Chlorogenic Acid and Quercetin in a Diet with Fermentable Fiber Influence Multiple Processes Involved in DSS-Induced Ulcerative Colitis but Do Not Reduce Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Diets and Study Design
2.2. Body Weight and Diet Intake
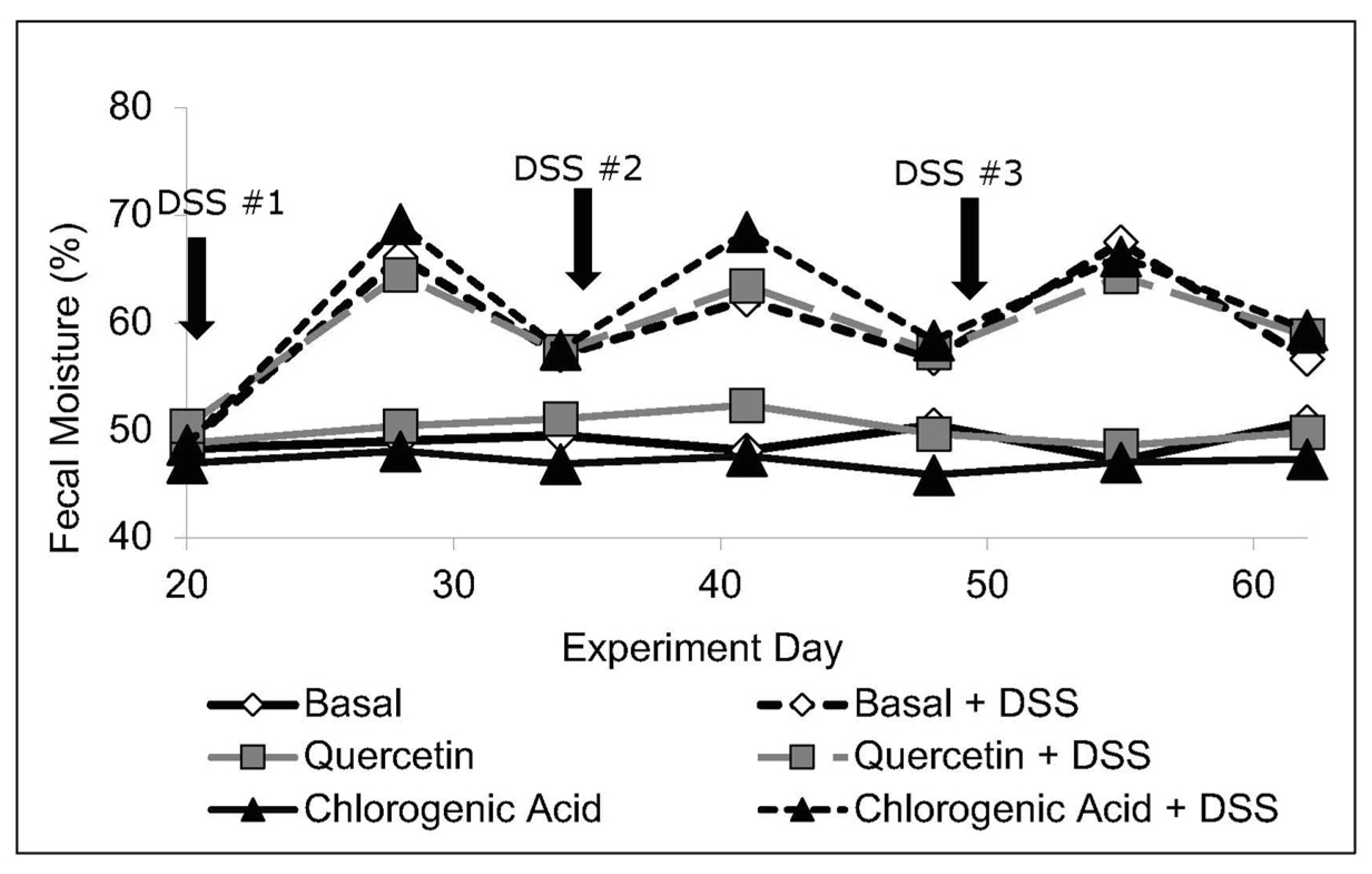
2.3. Fecal Output and Moisture Content
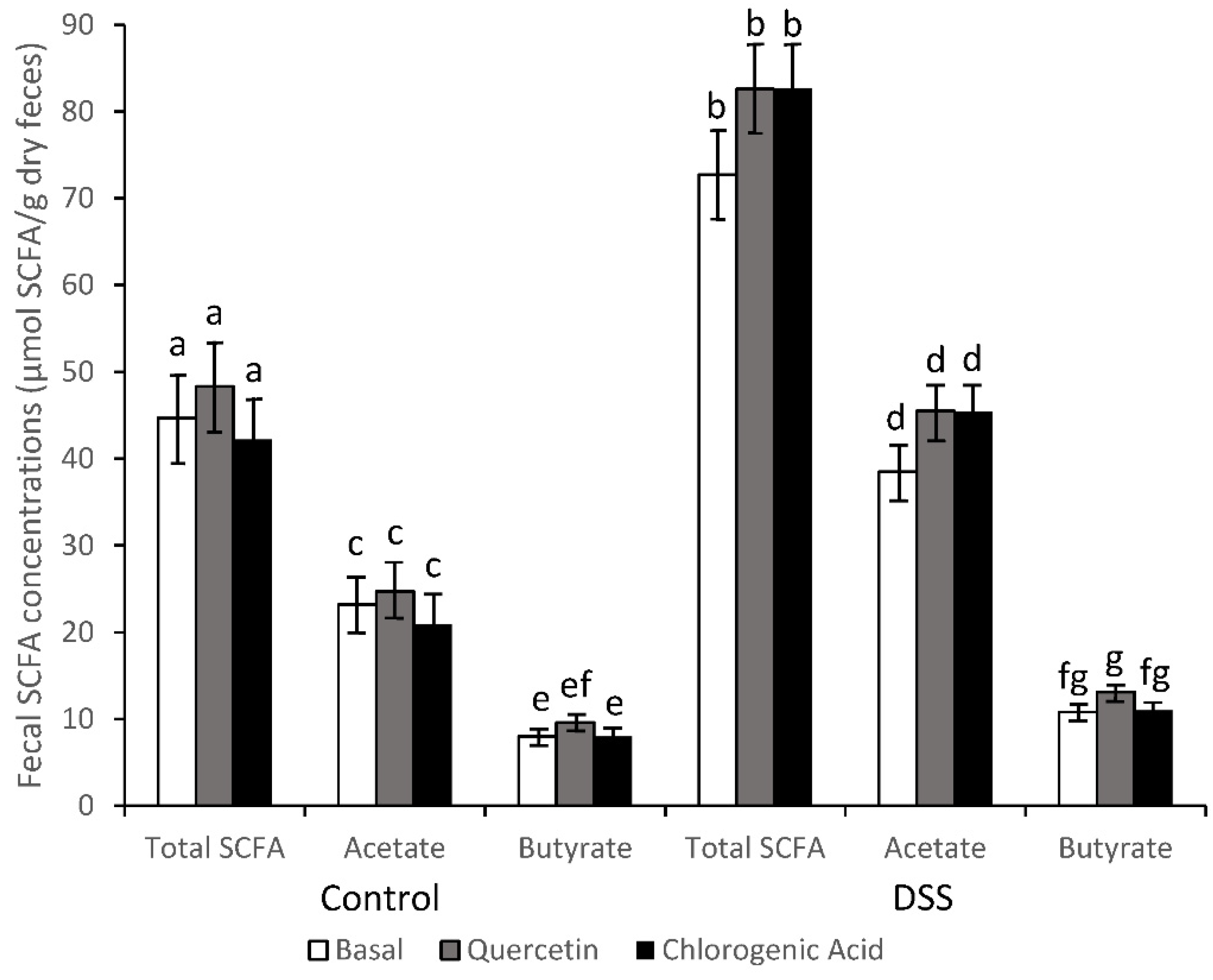
2.4. Short-Chain Fatty Acid Analysis
2.5. Tissue Fixation
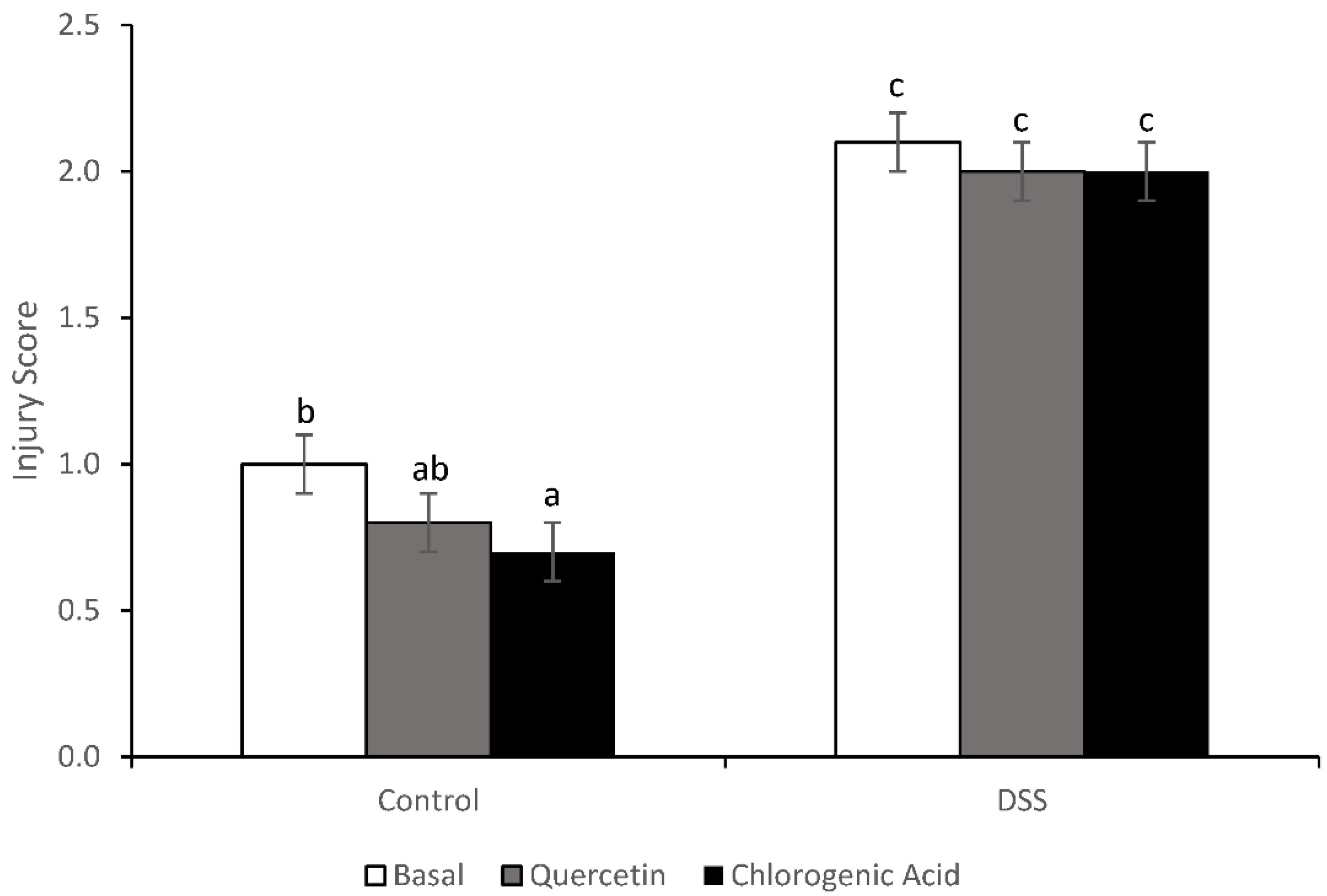
2.6. Injury and Inflammation Scores
2.7. Proliferative Index, Zone, and Crypt Height
2.8. Mucosal Samples
2.9. NF-κB Activity
2.10. Real Time RT-PCR
2.11. Statistics
3. Results
3.1. Body Weight, Intake, and Fecal Moisture Content
3.2. SCFA Concentrations
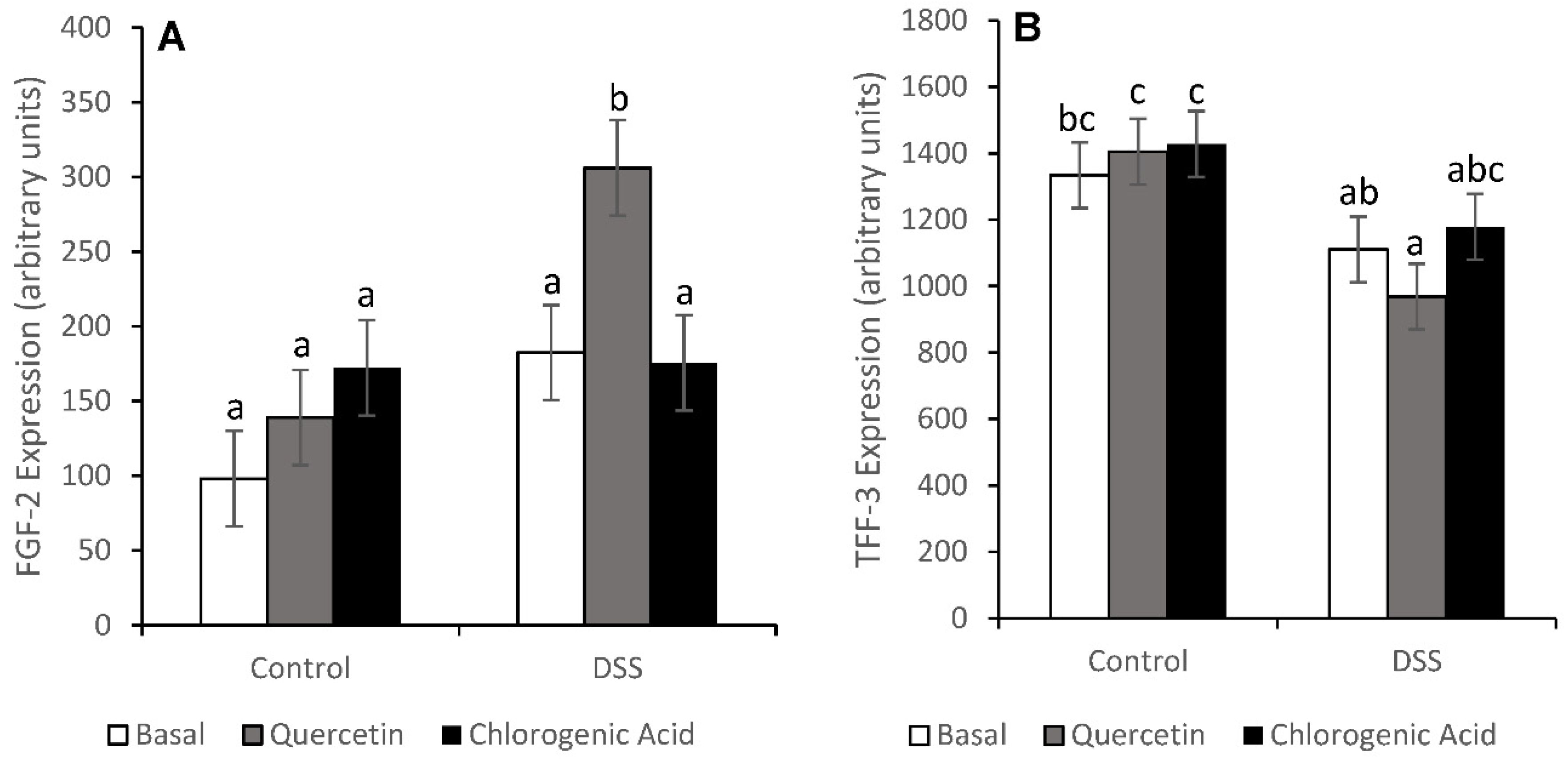
3.3. Injury and Injury Repair
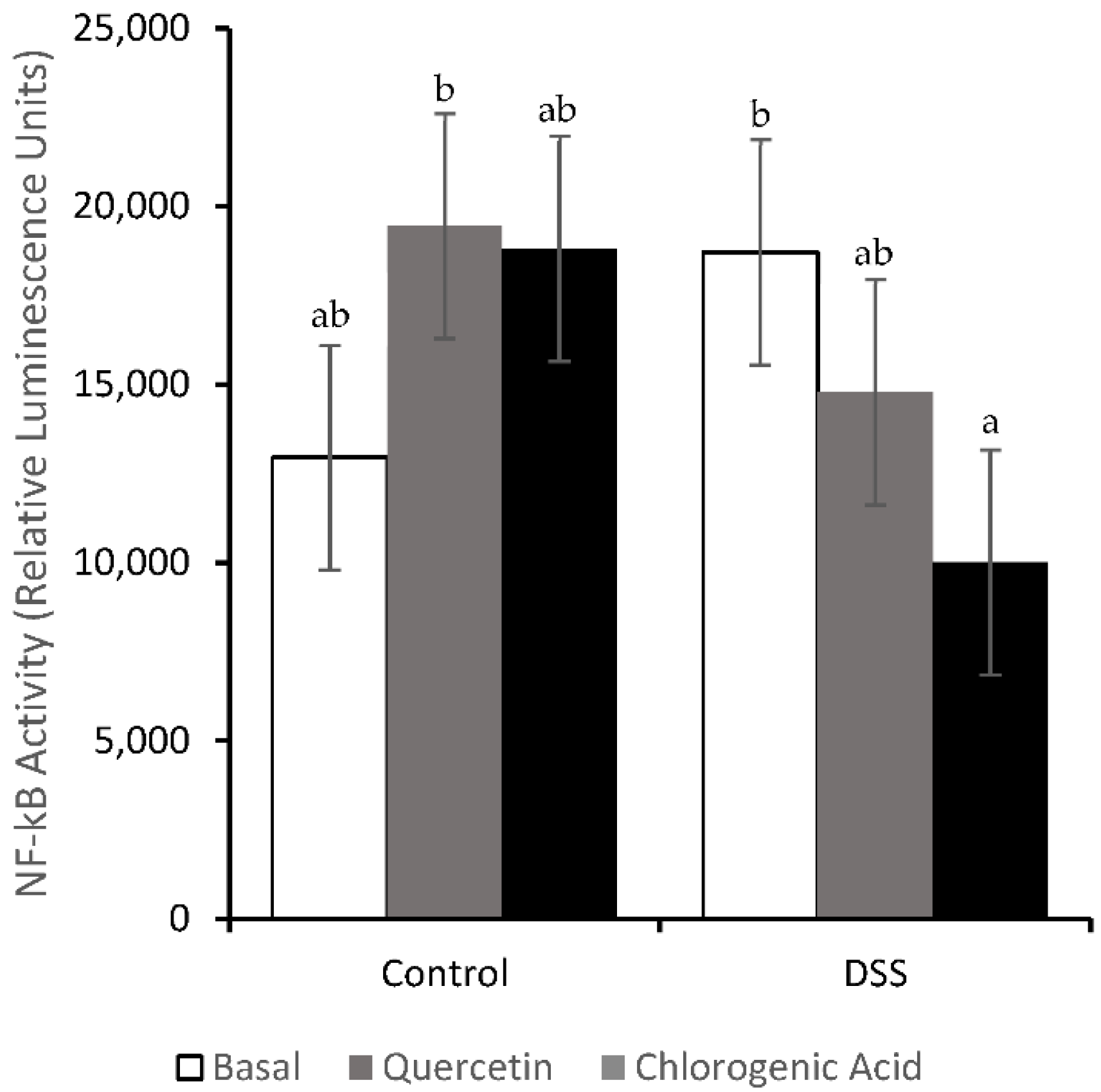
3.4. NF-κB Activity and Expression of Downstream Effectors
3.5. Toll like Receptor Pathways
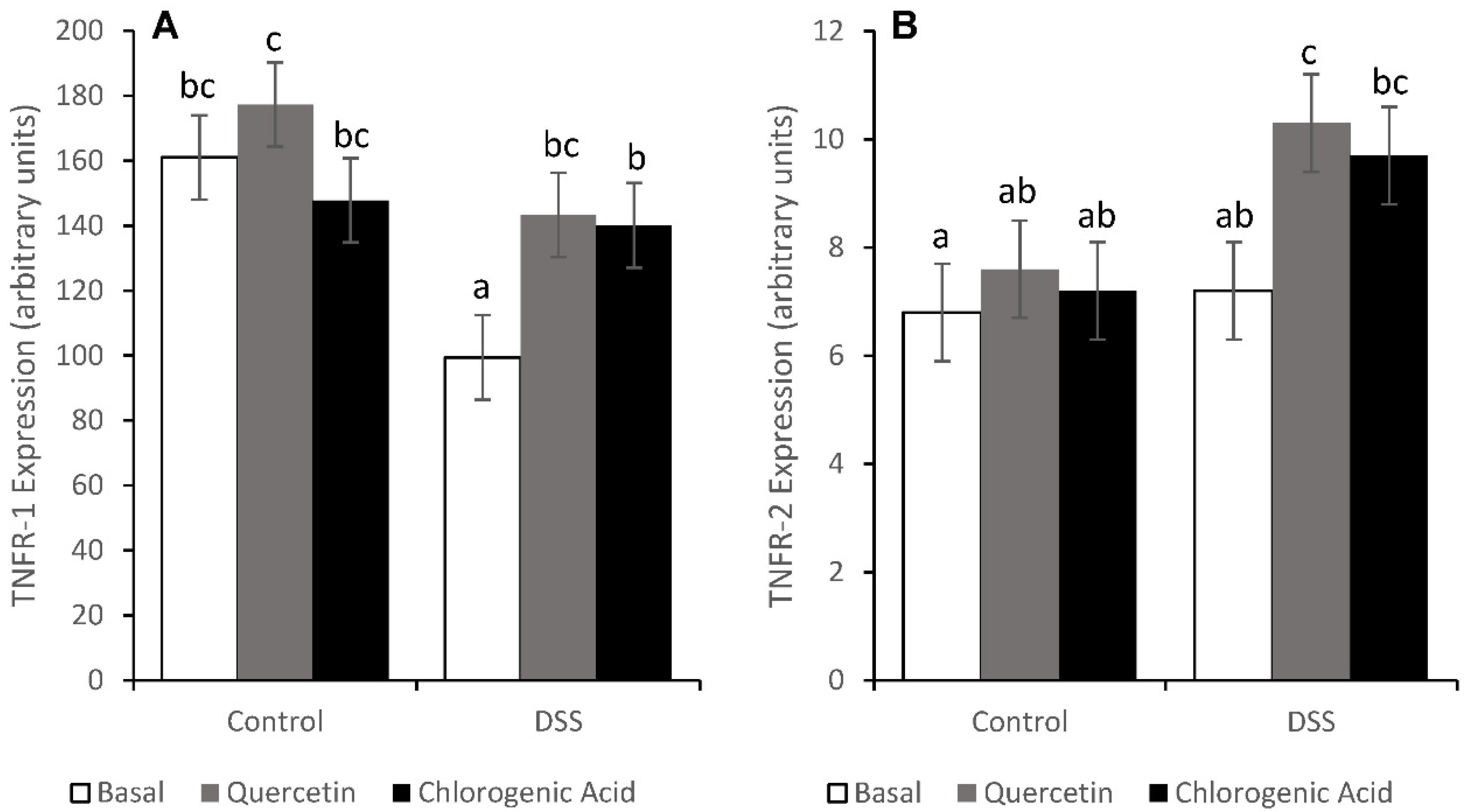
3.6. Tumor Necrosis Factor Receptor Pathways
3.7. Proliferation in the Distal Colon
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control. Inflammatory Bowel Disease (IBD) 2022. Available online: http://www.cdc.gov/ibd/-epidIBD (accessed on 15 March 2022).
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Seril, D.N.; Liao, J.; Yang, G.-Y.; Yang, C.S. Oxidative stress and ulcerative colitis-associated carcinogenesis: Studies in humans and animal models. Carcinogenesis 2003, 24, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Itzkowitz, S.H. Colorectal cancer in inflammatory bowel disease: Mechanisms and management. Gastroenterology 2022, 162, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.; Colombel, J.-F.; Feagan, B.C.; Fedorak, R.N.; Hanauer, S.B.; Kamm, M.A.; Mayer, L.; Regueiro, C.; Rutgeerts, P.; Sandborn, W.J.; et al. American Gastroentergological Association Consensus Development Conference on the use of biologics in the treatment of inflammatory bowel disease, June 21–23, 2006. Gastroenterology 2007, 133, 312–339. [Google Scholar] [CrossRef]
- Chan, A.T.; Giovannucci, E.L. Primary prevention of colorectal cancer. Gastroenterology 2010, 138, 2029–2043. [Google Scholar] [CrossRef]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit and cancer prevention: A review. J. Am. Diet. Assoc. 1996, 96, 1027–1039. [Google Scholar] [CrossRef]
- Reif, S.; Klein, I.; Lubin, F.; Farbstein, M.; Hallak, A.; Gilat, T. Pre-illness dietary factors in inflammatory bowel disease. Gut 1997, 40, 754–760. [Google Scholar] [CrossRef]
- Antoniussen, C.S.; Rasmussen, H.H.; Holst, M.; Lauridsen, C. Reducing disease activity of inflammatory bowel disease by consumption of plant-based foods and nutrients. Front. Nutr. 2021, 8, 733433. [Google Scholar] [CrossRef]
- Noratto, G.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Identifying peach and plum polyphenols with chemopreventative potential against estrogen-independent breast cancer cells. J. Agric. Food Chem. 2009, 57, 5219–5226. [Google Scholar] [CrossRef]
- Vizzotto, M.; Cisneros-Zevallos, L.; Byrne, D.H.; Ramming, D.W.; Okie, W.R. Large variation found in the phytochemical and antioxidant activity of peach and plum germplasm. J. Am. Soc. Hortic. Sci. 2007, 132, 334–340. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC-DAD-ESIMS analysis of phenolic compounds in nectarines, peaches, and plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gallaher, D.D. Effect of dried plums on colon cancer risk factors in rats. Nutr. Cancer 2005, 53, 117–125. [Google Scholar] [CrossRef]
- Tan, A.C.; Konczak, I.; Ramzam, I.; Zabaras, D.; Sze, D.M.-Y. Potential antioxidant, antiinflammatory, and proapoptotic anticancer activities of Kakuda plum and Illawarra plum polyphenolic fractions. Nutr. Cancer 2011, 63, 1074–1084. [Google Scholar] [CrossRef]
- Warren, C.A.; Paulhill, K.J.; Davidson, L.A.; Lupton, J.R.; Taddeo, S.S.; Hong, M.Y.; Carroll, R.J.; Chapkin, R.S.; Turner, N.D. Quercetin may suppress rat aberrant crypt foci formation by suppressing inflammatory mediators that influence proliferation and apoptosis. J. Nutr. 2009, 139, 101–105. [Google Scholar] [CrossRef]
- Amasheh, M.; Schlichter, S.; Amasheh, S.; Mankertz, J.; Zeitz, M.; Fromm, M.; Schulske, J.D. Quercetin enhances epithelial barrier function and increases claudin-4 expression in Caco-2 cells. J. Nutr. 2008, 138, 1067–1073. [Google Scholar]
- Comalada, M.; Camuesco, D.; Sierra, S.; Ballester, I.; Xaus, J.; Gálvez, J.; Zarzuelo, A. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-κB pathway. Eur. J. Immunol. 2005, 35, 584–592. [Google Scholar] [CrossRef]
- Rao, C.V.; Desai, D.; Simi, B.; Kulkarni, N.; Amin, S.; Reddy, B.S. Inhibitory effect of caffeic acid esters on azoxymethanie-induced biochemical changes and aberrant crypt foci formation in rat colon. Cancer Res. 1993, 53, 4182–4188. [Google Scholar]
- Larrosa, M.; Luceri, C.; Vivoli, E.; Pagliuca, C.; Lodovici, M.; Moneti, G.; Dolara, P. Polyphenol metabolites from colonic microbiota exert anti-inflammatory activity on different inflammation models. Mol. Nutr. Food Res. 2009, 53, 1044–1054. [Google Scholar] [CrossRef]
- Kajiura, T.; Takeda, T.; Sakata, S.; Sakamoto, M.; Hashimoto, M.; Suzuki, H.; Suzuki, M.; Benno, Y. Change of intestinal microbiota with elemental diet and its impact on therapeutic effects in a murine model of chronic colitis. Dig. Dis. Sci. 2009, 54, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Appunni, S.; Rubens, M.; Ramamoorthy, V.; Tonse, R.; Saxena, A.; McGranaghan, P.; Kaiser, A.; Kotecha, R. Emerging evidence on the effects of dietary factors on the gut microbiome in colorectal cancer. Front. Nutr. 2021, 8, 718389. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.A.; Takaishi, Y.; Shirotori, M.; Kawaguchi, Y.; Tsuchiya, K.; Shibata, H.; Higuti, T.; Tadokoro, T.; Takeuchi, M. Antibacterial and antioxidant activities of quercetin oxidation products from yellow onion (Allium cepa) skin. J. Agric. Food Chem. 2006, 54, 3551–3557. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Bosscher, D.; Breynaert, A.; Pieters, L.; Hermans, N. Food-based strategies to modulate the composition of the intestinal microbiota and their associated health effects. J. Physiol. Pharmacol. 2009, 60 (Suppl. 6), 5–11. [Google Scholar] [PubMed]
- Clifford, M.N. Chlorogenic acid and other cinnamates - nature, occurrence, and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar]
- Bhandarkar, N.S.; Brown, L.; Panchal, S.K. Chlorogenic acid attenuates high-carbohydrate, high-fat diet-induced cardiovascular, liver, and metabolic changes in rats. Nutr. Res. 2019, 62, 78–88. [Google Scholar] [CrossRef]
- Ritchie, L.E.; Taddeo, S.S.; Weeks, B.R.; Carroll, R.J.; Dykes, L.; Rooney, L.W.; Turner, N.D. Impact of novel sorghum bran diets on DSS-induced colitis. Nutrients 2017, 9, 330. [Google Scholar] [CrossRef]
- Kawada, M.; Arihiro, A.; Mizoguchi, E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 5581–5593. [Google Scholar] [CrossRef]
- Gaudio, E.; Taddei, G.; Vetuschi, A.; Sferra, R.; Frieri, G.; Ricciardi, G.; Caprilli, R. Dextran sulfate sodium (DSS) colitis in rats: Clinical, structural, and ultrastructural aspects. Dig. Dis. Sci. 1999, 44, 1458–1475. [Google Scholar] [CrossRef]
- Zoran, D.L.; Barhoumi, R.; Burghardt, R.C.; Chapkin, R.S.; Lupton, J.R. Diet and carcinogen alter luminal butyrate concentration and intracellular pH in isolated rat colonocytes. Nutr. Cancer 1997, 27, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Vanamala, J.; Leonardi, T.; Patil, B.S.; Taddeo, S.S.; Murphy, M.E.; Pike, L.M.; Chapkin, R.S.; Lupton, J.R.; Turner, N.D. Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis 2006, 27, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Lupton, J.R.; Smith, R.; Weeks, B.R.; Callaway, E.; Davidson, L.A.; Kim, W.; Fan, Y.-Y.; Yang, P.; Newman, R.A.; et al. Reduced colitis-associated colon cancer in Fat-1 (n-3 fatty acid desaturase) transgenic mice. Cancer Res. 2008, 68, 3985–3991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Q.; Ivanov, I.; Zlatev, Z.Z.; Alaniz, R.C.; Weeks, B.R.; Callaway, E.S.; Goldsby, J.S.; Davidson, L.A.; Fan, Y.-Y.; Zhou, L.; et al. Dietary fish oil and curcumin combine to modulate colonic cytokinetics and gene expression in dextran sodium sulphate-treated mice. Br. J. Nutr. 2011, 106, 519–529. [Google Scholar] [CrossRef]
- Leonardi, T.; Vanamala, J.; Taddeo, S.S.; Davidson, L.A.; Murphy, M.E.; Patil, B.S.; Wang, N.; Carroll, R.J.; Chapkin, R.S.; Lupton, J.R.; et al. Apigenin and naringenin suppress colon carcinogenesis through the aberrant crypt stage in azoxymethane-treated rats. Exp. Biol. Med. 2010, 235, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, D.K.; Gerken, G.; Eyking, A.; Cario, E. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology 2009, 137, 209–220. [Google Scholar] [CrossRef]
- Howarth, G.S. Commentary on prebiotic utility in colitis: Will inflammasomics hold the key? J. Nutr. 2012, 142, 1189–1190. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Rhodes, J.M. Nutrition and gut health: The impact of specific dietary component–it’s not just five-a-day. Proc. Nutr. Soc. 2021, 80, 9–18. [Google Scholar] [CrossRef]
- Thibault, R.; De Coppet, P.; Daly, K.; Bourreille, A.; Cuff, M.; Bonnet, C.; Mosneir, J.-F.; Galmiche, J.-P.; Shirazi-Beechey, S.; Segain, J.-P. Down-regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology 2007, 133, 1916–1927. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Bäumler, A.J. Colonocyte metabolism shapes the gut microbiota. Science 2018, 362, eaat9076. [Google Scholar] [CrossRef]
- Renes, I.B.; Verburg, M.; Van Nispen, D.J.P.M.; Taminiau, J.A.J.M.; Büller, H.A.; Dekker, J.; Einerhand, A.W.C. Epithelial proliferation, cell death, and gene expression in the experimental colitis: Alterations in carbonic anhydrase I, mucin MUC2, and trefoil factor 3 expression. Int. J. Colorectal. Dis. 2002, 17, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Greten, F.R. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.K.-M.; Shisler, J.; Bixby, J.G.; Felices, M.; Zheng, L.; Appel, M.; Orenstein, J.; Moss, B.; Lenardo, M.J. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J. Biol. Chem. 2003, 278, 51613–51621. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, E.; Mizoguchi, A.; Takedatsu, H.; Cario, E.; de Jong, Y.P.; Ooi, C.J.; Xavier, R.J.; Terhorst, C.; Podolsky, D.K.; Bhan, A.K. Role of tumor necrosis factor receptor 2 (TNFR2) in colonic epithelial hyperplasia and chronic intestinal inflammation in mice. Gastroenterology 2002, 122, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Karrasch, T.; Jobin, C. NF-kappaB and the intestine: Friend or foe? Inflamm. Bowel Dis. 2008, 14, 114–124. [Google Scholar] [CrossRef]
- Rogler, G.; Brand, K.; Vogl, D.; Page, S.; Hofmeister, R.; Andus, T.; Knuechel, R.; Baeuerle, P.A.; Schölmerich, J.; Gross, V. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 1998, 115, 357–369. [Google Scholar] [CrossRef]



| Basal Diet | Quercetin Diet | Chlorogenic Acid Diet | ||||
|---|---|---|---|---|---|---|
| Control | DSS | Control | DSS | Control | DSS | |
| IL-6 | 752 ± 168 a | 544 ± 168 a | 923 ± 168 ab | 1365 ± 180 b | 828 ± 168 a | 606 ± 168 a |
| TGF-Β | 152 ± 18 a | 147 ± 18 a | 179 ± 18 a | 177 ± 20 a | 161 ± 18 a | 170 ± 18 a |
| TNF-α | 1804 ± 354 a | 2090 ± 354 ab | 2596 ± 354 ab | 2991 ± 378 b | 2543 ± 354 ab | 1662 ± 354 a |
| Basal Diet | Quercetin Diet | Chlorogenic Acid Diet | ||||
|---|---|---|---|---|---|---|
| Control | DSS | Control | DSS | Control | DSS | |
| TLR-2 | 3.9 ± 1.0 a | 6.6 ± 1.0 ab | 6.9 ± 1.0 b | 7.7 ± 1.7 b | 6.2 ± 1.0 ab | 7.7 ± 1.0 b |
| TLR-4 | 61.4 ± 5.5 b | 30.3 ± 5.5 a | 55.0 ± 5.5 b | 34.9 ± 5.9 a | 55.7 ± 5.5 b | 36.5 ± 5.5 a |
| TLR-5 | 30.1 ± 3.6 ab | 25.3 ± 3.6 ab | 31.2 ± 3.6 ab | 22.7 ± 3.8 a | 35.1 ± 3.6 b | 26.1 ± 3.6 ab |
| TLR-9 | 7.0 ± 1.2 abc | 4.7 ± 1.2 ab | 7.6 ± 1.2 bc | 7.9 ± 1.3 bc | 8.9 ± 1.2 c | 3.9 ± 1.2 a |
| MyD88 | 99.7 ± 8.4 c | 60.4 ± 8.4 a | 104.8 ± 8.4 c | 70.5 ± 8.9 ab | 91.8 ± 8.6 bc | 74.2 ± 8.4 ab |
| Tollip | 58.1 ± 4.2 c | 42.7 ± 4.2 a | 57.0 ± 4.2 bc | 45.1 ± 4.4 ab | 56.7 ± 4.2 bc | 46.8 ± 4.2 abc |
| Basal Diet | Quercetin Diet | Chlorogenic Acid Diet | ||||
|---|---|---|---|---|---|---|
| Control | DSS | Control | DSS | Control | DSS | |
| CH 2 | 22.8 ± 1.0 ab | 24.8 ± 1.0 b | 20.9 ± 1.0 a | 22.2 ± 1.1 ab | 21.3 ± 1.0 a | 21.9 ± 1.0 a |
| PI 2 | 16.6 ± 2.4 ab | 15.0 ± 2.4 a | 20.0 ± 2.4 ab | 20.0 ± 2.7 ab | 22.2 ± 2.4 b | 18.0 ± 2.4 ab |
| PZ 2 | 39.0 ± 2.5 a | 37.5 ± 2.5 a | 41.3 ± 2.5 a | 39.3 ± 2.8 a | 43.8 ± 2.5 a | 40.6 ± 2.5 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslin, L.A.; Weeks, B.R.; Carroll, R.J.; Byrne, D.H.; Turner, N.D. Chlorogenic Acid and Quercetin in a Diet with Fermentable Fiber Influence Multiple Processes Involved in DSS-Induced Ulcerative Colitis but Do Not Reduce Injury. Nutrients 2022, 14, 3706. https://doi.org/10.3390/nu14183706
Maslin LA, Weeks BR, Carroll RJ, Byrne DH, Turner ND. Chlorogenic Acid and Quercetin in a Diet with Fermentable Fiber Influence Multiple Processes Involved in DSS-Induced Ulcerative Colitis but Do Not Reduce Injury. Nutrients. 2022; 14(18):3706. https://doi.org/10.3390/nu14183706
Chicago/Turabian StyleMaslin, Leigh Ann, Bradley R. Weeks, Raymond J. Carroll, David H. Byrne, and Nancy D. Turner. 2022. "Chlorogenic Acid and Quercetin in a Diet with Fermentable Fiber Influence Multiple Processes Involved in DSS-Induced Ulcerative Colitis but Do Not Reduce Injury" Nutrients 14, no. 18: 3706. https://doi.org/10.3390/nu14183706
APA StyleMaslin, L. A., Weeks, B. R., Carroll, R. J., Byrne, D. H., & Turner, N. D. (2022). Chlorogenic Acid and Quercetin in a Diet with Fermentable Fiber Influence Multiple Processes Involved in DSS-Induced Ulcerative Colitis but Do Not Reduce Injury. Nutrients, 14(18), 3706. https://doi.org/10.3390/nu14183706







