Megalin and Vitamin D Metabolism—Implications in Non-Renal Tissues and Kidney Disease
Abstract
:1. Introduction
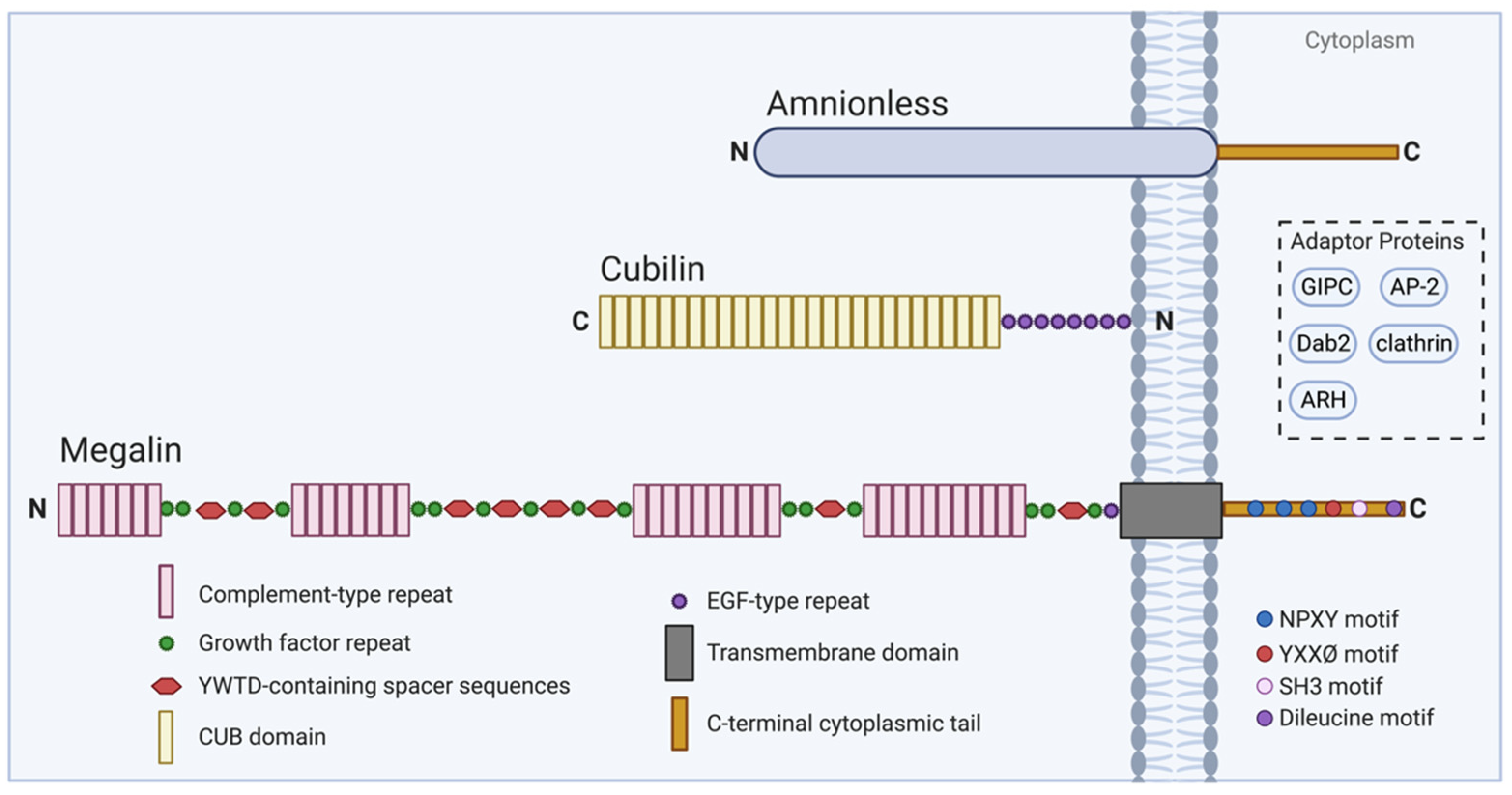
2. Megalin and Cubilin
2.1. Megalin Overview
2.2. Cubilin and Associated Molecules in Endocytosis
2.3. Megalin and Cubilin Interactions
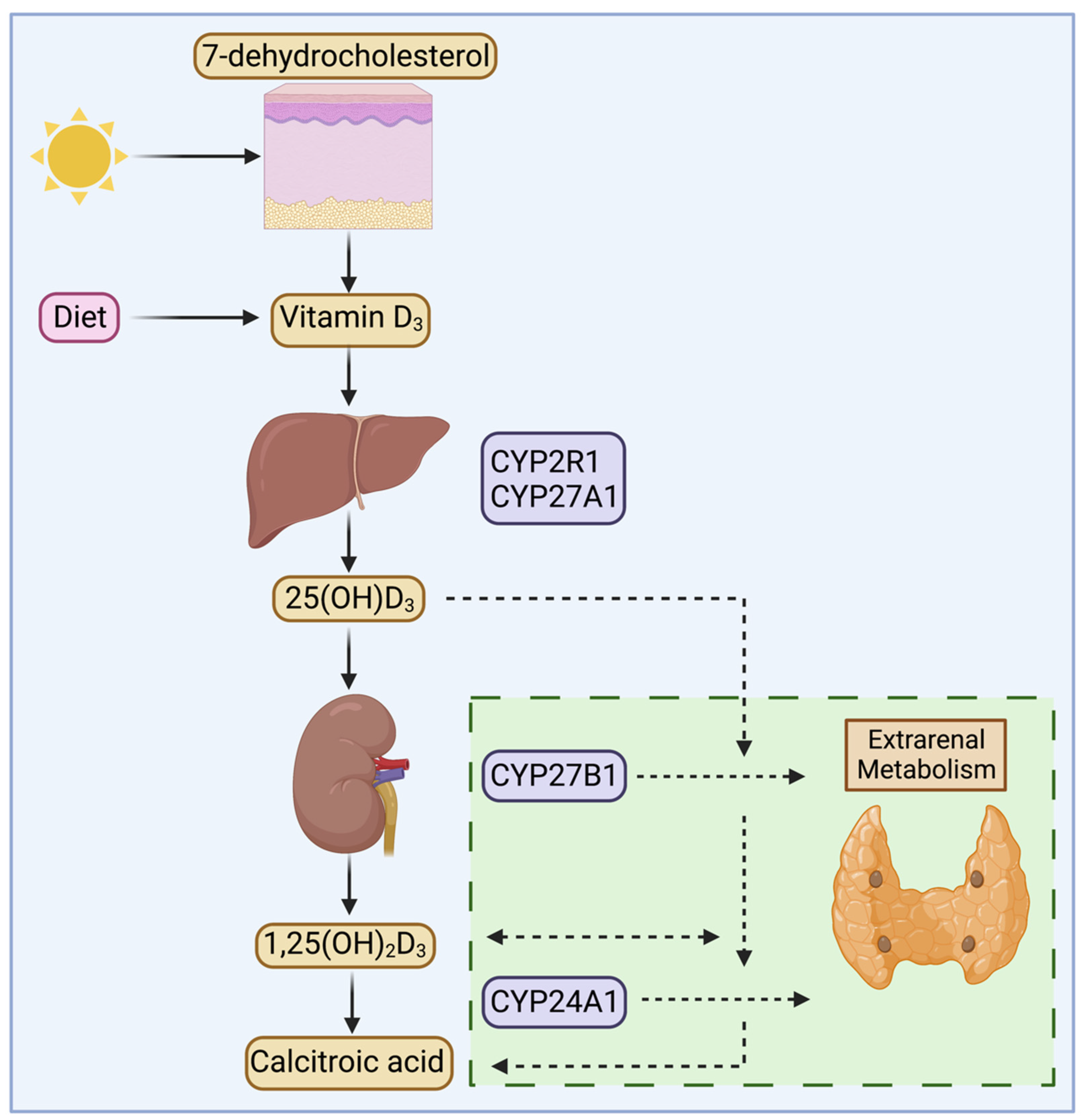
3. Megalin and Vitamin D Metabolism in the Kidney
4. Extrarenal Megalin and Vitamin D Metabolism
4.1. Parathyroid Gland
4.2. Mammary Gland, Prostate, and Colon
4.3. Muscle and Fat
4.4. Mesenchymal Stem Cells
4.5. Bone
5. Megalin in Chronic Kidney Disease
5.1. Chronic Kidney Disease Overview
5.2. Megalin in Chronic Kidney Disease
5.3. Vitamin D Regulation
5.4. Diabetic Nephropathy
6. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1,25(OH)2D3 | 1,25 dihydroxyvitamin D3 |
| 25(OH)D3 | 25 hydroxyvitamin D3 |
| 24,25(OH)2D3 | 24,25 dihydroxyvitamin D3 |
| 1,24,25(OH)3D3 | 1,24,25 trihydroxyvitamin D3 |
| ALP | Alkaline phosphatase |
| AMN | Amnionless |
| AP-2 | Adaptor protein-2 |
| ARH | Autosomal recessive hypercholesterolemia |
| AT1 | Angiotensin II type 1 |
| CaSR | Calcium-sensing receptor |
| CKD | Chronic kidney disease |
| CYP24A1 | Cytochrome P450 24A1 |
| CYP27B1 | Cytochrome P450 27B1 |
| Dab-2 | Disabled-2 |
| DBP | Vitamin D-binding protein |
| EGF | Epidermal growth factor |
| GIPC | GAIP interacting protein |
| GAIP | G alpha interacting protein |
| hMSC | Human mesenchymal stem cell |
| MegBP | Megalin binding protein |
| PTEC | Proximal tubular epithelial cells |
| PTH | Parathyroid hormone |
| RA | All-trans-retinoic acid |
| RAP | Receptor-associated protein |
| RBP | Retinol-binding protein |
| RIP | Regulated intramembrane proteolysis |
| RXR | Retinoid X receptor |
| SHPT | Secondary hyperparathyroidism |
| SKIP | SKI-interacting protein |
| VDR | Vitamin D receptor |
| VDRA | Vitamin D receptor agonist |
| VDRE | VDR response element |
References
- Wierzbicka, J.; Piotrowska, A.; Żmijewski, M.A. The Renaissance of Vitamin D. Acta Biochim. Pol. 2014, 61, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol.-Ren. Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef]
- Cooke, N.E.; Haddad, J.G. Vitamin D Binding Protein (Gc-Globulin)*. Endocr. Rev. 1989, 10, 294–307. [Google Scholar] [CrossRef]
- Bouillon, R.; Van Baelen, H.; Rombauts, W.; De Moor, P. The Purification and Characterisation of the Human-Serum Binding Protein for the 25-Hydroxycholecalciferol (Transcalciferin) Identity with Group-Specific Component. Eur. J. Biochem. 1976, 66, 285–291. [Google Scholar] [CrossRef] [PubMed]
- HADDAD, J.G.; HILLMAN, L.; ROJANASATHIT, S. Human Serum Binding Capacity and Affinity for 25-Hydroxyergocalciferol and 25-Hydroxycholecalciferol. J. Clin. Endocrinol. Metab. 1976, 43, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Gee, E.; Halloran, B.; Kowalski, M.A.; Ryzen, E.; Haddad, J.G. Assessment of the Free Fraction of 25-Hydroxyvitamin D in Serum and Its Regulation by Albumin and the Vitamin D-Binding Protein. J. Clin. Endocrinol. Metab. 1986, 63, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Van Assche, F.A.; Van Baelen, H.; Heyns, W.; De Moor, P. Influence of the Vitamin D-Binding Protein on the Serum Concentration of 1,25-Dihydroxyvitamin D3. J. Clin. Investig. 1981, 67, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2020, 10, 910. [Google Scholar] [CrossRef]
- Reddy, G.S.; Tserng, K.Y. Calcitroic Acid, End Product of Renal Metabolism of 1,25-Dihydroxyvitamin D3 through C-24 Oxidation Pathway. Biochemistry 1989, 28, 1763–1769. [Google Scholar] [CrossRef]
- Bikle, D.D. Extra Renal Synthesis of 1,25-Dihydroxyvitamin D and Its Health Implications. Clin. Rev. Bone Min. Metab. 2009, 7, 114–125. [Google Scholar] [CrossRef]
- Fraser, D.R.; Kodicek, E. Unique Biosynthesis by Kidney of a Biological Active Vitamin D Metabolite. Nature 1970, 228, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Patzek, S.; Wang, Y. Physiologic and Pathophysiologic Roles of Extra Renal CYP27b1: Case Report and Review. Bone Rep. 2018, 8, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Tiosano, D.; Wildbaum, G.; Gepstein, V.; Verbitsky, O.; Weisman, Y.; Karin, N.; Eztioni, A. The Role of Vitamin D Receptor in Innate and Adaptive Immunity: A Study in Hereditary Vitamin D-Resistant Rickets Patients. J. Clin. Endocrinol. Metab. 2013, 98, 1685–1693. [Google Scholar] [CrossRef]
- Kreutz, M.; Andreesen, R.; Krause, S.W.; Szabo, A.; Ritz, E.; Reichel, H. 1,25-Dihydroxyvitamin D3 Production and Vitamin D3 Receptor Expression Are Developmentally Regulated during Differentiation of Human Monocytes into Macrophages. Blood 1993, 82, 1300–1307. [Google Scholar] [CrossRef]
- Wiseman, H. Vitamin D Is a Membrane Antioxidant. Ability to Inhibit Iron-Dependent Lipid Peroxidation in Liposomes Compared to Cholesterol, Ergosterol and Tamoxifen and Relevance to Anticancer Action. FEBS Lett. 1993, 326, 285–288. [Google Scholar] [CrossRef]
- Tzilas, V.; Bouros, E.; Barbayianni, I.; Karampitsakos, T.; Kourtidou, S.; Ntassiou, M.; Ninou, I.; Aidinis, V.; Bouros, D.; Tzouvelekis, A. Vitamin D Prevents Experimental Lung Fibrosis and Predicts Survival in Patients with Idiopathic Pulmonary Fibrosis. Pulm. Pharm. 2019, 55, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Gorham, E.D.; Garland, C.F.; Garland, F.C.; Grant, W.B.; Mohr, S.B.; Lipkin, M.; Newmark, H.L.; Giovannucci, E.; Wei, M.; Holick, M.F. Vitamin D and Prevention of Colorectal Cancer. J. Steroid Biochem. Mol. Biol. 2005, 97, 179–194. [Google Scholar] [CrossRef]
- Lavie, C.J.; Dinicolantonio, J.J.; Milani, R.V.; O’Keefe, J.H. Vitamin D and Cardiovascular Health. Circulation 2013, 128, 2404–2406. [Google Scholar] [CrossRef]
- Harinarayan, C.V. Vitamin D and Diabetes Mellitus. Hormones 2014, 13, 163–181. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Qureshi, O.S.; Gardner, D.; Hou, T.Z.; Briggs, Z.; Soskic, B.; Baker, J.; Raza, K.; Sansom, D.M. Vitamin D Antagonises the Suppressive Effect of Inflammatory Cytokines on CTLA-4 Expression and Regulatory Function. PLoS ONE 2015, 10, e0131539. [Google Scholar] [CrossRef] [Green Version]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Kemmis, C.M.; Salvador, S.M.; Smith, K.M.; Welsh, J. Human Mammary Epithelial Cells Express CYP27B1 and Are Growth Inhibited by 25-Hydroxyvitamin D-3, the Major Circulating Form of Vitamin D-3. J. Nutr. 2006, 136, 887–892. [Google Scholar] [CrossRef]
- O’Brien, K.O.; Li, S.; Cao, C.; Kent, T.; Young, B.V.; Queenan, R.A.; Pressman, E.K.; Cooper, E.M. Placental CYP27B1 and CYP24A1 Expression in Human Placental Tissue and Their Association with Maternal and Neonatal Calcitropic Hormones. J. Clin. Endocrinol. Metab. 2014, 99, 1348–1356. [Google Scholar] [CrossRef]
- Anderson, P.H.; Iida, S.; Tyson, J.H.T.; Turner, A.G.; Morris, H.A. Bone CYP27B1 Gene Expression Is Increased with High Dietary Calcium and in Mineralising Osteoblasts. J. Steroid Biochem. Mol. Biol. 2010, 121, 71–75. [Google Scholar] [CrossRef]
- White, J.H. Vitamin D Metabolism and Signaling in the Immune System. Rev. Endocr. Metab. Disord. 2012, 13, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Chanakul, A.; Zhang, M.Y.H.; Louw, A.; Armbrecht, H.J.; Miller, W.L.; Portale, A.A.; Perwad, F. FGF-23 Regulates CYP27B1 Transcription in the Kidney and in Extra-Renal Tissues. PLoS ONE 2013, 8, e72816. [Google Scholar] [CrossRef]
- Nielsen, R.; Christensen, E.I.; Birn, H. Megalin and Cubilin in Proximal Tubule Protein Reabsorption: From Experimental Models to Human Disease. Kidney Int. 2016, 89, 58–67. [Google Scholar] [CrossRef]
- Raychowdhury, R.; Niles, J.L.; McCluskey, R.T.; Smith, J.A. Autoimmune Target in Heymann Nephritis Is a Glycoprotein with Homology to the LDL Receptor. Science 1989, 244, 1163–1165. [Google Scholar] [CrossRef] [PubMed]
- Hjälm, G.; Murray, E.; Crumley, G.; Harazim, W.; Lundgren, S.; Onyango, I.; Ek, B.; Larsson, M.; Juhlin, C.; Hellman, P.; et al. Cloning and Sequencing of Human Gp330, a Ca(2+)-Binding Receptor with Potential Intracellular Signaling Properties. Eur. J. Biochem. 1996, 239, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Pietromonaco, S.; Loo, A.K.; Farquhar, M.G. Complete Cloning and Sequencing of Rat Gp330/"megalin," a Distinctive Member of the Low Density Lipoprotein Receptor Gene Family. Proc. Natl. Acad. Sci. USA 1994, 91, 9725–9729. [Google Scholar] [CrossRef] [Green Version]
- Takeda, T.; Yamazaki, H.; Farquhar, M.G. Identification of an Apical Sorting Determinant in the Cytoplasmic Tail of Megalin. Am. J. Physiol. Cell Physiol. 2003, 284, C1105–C1113. [Google Scholar] [CrossRef]
- Nagai, M.; Meerloo, T.; Takeda, T.; Farquhar, M.G. The Adaptor Protein ARH Escorts Megalin to and through Endosomes. Mol. Biol. Cell 2003, 14, 4984–4996. [Google Scholar] [CrossRef]
- He, G.; Gupta, S.; Yi, M.; Michaely, P.; Hobbs, H.H.; Cohen, J.C. ARH Is a Modular Adaptor Protein That Interacts with the LDL Receptor, Clathrin, and AP-2. J. Biol. Chem. 2002, 277, 44044–44049. [Google Scholar] [CrossRef] [PubMed]
- Keyel, P.A.; Thieman, J.R.; Roth, R.; Erkan, E.; Everett, E.T.; Watkins, S.C.; Heuser, J.E.; Traub, L.M. The AP-2 Adaptor Β2 Appendage Scaffolds Alternate Cargo Endocytosis. Mol. Biol. Cell 2008, 19, 5309–5326. [Google Scholar] [CrossRef] [PubMed]
- Oleinikov, A.V.; Zhao, J.; Makker, S.P. Cytosolic Adaptor Protein Dab2 Is an Intracellular Ligand of Endocytic Receptor Gp600/Megalin. Biochem. J. 2000, 347, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; McQuistan, T.; Orlando, R.A.; Farquhar, M.G. GAIP, GIPC and Galphai3 Are Concentrated in Endocytic Compartments of Proximal Tubule Cells: Putative Role in Regulating Megalin’s Function. J. Am. Soc. Nephrol. 2002, 13, 918–927. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Farquhar, M.G. The Pathogenic Antigen of Heymann Nephritis Is a Membrane Glycoprotein of the Renal Proximal Tubule Brush Border. Proc. Natl. Acad. Sci. USA 1982, 79, 5557–5561. [Google Scholar] [CrossRef] [PubMed]
- Chatelet, F.; Brianti, E.; Ronco, P.; Roland, J.; Verroust, P. Ultrastructural Localization by Monoclonal Antibodies of Brush Border Antigens Expressed by Glomeruli. I. Renal Distribution. Am. J. Pathol. 1986, 122, 500–511. [Google Scholar]
- Christensen, E.I.; Nielsen, S.; Moestrup, S.K.; Borre, C.; Maunsbach, A.B.; de Heer, E.; Ronco, P.; Hammond, T.G.; Verroust, P. Segmental Distribution of the Endocytosis Receptor Gp330 in Renal Proximal Tubules. Eur. J. Cell Biol. 1995, 66, 349–364. [Google Scholar]
- Kerjaschki, D.; Farquhar, M.G. Immunocytochemical Localization of the Heymann Nephritis Antigen (GP330) in Glomerular Epithelial Cells of Normal Lewis Rats. J. Exp. Med. 1983, 157, 667–686. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wen, Y.; Tang, T.-T.; Lv, L.-L.; Tang, R.-N.; Liu, H.; Ma, K.-L.; Crowley, S.D.; Liu, B.-C. Megalin/Cubulin-Lysosome-Mediated Albumin Reabsorption Is Involved in the Tubular Cell Activation of NLRP3 Inflammasome and Tubulointerstitial Inflammation. J. Biol. Chem. 2015, 290, 18018–18028. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, S.; Carling, T.; Hjälm, G.; Juhlin, C.; Rastad, J.; Pihlgren, U.; Rask, L.; Akerström, G.; Hellman, P. Tissue Distribution of Human Gp330/Megalin, a Putative Ca(2+)-Sensing Protein. J. Histochem. Cytochem. 1997, 45, 383–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storm, T.; Heegaard, S.; Christensen, E.I.; Nielsen, R. Megalin–Deficiency Causes High Myopia, Retinal Pigment Epithelium-Macromelanosomes and Abnormal Development of the Ciliary Body in Mice. Cell Tissue Res. 2014, 358, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Erranz, B.; Miquel, J.F.; Argraves, W.S.; Barth, J.L.; Pimentel, F.; Marzolo, M.-P. Megalin and Cubilin Expression in Gallbladder Epithelium and Regulation by Bile Acids. J. Lipid Res. 2004, 45, 2185–2198. [Google Scholar] [CrossRef] [PubMed]
- Yammani, R.R.; Seetharam, S.; Seetharam, B. Cubilin and Megalin Expression and Their Interaction in the Rat Intestine: Effect of Thyroidectomy. Am. J. Physiol.-Endocrinol. Metab. 2001, 281, E900–E907. [Google Scholar] [CrossRef] [PubMed]
- Birn, H.; Verroust, P.J.; Nexo, E.; Hager, H.; Jacobsen, C.; Christensen, E.I.; Moestrup, S.K. Characterization of an Epithelial Approximately 460-KDa Protein That Facilitates Endocytosis of Intrinsic Factor-Vitamin B12 and Binds Receptor-Associated Protein. J. Biol. Chem. 1997, 272, 26497–26504. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Verroust, P.J.; Moestrup, S.K.; Christensen, E.I. Megalin/Gp330 Mediates Uptake of Albumin in Renal Proximal Tubule. Am. J. Physiol. 1996, 271, F900–F907. [Google Scholar] [CrossRef]
- Gburek, J.; Verroust, P.J.; Willnow, T.E.; Fyfe, J.C.; Nowacki, W.; Jacobsen, C.; Moestrup, S.K.; Christensen, E.I. Megalin and Cubilin Are Endocytic Receptors Involved in Renal Clearance of Hemoglobin. J. Am. Soc. Nephrol. 2002, 13, 423–430. [Google Scholar] [CrossRef]
- Orlando, R.A.; Rader, K.; Authier, F.; Yamazaki, H.; Posner, B.I.; Bergeron, J.J.; Farquhar, M.G. Megalin Is an Endocytic Receptor for Insulin. J. Am. Soc. Nephrol. 1998, 9, 1759–1766. [Google Scholar] [CrossRef]
- Christensen, E.I.; Moskaug, J.O.; Vorum, H.; Jacobsen, C.; Gundersen, T.E.; Nykjaer, A.; Blomhoff, R.; Willnow, T.E.; Moestrup, S.K. Evidence for an Essential Role of Megalin in Transepithelial Transport of Retinol. J. Am. Soc. Nephrol. 1999, 10, 685–695. [Google Scholar] [CrossRef]
- Nykjaer, A.; Dragun, D.; Walther, D.; Vorum, H.; Jacobsen, C.; Herz, J.; Melsen, F.; Christensen, E.I.; Willnow, T.E. An Endocytic Pathway Essential for Renal Uptake and Activation of the Steroid 25-(OH) Vitamin D3. Cell 1999, 96, 507–515. [Google Scholar] [CrossRef]
- Zhai, X.Y.; Nielsen, R.; Birn, H.; Drumm, K.; Mildenberger, S.; Freudinger, R.; Moestrup, S.K.; Verroust, P.J.; Christensen, E.I.; Gekle, M. Cubilin- and Megalin-Mediated Uptake of Albumin in Cultured Proximal Tubule Cells of Opossum Kidney. Kidney Int. 2000, 58, 1523–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahali, D.; Mulliez, N.; Chatelet, F.; Dupuis, R.; Ronco, P.; Verroust, P. Characterization of a 280-KD Protein Restricted to the Coated Pits of the Renal Brush Border and the Epithelial Cells of the Yolk Sac. Teratogenic Effect of the Specific Monoclonal Antibodies. J. Exp. Med. 1988, 167, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Sahali, D.; Mulliez, N.; Chatelet, F.; Laurent-Winter, C.; Citadelle, D.; Sabourin, J.C.; Roux, C.; Ronco, P.; Verroust, P. Comparative Immunochemistry and Ontogeny of Two Closely Related Coated Pit Proteins. The 280-Kd Target of Teratogenic Antibodies and the 330-Kd Target of Nephritogenic Antibodies. Am. J. Pathol. 1993, 142, 1654–1667. [Google Scholar] [PubMed]
- Fyfe, J.C.; Madsen, M.; Højrup, P.; Christensen, E.I.; Tanner, S.M.; de la Chapelle, A.; He, Q.; Moestrup, S.K. The Functional Cobalamin (Vitamin B12)-Intrinsic Factor Receptor Is a Novel Complex of Cubilin and Amnionless. Blood 2004, 103, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Coudroy, G.; Gburek, J.; Kozyraki, R.; Madsen, M.; Trugnan, G.; Moestrup, S.K.; Verroust, P.J.; Maurice, M. Contribution of Cubilin and Amnionless to Processing and Membrane Targeting of Cubilin–Amnionless Complex. J. Am. Soc. Nephrol. 2005, 16, 2330–2337. [Google Scholar] [CrossRef]
- Ahuja, R.; Yammani, R.; Bauer, J.A.; Kalra, S.; Seetharam, S.; Seetharam, B. Interactions of Cubilin with Megalin and the Product of the Amnionless Gene (AMN): Effect on Its Stability. Biochem. J. 2008, 410, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Tauris, J.; Christensen, E.I.; Nykjaer, A.; Jacobsen, C.; Petersen, C.M.; Ovesen, T. Cubilin and Megalin Co-Localize in the Neonatal Inner Ear. Audiol. Neurootol. 2009, 14, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Kozyraki, R.; Fyfe, J.; Kristiansen, M.; Gerdes, C.; Jacobsen, C.; Cui, S.; Christensen, E.I.; Aminoff, M.; de la Chapelle, A.; Krahe, R.; et al. The Intrinsic Factor–Vitamin B12 Receptor, Cubilin, Is a High-Affinity Apolipoprotein A-I Receptor Facilitating Endocytosis of High-Density Lipoprotein. Nat. Med. 1999, 5, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Storm, T.; Emma, F.; Verroust, P.J.; Hertz, J.M.; Nielsen, R.; Christensen, E.I. A Patient with Cubilin Deficiency. N. Engl. J. Med. 2011, 364, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Amsellem, S.; Gburek, J.; Hamard, G.; Nielsen, R.; Willnow, T.E.; Devuyst, O.; Nexo, E.; Verroust, P.J.; Christensen, E.I.; Kozyraki, R. Cubilin Is Essential for Albumin Reabsorption in the Renal Proximal Tubule. J. Am. Soc. Nephrol. 2010, 21, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Nykjaer, A.; Fyfe, J.C.; Kozyraki, R.; Leheste, J.-R.; Jacobsen, C.; Nielsen, M.S.; Verroust, P.J.; Aminoff, M.; de la Chapelle, A.; Moestrup, S.K.; et al. Cubilin Dysfunction Causes Abnormal Metabolism of the Steroid Hormone 25(OH) Vitamin D3. Proc. Natl. Acad. Sci. USA 2001, 98, 13895–13900. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Lopez, E.; Lange, B.; Penna-Martinez, M.; Brück, P.; Swiech, K.; Mauf, S.; Kahles, H.; Badenhoop, K. The Role of Cubilin Gene Polymorphisms and Their Influence on 25(OH)D3 and 1,25(OH)2D3 Plasma Levels in Type 1 Diabetes Patients. J. Steroid Biochem. Mol. Biol. 2010, 121, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Armbrecht, H.J.; Zenser, T.V.; Davis, B.B. Conversion of 25-Hydroxyvitamin D3 to 1,25-Dihydroxyvitamin D3 and 24,25-Dihydroxyvitamin D3 in Renal Slices from the Rat. Endocrinology 1981, 109, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, K.; Kitanaka, S.; Sato, T.; Kobori, M.; Yanagisawa, J.; Kato, S. 25-Hydroxyvitamin D3 1alpha-Hydroxylase and Vitamin D Synthesis. Science 1997, 277, 1827–1830. [Google Scholar] [CrossRef] [PubMed]
- Mendel, C.M. The Free Hormone Hypothesis: A Physiologically Based Mathematical Model. Endocr. Rev. 1989, 10, 232–274. [Google Scholar] [CrossRef]
- Zella, L.A.; Shevde, N.K.; Hollis, B.W.; Cooke, N.E.; Pike, J.W. Vitamin D-Binding Protein Influences Total Circulating Levels of 1,25-Dihydroxyvitamin D3 but Does Not Directly Modulate the Bioactive Levels of the Hormone in Vivo. Endocrinology 2008, 149, 3656–3667. [Google Scholar] [CrossRef] [PubMed]
- Moestrup, S.K.; Cui, S.; Vorum, H.; Bregengård, C.; Bjørn, S.E.; Norris, K.; Gliemann, J.; Christensen, E.I. Evidence That Epithelial Glycoprotein 330/Megalin Mediates Uptake of Polybasic Drugs. J. Clin. Investig. 1995, 96, 1404–1413. [Google Scholar] [CrossRef]
- Moestrup, S.K.; Birn, H.; Fischer, P.B.; Petersen, C.M.; Verroust, P.J.; Sim, R.B.; Christensen, E.I.; Nexø, E. Megalin-Mediated Endocytosis of Transcobalamin-Vitamin-B12 Complexes Suggests a Role of the Receptor in Vitamin-B12 Homeostasis. Proc. Natl. Acad. Sci. USA 1996, 93, 8612–8617. [Google Scholar] [CrossRef]
- Leheste, J.R.; Rolinski, B.; Vorum, H.; Hilpert, J.; Nykjaer, A.; Jacobsen, C.; Aucouturier, P.; Moskaug, J.O.; Otto, A.; Christensen, E.I.; et al. Megalin Knockout Mice as an Animal Model of Low Molecular Weight Proteinuria. Am. J. Pathol. 1999, 155, 1361–1370. [Google Scholar] [CrossRef]
- Leheste, J.R.; Melsen, F.; Wellner, M.; Jansen, P.; Schlichting, U.; Renner-Müller, I.; Andreassen, T.T.; Wolf, E.; Bachmann, S.; Nykjaer, A.; et al. Hypocalcemia and Osteopathy in Mice with Kidney-Specific Megalin Gene Defect. FASEB J. 2003, 17, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Petersen, H.H.; Hilpert, J.; Militz, D.; Zandler, V.; Jacobsen, C.; Roebroek, A.J.M.; Willnow, T.E. Functional Interaction of Megalin with the Megalinbinding Protein (MegBP), a Novel Tetratrico Peptide Repeat-Containing Adaptor Molecule. J. Cell Sci. 2003, 116, 453–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, P.; Bock, H.H.; Herz, J. Integration of Endocytosis and Signal Transduction by Lipoprotein Receptors. Sci. STKE 2003, 2003, PE12. [Google Scholar] [CrossRef]
- Biemesderfer, D. Regulated Intramembrane Proteolysis of Megalin: Linking Urinary Protein and Gene Regulation in Proximal Tubule? Kidney Int. 2006, 69, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Dickson, L.E.; Wagner, M.C.; Sandoval, R.M.; Molitoris, B.A. The Proximal Tubule and Albuminuria: Really! J. Am. Soc. Nephrol. 2014, 25, 443–453. [Google Scholar] [CrossRef]
- Chapron, B.D.; Chapron, A.; Phillips, B.; Okoli, M.C.; Shen, D.D.; Kelly, E.J.; Himmelfarb, J.; Thummel, K.E. Reevaluating the Role of Megalin in Renal Vitamin D Homeostasis Using a Human Cell-Derived Microphysiological System. ALTEX 2018, 35, 504–515. [Google Scholar] [CrossRef]
- Berkowska, K.; Corcoran, A.; Grudzień, M.; Jakuszak, A.; Chodyński, M.; Kutner, A.; Marcinkowska, E. Investigating the Role of VDR and Megalin in Semi-Selectivity of Side-Chain Modified 19-nor Analogs of Vitamin D. Int. J. Mol. Sci. 2019, 20, 4183. [Google Scholar] [CrossRef] [PubMed]
- Willnow, T.E.; Hilpert, J.; Armstrong, S.A.; Rohlmann, A.; Hammer, R.E.; Burns, D.K.; Herz, J. Defective Forebrain Development in Mice Lacking Gp330/Megalin. Proc. Natl. Acad. Sci. USA 1996, 93, 8460–8464. [Google Scholar] [CrossRef]
- Hammes, A.; Andreassen, T.K.; Spoelgen, R.; Raila, J.; Hubner, N.; Schulz, H.; Metzger, J.; Schweigert, F.J.; Luppa, P.B.; Nykjaer, A.; et al. Role of Endocytosis in Cellular Uptake of Sex Steroids. Cell 2005, 122, 751–762. [Google Scholar] [CrossRef]
- Marinò, M.; Zheng, G.; Chiovato, L.; Pinchera, A.; Brown, D.; Andrews, D.; McCluskey, R.T. Role of Megalin (Gp330) in Transcytosis of Thyroglobulin by Thyroid Cells: A novel function in the control of thyroid hormone release. J. Biol. Chem. 2000, 275, 7125–7137. [Google Scholar] [CrossRef]
- Marinò, M.; Zheng, G.; McCluskey, R.T. Megalin (Gp330) Is an Endocytic Receptor for Thyroglobulin on Cultured Fisher Rat Thyroid Cells. J. Biol. Chem. 1999, 274, 12898–12904. [Google Scholar] [CrossRef] [PubMed]
- Hermo, L.; Lustig, M.; Lefrancois, S.; Argraves, W.S.; Morales, C.R. Expression and Regulation of LRP-2/Megalin in Epithelial Cells Lining the Efferent Ducts and Epididymis during Postnatal Development. Mol. Reprod. Dev. 1999, 53, 282–293. [Google Scholar] [CrossRef]
- Argraves, W.S.; Morales, C.R. Immunolocalization of Cubilin, Megalin, Apolipoprotein J, and Apolipoprotein A-I in the Uterus and Oviduct. Mol. Reprod. Dev. 2004, 69, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, S.; Hjälm, G.; Hellman, P.; Ek, B.; Junlin, C.; Rastad, J.; Klareskog, L.; Åkerström, G.; Rask, L. A Protein Involved in Calcium Sensing of the Human Parathyroid and Placental Cytotrophoblast Cells Belongs to the LDL-Receptor Protein Superfamily. Exp. Cell Res. 1994, 212, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Herz, J.; Hamann, U.; Rogne, S.; Myklebost, O.; Gausepohl, H.; Stanley, K.K. Surface Location and High Affinity for Calcium of a 500-Kd Liver Membrane Protein Closely Related to the LDL-Receptor Suggest a Physiological Role as Lipoprotein Receptor. EMBO J. 1988, 7, 4119–4127. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.I.; Gliemann, J.; Moestrup, S.K. Renal Tubule Gp330 Is a Calcium Binding Receptor for Endocytic Uptake of Protein. J. Histochem. Cytochem. 1992, 40, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. A Receptor-Mediated Pathway for Cholesterol Homeostasis. Science 1986, 232, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M. Role of the Calcium-Sensing Receptor in Extracellular Calcium Homeostasis. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.-L.; Crumrine, D.A.; Man, M.-Q.; Chang, W.; Elalieh, H.; You, M.; Elias, P.M.; Bikle, D.D. Ablation of the Calcium-Sensing Receptor in Keratinocytes Impairs Epidermal Differentiation and Barrier Function. J. Investig. Dermatol. 2012, 132, 2350–2359. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Kállay, E. Cross Talk between the Calcium-Sensing Receptor and the Vitamin D System in Prevention of Cancer. Front. Physiol. 2016, 7, 451. [Google Scholar] [CrossRef]
- Juhlin, C.; Johansson, H.; Holmdahl, R.; Gylfe, E.; Larsson, R.; Rastad, J.; Akerström, G.; Klareskog, L. Monoclonal Anti-Parathyroid Antibodies Interfering with a Ca2+-Sensor of Human Parathyroid Cells. Biochem. Biophys. Res. Commun. 1987, 143, 570–574. [Google Scholar] [CrossRef]
- Juhlin, C.; Holmdahl, R.; Johansson, H.; Rastad, J.; Akerström, G.; Klareskog, L. Monoclonal Antibodies with Exclusive Reactivity against Parathyroid Cells and Tubule Cells of the Kidney. Proc. Natl. Acad. Sci. USA 1987, 84, 2990–2994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellman, P.; Ridefelt, P.; Juhlin, C.; Akerström, G.; Rastad, J.; Gylfe, E. Parathyroid-like Regulation of Parathyroid-Hormone-Related Protein Release and Cytoplasmic Calcium in Cytotrophoblast Cells of Human Placenta. Arch. Biochem. Biophys. 1992, 293, 174–180. [Google Scholar] [CrossRef]
- Juhlin, C.; Klareskog, L.; Nygren, P.; Ljunghall, S.; Gylfe, E.; Rastad, J.; Akerström, G. Hyperparathyroidism Is Associated with Reduced Expression of a Parathyroid Calcium Receptor Mechanism Defined by Monoclonal Antiparathyroid Antibodies. Endocrinology 1988, 122, 2999–3001. [Google Scholar] [CrossRef]
- Welsh, J.; Wietzke, J.A.; Zinser, G.M.; Byrne, B.; Smith, K.; Narvaez, C.J. Vitamin D-3 Receptor as a Target for Breast Cancer Prevention. J. Nutr. 2003, 133, 2425S–2433S. [Google Scholar] [CrossRef] [PubMed]
- Rowling, M.J.; Kemmis, C.M.; Taffany, D.A.; Welsh, J. Megalin-Mediated Endocytosis of Vitamin D Binding Protein Correlates with 25-Hydroxycholecalciferol Actions in Human Mammary Cells. J. Nutr. 2006, 136, 2754–2759. [Google Scholar] [CrossRef] [PubMed]
- Chlon, T.M.; Taffany, D.A.; Welsh, J.; Rowling, M.J. Retinoids Modulate Expression of the Endocytic Partners Megalin, Cubilin, and Disabled-2 and Uptake of Vitamin D-Binding Protein in Human Mammary Cells. J. Nutr. 2008, 138, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.M.; Tallquist, M.D.; Rock, C.O.; Cooper, J.A. Dual Roles for the Dab2 Adaptor Protein in Embryonic Development and Kidney Transport. EMBO J. 2002, 21, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Ternes, S.B.; Rowling, M.J. Vitamin D Transport Proteins Megalin and Disabled-2 Are Expressed in Prostate and Colon Epithelial Cells and Are Induced and Activated by All-Trans-Retinoic Acid. Nutr. Cancer 2013, 65, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Abboud, M.; Puglisi, D.A.; Davies, B.N.; Rybchyn, M.; Whitehead, N.P.; Brock, K.E.; Cole, L.; Gordon-Thomson, C.; Fraser, D.R.; Mason, R.S. Evidence for a Specific Uptake and Retention Mechanism for 25-Hydroxyvitamin D (25OHD) in Skeletal Muscle Cells. Endocrinology 2013, 154, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- Abboud, M.; Gordon-Thomson, C.; Hoy, A.J.; Balaban, S.; Rybchyn, M.S.; Cole, L.; Su, Y.; Brennan-Speranza, T.C.; Fraser, D.R.; Mason, R.S. Uptake of 25-Hydroxyvitamin D by Muscle and Fat Cells. J. Steroid Biochem. Mol. Biol. 2014, 144, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, S.; Luu, S.; Glowacki, J. Megalin Mediates 25-Hydroxyvitamin D3 Actions in Human Mesenchymal Stem Cells. FASEB J. 2019, 33, 7684–7693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkins, G.J.; Anderson, P.H.; Findlay, D.M.; Welldon, K.J.; Vincent, C.; Zannettino, A.C.W.; O’Loughlin, P.D.; Morris, H.A. Metabolism of Vitamin D3 in Human Osteoblasts: Evidence for Autocrine and Paracrine Activities of 1α,25-Dihydroxyvitamin D3. Bone 2007, 40, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Esteban, C.; Geuskens, M.; Ena, J.M.; Mishal, Z.; Macho, A.; Torres, J.M.; Uriel, J. Receptor-Mediated Uptake and Processing of Vitamin D-Binding Protein in Human B-Lymphoid Cells. J. Biol. Chem. 1992, 267, 10177–10183. [Google Scholar] [CrossRef]
- Bolasco, P. Treatment Options of Secondary Hyperparathyroidism (SHPT) in Patients with Chronic Kidney Disease Stages 3 and 4: An Historic Review. Clin. Cases Min. Bone Metab. 2009, 6, 210–219. [Google Scholar]
- Grabner, A.; Schramm, K.; Silswal, N.; Hendrix, M.; Yanucil, C.; Czaya, B.; Singh, S.; Wolf, M.; Hermann, S.; Stypmann, J.; et al. FGF23/FGFR4-Mediated Left Ventricular Hypertrophy Is Reversible. Sci. Rep. 2017, 7, 1993. [Google Scholar] [CrossRef]
- Negri, A.L.; Brandenburg, V.M.; Brandemburg, V.M. Calcitriol Resistance in Hemodialysis Patients with Secondary Hyperparathyroidism. Int. Urol. Nephrol. 2014, 46, 1145–1151. [Google Scholar] [CrossRef]
- Dusso, A.S. Vitamin D Receptor: Mechanisms for Vitamin D Resistance in Renal Failure. Kidney Int. Suppl. 2003, 63, S6–S9. [Google Scholar] [CrossRef]
- Lemke, D.; Klement, R.J.; Schweiger, F.; Schweiger, B.; Spitz, J. Vitamin D Resistance as a Possible Cause of Autoimmune Diseases: A Hypothesis Confirmed by a Therapeutic High-Dose Vitamin D Protocol. Front. Immunol. 2021, 12, 1110. [Google Scholar] [CrossRef]
- Takemoto, F.; Shinki, T.; Yokoyama, K.; Inokami, T.; Hara, S.; Yamada, A.; Kurokawa, K.; Uchida, S. Gene Expression of Vitamin D Hydroxylase and Megalin in the Remnant Kidney of Nephrectomized Rats. Kidney Int. 2003, 64, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, D.; Bland, R.; Walker, E.A.; Bradwell, A.R.; Howie, A.J.; Hewison, M.; Stewart, P.M. Expression of 25-Hydroxyvitamin D3-1alpha-Hydroxylase in the Human Kidney. J. Am. Soc. Nephrol. 1999, 10, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Bell, N.H. Renal and Nonrenal 25-Hydroxyvitamin D-1alpha-Hydroxylases and Their Clinical Significance. J. Bone Min. Res. 1998, 13, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Huby, A.-C.; Rastaldi, M.-P.; Caron, K.; Smithies, O.; Dussaule, J.-C.; Chatziantoniou, C. Restoration of Podocyte Structure and Improvement of Chronic Renal Disease in Transgenic Mice Overexpressing Renin. PLoS ONE 2009, 4, e6721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosojima, M.; Sato, H.; Yamamoto, K.; Kaseda, R.; Soma, T.; Kobayashi, A.; Suzuki, A.; Kabasawa, H.; Takeyama, A.; Ikuyama, K.; et al. Regulation of Megalin Expression in Cultured Proximal Tubule Cells by Angiotensin II Type 1A Receptor- and Insulin-Mediated Signaling Cross Talk. Endocrinology 2009, 150, 871–878. [Google Scholar] [CrossRef]
- Xu, Z.; Li, W.; Han, J.; Zou, C.; Huang, W.; Yu, W.; Shan, X.; Lum, H.; Li, X.; Liang, G. Angiotensin II Induces Kidney Inflammatory Injury and Fibrosis through Binding to Myeloid Differentiation Protein-2 (MD2). Sci. Rep. 2017, 7, 44911. [Google Scholar] [CrossRef]
- Benetti, A.; Martins, F.L.; Sene, L.B.; Shimizu, M.H.M.; Seguro, A.C.; Luchi, W.M.; Girardi, A.C.C. Urinary DPP4 Correlates with Renal Dysfunction, and DPP4 Inhibition Protects against the Reduction in Megalin and Podocin Expression in Experimental CKD. Am. J. Physiol.-Ren. Physiol. 2021, 320, F285–F296. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, H.; Ceol, M.; Gianesello, L.; Priante, G.; Iotti, A.; Del Prete, D. Downregulation of Megalin, Cubilin, ClC-5 and Podocin in Fabry Nephropathy: Potential Implications in the Decreased Effectiveness of Enzyme Replacement Therapy. J. Nephrol. 2021, 34, 1307–1314. [Google Scholar] [CrossRef]
- Kim, H.J.; Moradi, H.; Yuan, J.; Norris, K.; Vaziri, N.D. Renal Mass Reduction Results in Accumulation of Lipids and Dysregulation of Lipid Regulatory Proteins in the Remnant Kidney. Am. J. Physiol.-Ren. Physiol. 2009, 296, F1297–F1306. [Google Scholar] [CrossRef] [PubMed]
- Charlton, J.R.; Tan, W.; Daouk, G.; Teot, L.; Rosen, S.; Bennett, K.M.; Cwiek, A.; Nam, S.; Emma, F.; Jouret, F.; et al. Beyond the Tubule: Pathological Variants of LRP2, Encoding the Megalin Receptor, Result in Glomerular Loss and Early Progressive Chronic Kidney Disease. Am. J. Physiol. Ren. Physiol. 2020, 319, F988–F999. [Google Scholar] [CrossRef]
- Baumgarten, M.; Gehr, T. Chronic Kidney Disease: Detection and Evaluation. AFP 2011, 84, 1138–1148. [Google Scholar]
- Storm, T.; Tranebjærg, L.; Frykholm, C.; Birn, H.; Verroust, P.J.; Nevéus, T.; Sundelin, B.; Hertz, J.M.; Holmström, G.; Ericson, K.; et al. Renal Phenotypic Investigations of Megalin-Deficient Patients: Novel Insights into Tubular Proteinuria and Albumin Filtration. Nephrol. Dial. Transpl. 2013, 28, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, W.R.; Carling, T.; Juhlin, C.; Rastad, J.; Ridefelt, P.; Akerström, G.; Hellman, P. Regulation of Gp330/Megalin Expression by Vitamins A and D. Eur. J. Clin. Investig. 1998, 28, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S.; Tokumoto, M. Defective Renal Maintenance of the Vitamin D Endocrine System Impairs Vitamin D Renoprotection: A Downward Spiral in Kidney Disease. Kidney Int. 2011, 79, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-C.; Lu, C.-L.; Lu, K.-C. Mineral Bone Disorders in Chronic Kidney Disease. Nephrology 2018, 23, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dusso, A.; González, E.A.; Martin, K.J. Vitamin D in Chronic Kidney Disease. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S. Kidney Disease and Vitamin D Levels: 25-Hydroxyvitamin D, 1,25-Dihydroxyvitamin D, and VDR Activation. Kidney Int. Suppl. 2011, 1, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Kim, S.W. Vitamin D and Chronic Kidney Disease. Korean J. Intern. Med. 2014, 29, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, C.; de Boer, I.H. Impaired Vitamin D Metabolism in CKD. Semin. Nephrol. 2013, 33, 158. [Google Scholar] [CrossRef]
- Milani, C.; Katayama, M.L.H.; de Lyra, E.C.; Welsh, J.; Campos, L.T.; Brentani, M.M.; Maciel, M.D.S.; Roela, R.A.; del Valle, P.R.; Góes, J.C.G.S.; et al. Transcriptional Effects of 1,25 Dihydroxyvitamin D3 Physiological and Supra-Physiological Concentrations in Breast Cancer Organotypic Culture. BMC Cancer 2013, 13, 119. [Google Scholar] [CrossRef]
- van den Bemd, G.J.; Pols, H.A.; van Leeuwen, J.P. Anti-Tumor Effects of 1,25-Dihydroxyvitamin D3 and Vitamin D Analogs. Curr. Pharm. Des. 2000, 6, 717–732. [Google Scholar] [CrossRef]
- Nakagawa, K.; Sasaki, Y.; Kato, S.; Kubodera, N.; Okano, T. 22-Oxa-1alpha,25-Dihydroxyvitamin D3 Inhibits Metastasis and Angiogenesis in Lung Cancer. Carcinogenesis 2005, 26, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Swami, S.; Raghavachari, N.; Muller, U.R.; Bao, Y.P.; Feldman, D. Vitamin D Growth Inhibition of Breast Cancer Cells: Gene Expression Patterns Assessed by CDNA Microarray. Breast Cancer Res. Treat. 2003, 80, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.L.; de Azevedo, M.J.; Silveiro, S.P.; Canani, L.H.; Caramori, M.L.; Zelmanovitz, T. Diabetic Nephropathy: Diagnosis, Prevention, and Treatment. Diabetes Care 2005, 28, 164–176. [Google Scholar] [CrossRef]
- Anderson, R.L.; Ternes, S.B.; Strand, K.A.; Rowling, M.J. Vitamin D Homeostasis Is Compromised Due to Increased Urinary Excretion of the 25-Hydroxycholecalciferol-Vitamin D-Binding Protein Complex in the Zucker Diabetic Fatty Rat. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E959–E967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowlkes, J.L.; Bunn, R.C.; Cockrell, G.E.; Clark, L.M.; Wahl, E.C.; Lumpkin, C.K.; Thrailkill, K.M. Dysregulation of the Intrarenal Vitamin D Endocytic Pathway in a Nephropathy-Prone Mouse Model of Type 1 Diabetes. Exp. Diabetes Res. 2011, 2011, 269378. [Google Scholar] [CrossRef]
- Russo, L.M.; del Re, E.; Brown, D.; Lin, H.Y. Evidence for a Role of Transforming Growth Factor (TGF)-Beta1 in the Induction of Postglomerular Albuminuria in Diabetic Nephropathy: Amelioration by Soluble TGF-Beta Type II Receptor. Diabetes 2007, 56, 380–388. [Google Scholar] [CrossRef]
- Tojo, A.; Onozato, M.L.; Ha, H.; Kurihara, H.; Sakai, T.; Goto, A.; Fujita, T.; Endou, H. Reduced Albumin Reabsorption in the Proximal Tubule of Early-Stage Diabetic Rats. Histochem. Cell Biol. 2001, 116, 269–276. [Google Scholar] [CrossRef]
- Thrailkill, K.M.; Jo, C.-H.; Cockrell, G.E.; Moreau, C.S.; Fowlkes, J.L. Enhanced Excretion of Vitamin D Binding Protein in Type 1 Diabetes: A Role in Vitamin D Deficiency? J. Clin. Endocrinol. Metab. 2011, 96, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Thrailkill, K.M.; Nimmo, T.; Bunn, R.C.; Cockrell, G.E.; Moreau, C.S.; Mackintosh, S.; Edmondson, R.D.; Fowlkes, J.L. Microalbuminuria in Type 1 Diabetes Is Associated with Enhanced Excretion of the Endocytic Multiligand Receptors Megalin and Cubilin. Diabetes Care 2009, 32, 1266–1268. [Google Scholar] [CrossRef]
- Ogasawara, S.; Hosojima, M.; Kaseda, R.; Kabasawa, H.; Yamamoto-Kabasawa, K.; Kurosawa, H.; Sato, H.; Iino, N.; Takeda, T.; Suzuki, Y.; et al. Significance of Urinary Full-Length and Ectodomain Forms of Megalin in Patients with Type 2 Diabetes. Diabetes Care 2012, 35, 1112–1118. [Google Scholar] [CrossRef]
- De, S.; Kuwahara, S.; Hosojima, M.; Ishikawa, T.; Kaseda, R.; Sarkar, P.; Yoshioka, Y.; Kabasawa, H.; Iida, T.; Goto, S.; et al. Exocytosis-Mediated Urinary Full-Length Megalin Excretion Is Linked With the Pathogenesis of Diabetic Nephropathy. Diabetes 2017, 66, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Toi, N.; Inaba, M.; Ishimura, E.; Tsugawa, N.; Imanishi, Y.; Emoto, M.; Hirayama, Y.; Nakatani, S.; Saito, A.; Yamada, S. Significance of Urinary C-Megalin Excretion in Vitamin D Metabolism in Pre-Dialysis CKD Patients. Sci. Rep. 2019, 9, 2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.S.; Petkovich, M.; Holden, R.M.; Adams, M.A. Megalin and Vitamin D Metabolism—Implications in Non-Renal Tissues and Kidney Disease. Nutrients 2022, 14, 3690. https://doi.org/10.3390/nu14183690
Khan SS, Petkovich M, Holden RM, Adams MA. Megalin and Vitamin D Metabolism—Implications in Non-Renal Tissues and Kidney Disease. Nutrients. 2022; 14(18):3690. https://doi.org/10.3390/nu14183690
Chicago/Turabian StyleKhan, Sono S., Martin Petkovich, Rachel M. Holden, and Michael A. Adams. 2022. "Megalin and Vitamin D Metabolism—Implications in Non-Renal Tissues and Kidney Disease" Nutrients 14, no. 18: 3690. https://doi.org/10.3390/nu14183690
APA StyleKhan, S. S., Petkovich, M., Holden, R. M., & Adams, M. A. (2022). Megalin and Vitamin D Metabolism—Implications in Non-Renal Tissues and Kidney Disease. Nutrients, 14(18), 3690. https://doi.org/10.3390/nu14183690




