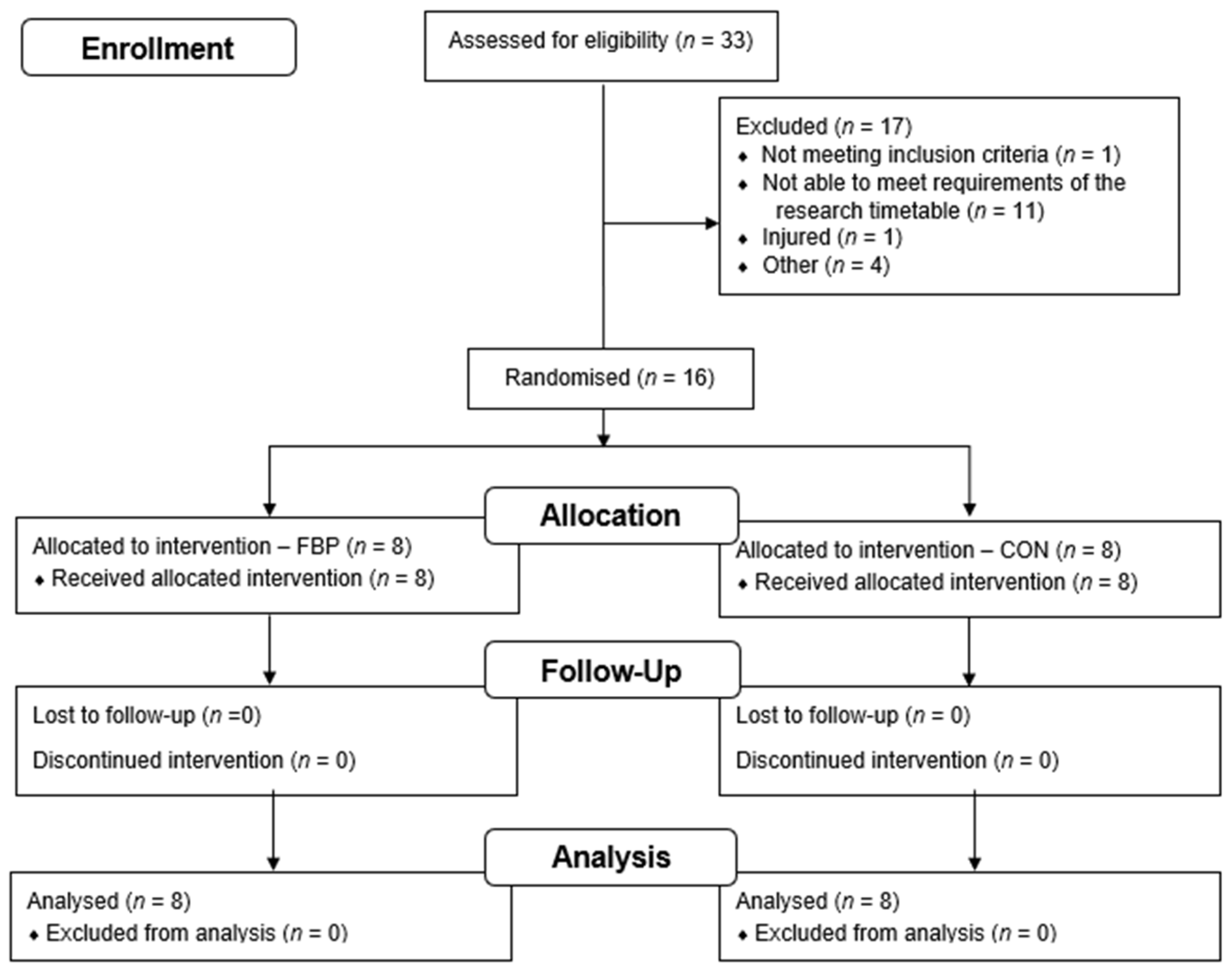
2.2. Participants
2.3. Preliminary Testing
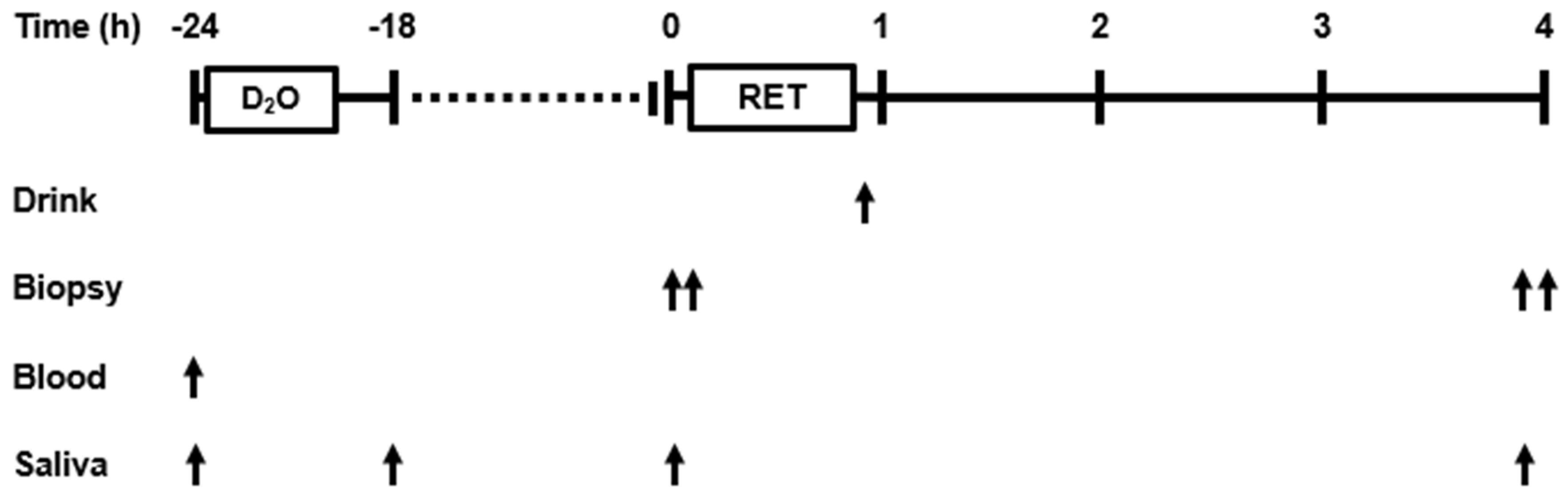
2.4. Experimental Protocol
2.5. Resistance Exercise Training Session
2.6. Body Water Enrichment
2.7. Muscle Analysis
2.8. Calculations
2.9. Statistical Analysis
3. Results
3.1. Participant Characteristics
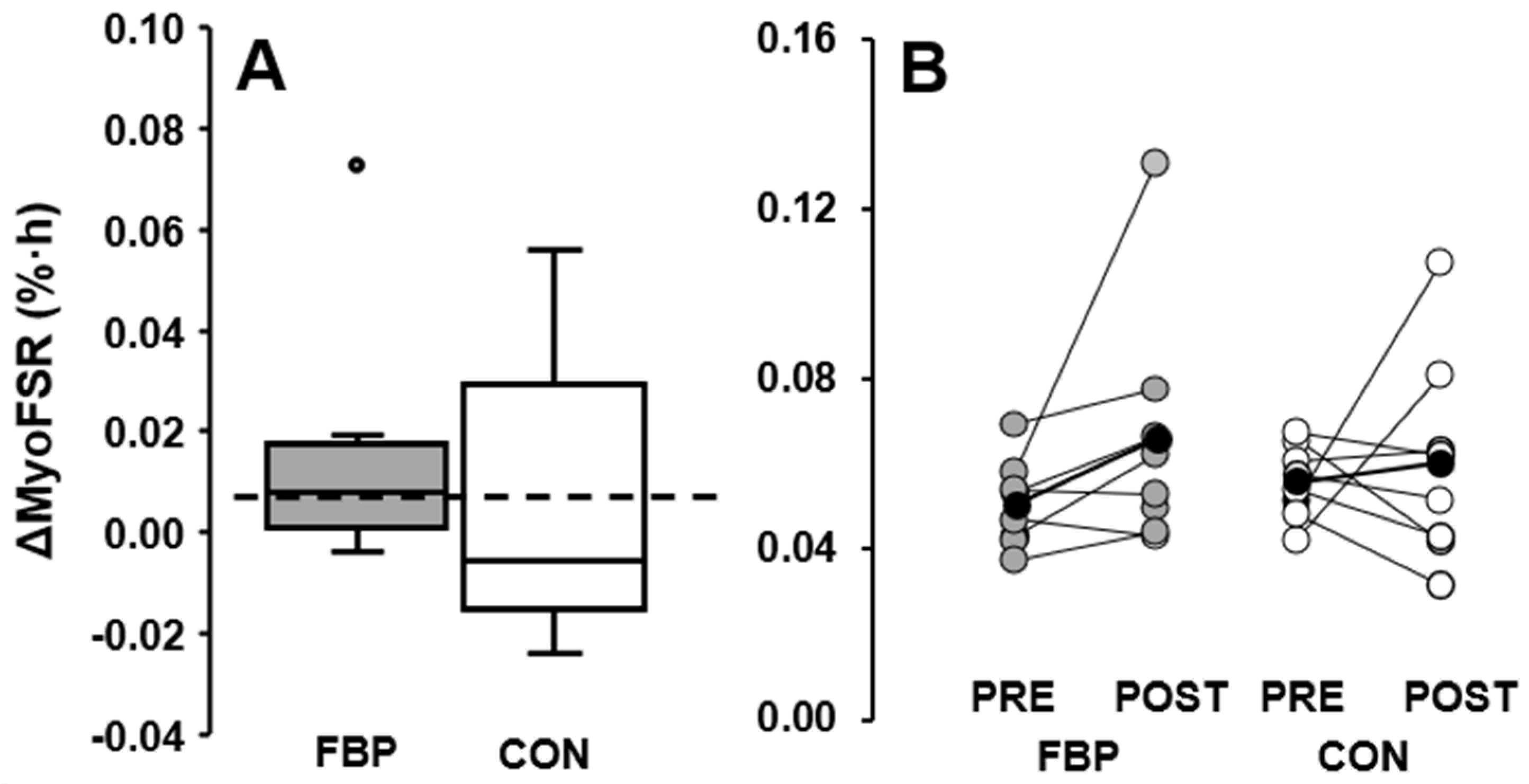
3.2. Resting Limb
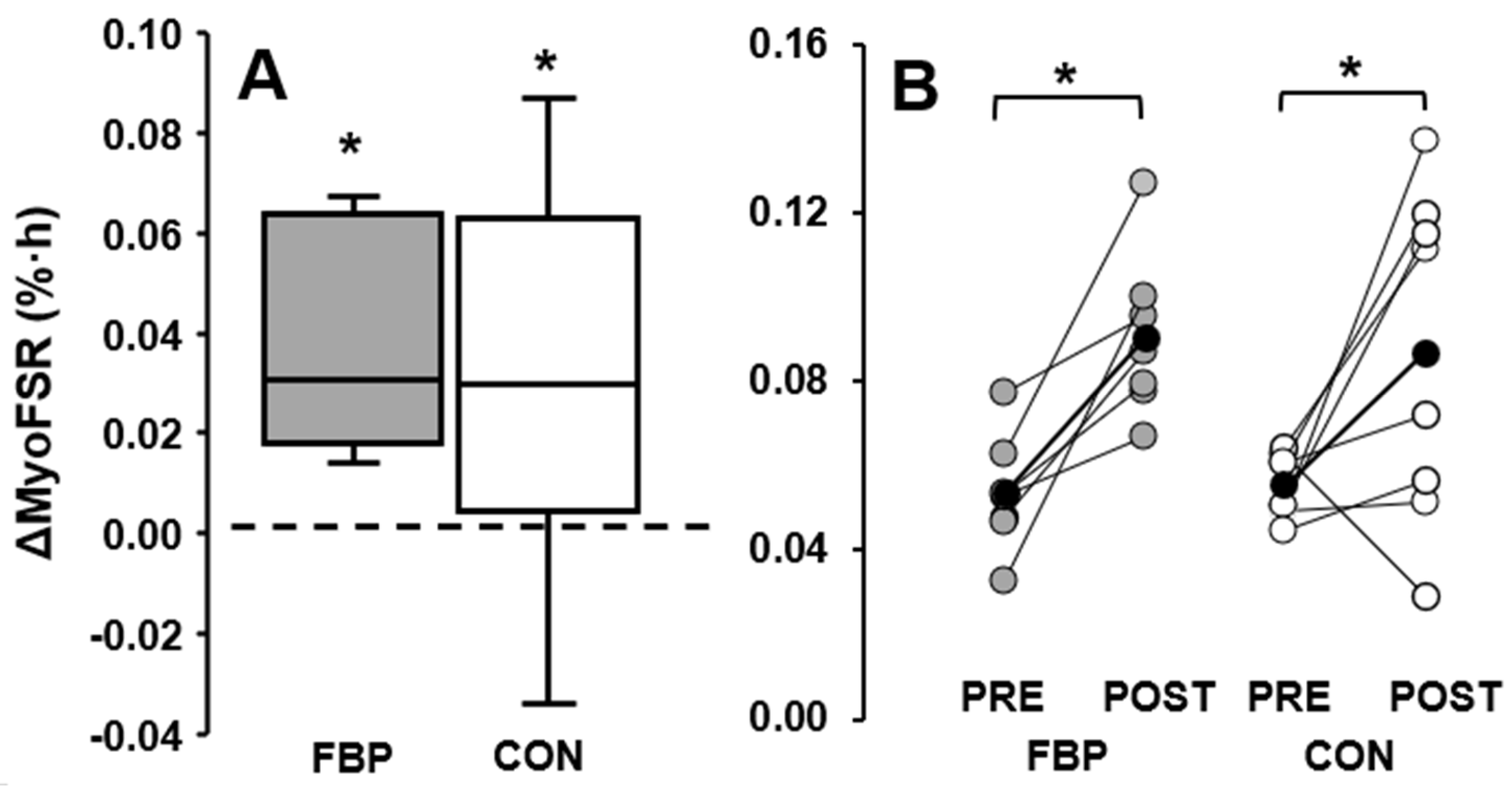
3.3. Exercised Limb
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atherton, P.J.; Smith, K. Muscle protein synthesis in response to nutrition and exercise. J. Physiol. 2012, 590, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Witard, O.C.; Jackman, S.R.; Breen, L.; Smith, K.; Selby, A.; Tipton, K.D. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 2014, 99, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. Regulation of muscle protein by amino acids. J. Nutr. 2002, 132, 3219S–3224S. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.W.; Jakeman, P.M. Separating the wheat from the chaff: Nutritional value of plant proteins and their potential contribution to human health. Nutrients 2020, 12, 2410. [Google Scholar] [CrossRef] [PubMed]
- Sá, A.G.; Moreno, Y.M.; Carciofi, B.A. Food processing for the improvement of plant proteins digestibility. Crit. Rev. Food Sci. Nutr. 2020, 60, 3367–3386. [Google Scholar] [CrossRef] [PubMed]
- Pinckaers, P.J.; Weijzen, M.E.; Houben, L.H.; Zorenc, A.H.; Kouw, I.W.; Groot, L.C.; Verdijk, L.B.; Snijders, T.; Loon, L.J. The muscle protein synthetic response following ingestion of corn protein, milk protein and their protein blend in young males. Curr. Dev. Nutr. 2020, 4 (Suppl. 2), 651. [Google Scholar] [CrossRef]
- Gorissen, S.H.; Horstman, A.M.; Franssen, R.; Crombag, J.J.; Langer, H.; Bierau, J.; Respondek, F.; Van Loon, L.J. Ingestion of wheat protein increases in vivo muscle protein synthesis rates in healthy older men in a randomized trial. J. Nutr. 2016, 146, 1651–1659. [Google Scholar] [CrossRef]
- Pinckaers, P.J.; Hendriks, F.K.; Hermans, W.J.; Goessens, J.P.; Senden, J.M.; van Kranenburg, J.M.; Wodzig, W.K.; Snijders, T.; van Loon, L.J. Potato Protein Ingestion Increases Muscle Protein Synthesis Rates at Rest and during Recovery from Exercise in Humans. Med. Sci. Sports Exerc. 2022, 54, 1572–1581. [Google Scholar] [CrossRef]
- Yang, Y.; Churchward-Venne, T.A.; Burd, N.A.; Breen, L.; Tarnopolsky, M.A.; Phillips, S.M. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr. Metab. 2012, 9, 57. [Google Scholar] [CrossRef]
- Multari, S.; Stewart, D.; Russell, W.R. Potential of fava bean as future protein supply to partially replace meat intake in the human diet. Comp. Rev. Food Sci. 2015, 14, 511–522. [Google Scholar] [CrossRef]
- Sharan, S.; Zanghelini, G.; Zotzel, J.; Bonerz, D.; Aschoff, J.; Saint-Eve, A.; Maillard, M.N. Fava bean (Vicia faba L.) for food applications: From seed to ingredient processing and its effect on functional properties, antinutritional factors, flavor, and color. Comp. Rev. Food Sci. 2021, 20, 401–428. [Google Scholar] [CrossRef]
- Patel, B.; Pauk, M.; Amigo-Benavent, M.; Nongonierma, A.B.; Fitzgerald, R.J.; Jakeman, P.M.; Carson, B.P. A cell-based evaluation of a non-essential amino acid formulation as a non-bioactive control for activation and stimulation of muscle protein synthesis using ex vivo human serum. PLoS ONE 2019, 14, e0220757. [Google Scholar] [CrossRef]
- Davies, R.W.; Bass, J.J.; Carson, B.P.; Norton, C.; Kozior, M.; Amigo-Benavent, M.; Wilkinson, D.J.; Brook, M.S.; Atherton, P.J.; Smith, K.; et al. Differential stimulation of post-exercise myofibrillar protein synthesis in humans following isonitrogenous, isocaloric pre-exercise feeding. Nutrients 2019, 11, 1657. [Google Scholar] [CrossRef]
- Davies, R.W.; Carson, B.P.; Bass, J.J.; Holohan, S.; Jakeman, P.M. Acute reduction of lower-body contractile function following a microbiopsy of m. vastus lateralis. Scand. J. Med. Sci. Sports 2018, 28, 2638–2642. [Google Scholar] [CrossRef]
- Narici, M.V.; Roi, G.S.; Landoni, L.; Minetti, A.E.; Cerretelli, P. Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps. Eur. J. Appl. Physiol. Occup. Physiol. 1989, 59, 310–319. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155. [Google Scholar] [CrossRef]
- Pinckaers, P.J.; Trommelen, J.; Snijders, T.; van Loon, L.J. The anabolic response to plant-based protein ingestion. Sports Med. 2021, 51, 59–74. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef]
- Jackman, S.R.; Witard, O.C.; Philp, A.; Wallis, G.A.; Baar, K.; Tipton, K.D. Branched-chain amino acid ingestion stimulates muscle myofibrillar protein synthesis following resistance exercise in humans. Front. Physiol. 2017, 8, 390. [Google Scholar] [CrossRef]
- Pinckaers, P.J.; Kouw, I.W.; Hendriks, F.K.; Van Kranenburg, J.M.; De Groot, L.C.; Verdijk, L.B.; Snijders, T.; Van Loon, L.J. No differences in muscle protein synthesis rates following ingestion of wheat protein, milk protein, and their protein blend in healthy, young males. Br. J. Nutr. 2021, 126, 1832–1842. [Google Scholar] [CrossRef]
- Smith, K.; Reynolds, N.; Downie, S.; Patel, A.; Rennie, M.J. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am. J. Physiol. 1998, 275, E73–E78. [Google Scholar] [CrossRef] [PubMed]
- Reidy, P.T.; Walker, D.K.; Dickinson, J.M.; Gundermann, D.M.; Drummond, M.J.; Timmerman, K.L.; Cope, M.B.; Mukherjea, R.; Jennings, K.; Volpi, E.; et al. Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle. J. Appl. Physiol. 2014, 116, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.W.; Bass, J.J.; Carson, B.P.; Norton, C.; Kozior, M.; Wilkinson, D.J.; Brook, M.S.; Atherton, P.J.; Smith, K.; Jakeman, P.M. The effect of whey protein supplementation on myofibrillar protein synthesis and performance recovery in resistance-trained men. Nutrients 2020, 12, 845. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Franchi, M.V.; Brook, M.S.; Narici, M.V.; Williams, J.P.; Mitchell, W.K.; Szewczyk, N.J.; Greenhaff, P.L.; Atherton, P.J.; Smith, K. A validation of the application of D2O stable isotope tracer techniques for monitoring day-to-day changes in muscle protein subfraction synthesis in humans. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E571–E579. [Google Scholar] [CrossRef]
- Burd, N.A.; Tang, J.E.; Moore, D.R.; Phillips, S.M. Exercise training and protein metabolism: Influences of contraction, protein intake, and sex-based differences. J. Appl. Physiol. 2009, 106, 1692–1701. [Google Scholar] [CrossRef]
- Trommelen, J.; Betz, M.W.; van Loon, L.J. The muscle protein synthetic response to meal ingestion following resistance-type exercise. Sports Med. 2019, 49, 185–197. [Google Scholar] [CrossRef]
- Smith, G.I.; Reeds, D.N.; Hall, A.M.; Chambers, K.T.; Finck, B.N.; Mittendorfer, B. Sexually dimorphic effect of aging on skeletal muscle protein synthesis. Biol. Sex Differ. 2012, 3, 11. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Jaffery, H.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. No major sex differences in muscle protein synthesis rates in the postabsorptive state and during hyperinsulinemia-hyperaminoacidemia in middle-aged adults. J. Appl. Physiol. 2009, 107, 1308–1315. [Google Scholar] [CrossRef]
- Knowles, O.E.; Aisbett, B.; Main, L.C.; Drinkwater, E.J.; Orellana, L.; Lamon, S. Resistance Training and Skeletal Muscle Protein Metabolism in Eumenorrheic Females: Implications for Researchers and Practitioners. Sports Med. 2019, 49, 1637–1650. [Google Scholar] [CrossRef]
| FBP | CON | FBP | CON | |
|---|---|---|---|---|
| AA Profile | g per 100 g | g per kg Body Mass | ||
| Alanine | 3.0 | 10.7 | 0.014 | 0.035 |
| Arginine | 6.5 | 0.0 | 0.031 | 0.000 |
| Aspartate | 8.4 | 12.9 | 0.040 | 0.043 |
| Cysteine | 0.8 | 0.0 | 0.004 | 0.000 |
| Glutamate | 12.4 | 36.4 | 0.059 | 0.120 |
| Glycine | 2.9 | 3.9 | 0.014 | 0.013 |
| Histidine | 1.9 | 0.0 | 0.009 | 0.000 |
| Isoleucine | 3.0 | 0.0 | 0.014 | 0.000 |
| Leucine | 5.7 | 0.0 | 0.027 | 0.000 |
| Lysine | 4.8 | 0.0 | 0.023 | 0.000 |
| Methionine | 0.5 | 0.0 | 0.002 | 0.000 |
| Phenylalanine | 3.4 | 0.0 | 0.016 | 0.000 |
| Proline | 3.2 | 15.6 | 0.015 | 0.051 |
| Serine | 3.8 | 13.5 | 0.018 | 0.045 |
| Threonine | 2.6 | 0.0 | 0.012 | 0.000 |
| Tryptophan | 0.6 | 0.0 | 0.003 | 0.000 |
| Tyrosine | 2.5 | 6.9 | 0.012 | 0.023 |
| Valine | 3.4 | 0.0 | 0.016 | 0.000 |
| EAA | 25.8 | 0.0 | 0.123 | 0.000 |
| TAA | 69.3 | 100 | 0.330 | 0.330 |
| FBP | CON | |
|---|---|---|
| Group Size | 8 | 8 |
| Sex (M:F) | 4:4 | 4:4 |
| Age (years) | 25 (3) | 25 (6) |
| Stature (m) | 1.73 (0.11) | 1.71 (0.10) |
| Body Mass (kg) | 72 (19) | 68 (10) |
| LTMI (kg·m−2) | 17 (2) | 18 (3) |
| % Fat Mass | 21 (7) | 23 (7) |
| Energy Intake (kcal·kg−1·d−1) | 32 (6) | 37 (10) |
| Protein Intake (g·kg−1·d−1) | 1.4 (0.4) | 1.5 (0.5) |
| REST | RET | |||||||
|---|---|---|---|---|---|---|---|---|
| PRE | POST | Δ | Δ% | PRE | POST | Δ | Δ% | |
| FBP | 0.050 (0.010) | 0.066 (0.020) | 0.016 (0.020) | 29 (34) | 0.054 (0.014) | 0.091 (0.020) * | 0.037 (0.016) * | 78 (45) * |
| CON | 0.056 (0.009) | 0.060 (0.024) | 0.004 (0.018) | 12 (37) | 0.055 (0.008) | 0.087 (0.039) * | 0.031 (0.027) * | 58(49) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davies, R.W.; Kozior, M.; Lynch, A.E.; Bass, J.J.; Atherton, P.J.; Smith, K.; Jakeman, P.M. The Effect of Fava Bean (Vicia faba L.) Protein Ingestion on Myofibrillar Protein Synthesis at Rest and after Resistance Exercise in Healthy, Young Men and Women: A Randomised Control Trial. Nutrients 2022, 14, 3688. https://doi.org/10.3390/nu14183688
Davies RW, Kozior M, Lynch AE, Bass JJ, Atherton PJ, Smith K, Jakeman PM. The Effect of Fava Bean (Vicia faba L.) Protein Ingestion on Myofibrillar Protein Synthesis at Rest and after Resistance Exercise in Healthy, Young Men and Women: A Randomised Control Trial. Nutrients. 2022; 14(18):3688. https://doi.org/10.3390/nu14183688
Chicago/Turabian StyleDavies, Robert W., Marta Kozior, Arthur E. Lynch, Joseph J. Bass, Philip J. Atherton, Ken Smith, and Philip M. Jakeman. 2022. "The Effect of Fava Bean (Vicia faba L.) Protein Ingestion on Myofibrillar Protein Synthesis at Rest and after Resistance Exercise in Healthy, Young Men and Women: A Randomised Control Trial" Nutrients 14, no. 18: 3688. https://doi.org/10.3390/nu14183688








