Relevance of Vitamin D and Its Deficiency for the Ovarian Follicle and the Oocyte: An Update
Abstract
:1. Introduction
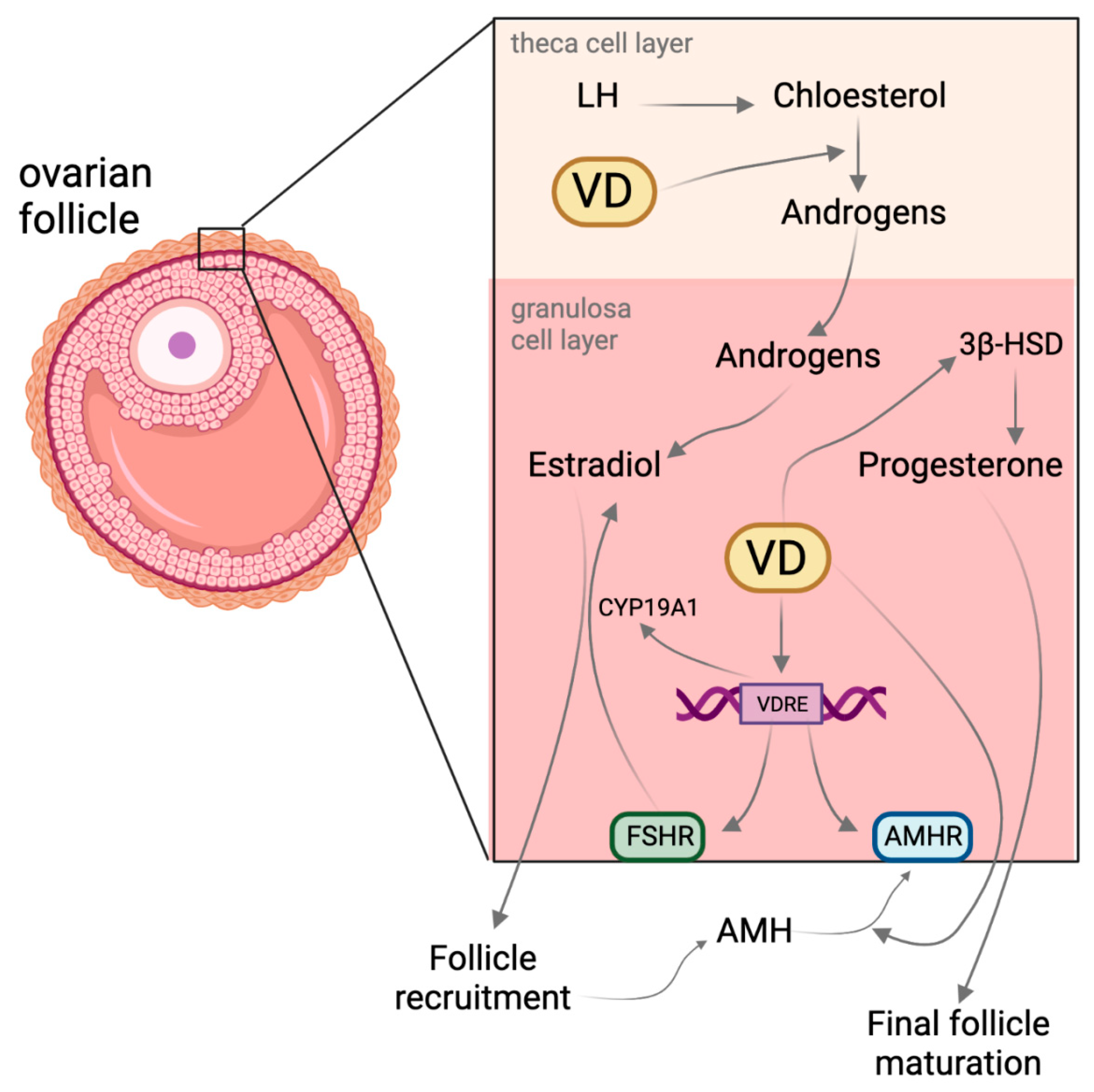
2. Vitamin D and Its Effect on Follicle Maturation
3. Vitamin D and Its Effect on Oocyte and Embryo Quality

4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Malmstroem, S.; Schwartz, J. Current Controversies: Are Free Vitamin Metabolite Levels a More Accurate Assessment of Vitamin D Status than Total Levels? Endocrinol. Metab. Clin. N. Am. 2017, 46, 901–918. [Google Scholar] [CrossRef] [PubMed]
- Donati, S.; Palmini, G.; Aurilia, C.; Falsetti, I.; Miglietta, F.; Iantomasi, T.; Brandi, M.L. Rapid Nontranscriptional Effects of Calcifediol and Calcitriol. Nutrients 2022, 14, 1291. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ren, S.; Chen, H.; Chun, R.F.; Gacad, M.A.; Adams, J.S. Intracellular Vitamin D Binding Proteins: Novel Facilitators of Vitamin D-Directed Transactivation. Mol. Endocrinol. 2000, 14, 1387–1397. [Google Scholar] [CrossRef]
- Franasiak, J.M.; Lara, E.E.; Pellicer, A. Vitamin D in Human Reproduction. Curr. Opin. Obstet. Gynecol. 2017, 29, 189–194. [Google Scholar] [CrossRef]
- Xu, F.; Wolf, S.; Green, O.; Xu, J. Vitamin D in Follicular Development and Oocyte Maturation. Reproduction 2021, 161, R129–R137. [Google Scholar] [CrossRef]
- Zhao, G.; Ford, E.S.; Tsai, J.; Li, C.; Croft, J.B. Factors Associated with Vitamin D Deficiency and Inadequacy among Women of Childbearing Age in the United States. ISRN Obstet. Gynecol. 2012, 2012, 691486. [Google Scholar] [CrossRef]
- LeBoff, M.S.; Chou, S.H.; Ratliff, K.A.; Cook, N.R.; Khurana, B.; Kim, E.; Cawthon, P.M.; Bauer, D.C.; Black, D.; Gallagher, J.C. Supplemental Vitamin D and Incident Fractures in Midlife and Older Adults. N. Engl. J. Med. 2022, 387, 299–309. [Google Scholar] [CrossRef]
- Xu, J.; Lawson, M.S.; Xu, F.; Du, Y.; Tkachenko, O.Y.; Bishop, C.V.; Pejovic-Nezhat, L.; Seifer, D.B.; Hennebold, J.D. Vitamin D3 Regulates Follicular Development and Intrafollicular Vitamin D Biosynthesis and Signaling in the Primate Ovary. Front. Physiol. 2018, 9, 1600. [Google Scholar] [CrossRef]
- la Cour Poulsen, L.; Pla, I.; Sanchez, A.; Grøndahl, M.L.; Marko-Varga, G.; Andersen, C.Y.; Englund, A.L.M.; Malm, J. Progressive Changes in Human Follicular Fluid Composition over the Course of Ovulation: Quantitative Proteomic Analyses. Mol. Cell. Endocrinol. 2019, 495, 110522. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Song, J.; Deng, J.; Sun, Z. Study on Follicular Fluid Metabolomics Components at Different Ages Based on Lipid Metabolism. Reprod. Biol. Endocrinol. 2020, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Da Broi, M.G.; Giorgi, V.S.I.; Wang, F.; Keefe, D.L.; Albertini, D.; Navarro, P.A. Influence of Follicular Fluid and Cumulus Cells on Oocyte Quality: Clinical Implications. J. Assist. Reprod. Genet. 2018, 35, 735–751. [Google Scholar] [PubMed]
- Zamah, A.M.; Hassis, M.E.; Albertolle, M.E.; Williams, K.E. Proteomic Analysis of Human Follicular Fluid from Fertile Women. Clin. Proteom. 2015, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Wissing, M.L.; Kristensen, S.G.; Andersen, C.Y.; Mikkelsen, A.L.; Høst, T.; Borup, R.; Grøndahl, M.L. Identification of New Ovulation-Related Genes in Humans by Comparing the Transcriptome of Granulosa Cells before and after Ovulation Triggering in the Same Controlled Ovarian Stimulation Cycle. Hum. Reprod. 2014, 29, 997–1010. [Google Scholar] [CrossRef]
- Ciepiela, P.; Dulęba, A.J.; Kowaleczko, E.; Chełstowski, K.; Kurzawa, R. Vitamin D as a Follicular Marker of Human Oocyte Quality and a Serum Marker of in Vitro Fertilization Outcome. J. Assist. Reprod. Genet. 2018, 35, 1265–1276. [Google Scholar] [CrossRef]
- Di Filippo, L.; De Lorenzo, R.; Giustina, A.; Rovere-Querini, P.; Conte, C. Vitamin D in Osteosarcopenic Obesity. Nutrients 2022, 14, 1816. [Google Scholar] [CrossRef]
- Bedenk, J.; Vrtačnik-Bokal, E.; Virant-Klun, I. The Role of Anti-Müllerian Hormone (AMH) in Ovarian Disease and Infertility. J. Assist. Reprod. Genet. 2020, 37, 89–100. [Google Scholar] [CrossRef]
- Oh, S.R.; Choe, S.Y.; Cho, Y.J. Clinical Application of Serum Anti-Müllerian Hormone in Women. Clin. Exp. Reprod. Med. 2019, 46, 50. [Google Scholar] [CrossRef]
- Victoria, M.; Labrosse, J.; Krief, F.; Cédrin-Durnerin, I.; Comtet, M.; Grynberg, M. Anti Müllerian Hormone: More than a Biomarker of Female Reproductive Function. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 19–24. [Google Scholar] [CrossRef]
- Malloy, P.J.; Peng, L.; Wang, J.; Feldman, D. Interaction of the Vitamin D Receptor with a Vitamin D Response Element in the Müllerian-Inhibiting Substance (MIS) Promoter: Regulation of MIS Expression by Calcitriol in Prostate Cancer Cells. Endocrinology 2009, 150, 1580–1587. [Google Scholar] [CrossRef] [Green Version]
- Grzesiak, M. Vitamin D3 Action within the Ovary—An Updated Review. Physiol. Res. 2020, 69, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Merhi, Z.; Doswell, A.; Krebs, K.; Cipolla, M. Vitamin D Alters Genes Involved in Follicular Development and Steroidogenesis in Human Cumulus Granulosa Cells. J. Clin. Endocrinol. Metab. 2014, 99, E1137–E1145. [Google Scholar] [CrossRef] [PubMed]
- Bednarska-Czerwińska, A.; Olszak-Wąsik, K.; Olejek, A.; Czerwiński, M.; Tukiendorf, A. Vitamin D and Anti-Müllerian Hormone Levels in Infertility Treatment: The Change-Point Problem. Nutrients 2019, 11, 1053. [Google Scholar] [CrossRef]
- Wong, H.Y.Q.; Li, H.W.R.; Lam, K.S.L.; Tam, S.; Shek, C.C.; Lee, C.Y.V.; Yeung, W.S.B.; Ho, P.C.; Ng, E.H.Y. Independent Association of Serum Vitamin D with Anti-Mullerian Hormone Levels in Women with Polycystic Ovary Syndrome. Clin. Endocrinol. 2018, 89, 634–641. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Q.; Zhu, L.; Ma, L.; Ye, X.; Li, C.; Lan, Y.; Huang, Y.; Liu, J.; Zhou, J. Correlation of Serum Vitamin d Levels with Ovarian Reserve Markers in Patients with Primary Ovarian Insufficiency. Int. J. Clin. Exp. Med. 2019, 12, 4147–4153. [Google Scholar]
- Parikh, G.; Varadinova, M.; Suwandhi, P.; Araki, T.; Rosenwaks, Z.; Poretsky, L.; Seto-Young, D. Vitamin D Regulates Steroidogenesis and Insulin-like Growth Factor Binding Protein-1 (IGFBP-1) Production in Human Ovarian Cells. Horm. Metab. Res. 2010, 42, 754–757. [Google Scholar] [CrossRef]
- Monastra, G.; De Grazia, S.; De Luca, L.; Vittorio, S.; Unfer, V. Vitamin D: A Steroid Hormone with Progesterone-like Activity. Eur. Rev. Med. Pharm. Sci. 2018, 22, 2502–2512. [Google Scholar]
- Makieva, S.; Reschini, M.; Ferrari, S.; Bonesi, F.; Polledri, E.; Fustinoni, S.; Restelli, L.; Sarais, V.; Somigliana, E.; Viganò, P. Oral Vitamin D Supplementation Impacts Gene Expression in Granulosa Cells in Women Undergoing IVF. Hum. Reprod. 2021, 36, 130–144. [Google Scholar] [CrossRef]
- Muyayalo, K.P.; Song, S.; Zhai, H.; Liu, H.; Huang, D.-H.; Zhou, H.; Chen, Y.-J.; Liao, A.-H. Low Vitamin D Levels in Follicular Fluid, but Not in Serum, Are Associated with Adverse Outcomes in Assisted Reproduction. Arch. Gynecol. Obs. 2022, 305, 505–517. [Google Scholar] [CrossRef]
- Safaei, Z.; Bakhshalizadeh, S.; Esfahani, M.H.N.; Sene, A.A.; Najafzadeh, V.; Soleimani, M.; Shirazi, R. Effect of Vitamin D3 on Mitochondrial Biogenesis in Granulosa Cells Derived from Polycystic Ovary Syndrome. Int. J. Fertil. Steril. 2020, 14, 143. [Google Scholar]
- van der Reest, J.; Cecchino, G.N.; Haigis, M.C.; Kordowitzki, P. Mitochondria: Their Relevance during Oocyte Ageing. Ageing Res. Rev. 2021, 70, 101378. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Kim, S.W.; Kim, H.; Ku, S.-Y. The Level of Vitamin D in Follicular Fluid and Ovarian Reserve in an in Vitro Fertilization Program: A Pilot Study. Sci. Prog. 2022, 105, 00368504221103782. [Google Scholar] [CrossRef]
- Neysanian, G.; Taebi, M.; Rezaeian, A.; Nasr-Esfahani, M.H.; Jahangirifar, M. The Effects of Serum and Follicular Fluid Vitamin D Levels on Assisted Reproductive Techniques: A Prospective Cohort Study. Int. J. Fertil. Steril. 2021, 15, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.; Christakos, S. New Aspects of Vitamin D Metabolism and Action—Addressing the Skin as Source and Target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D Receptor (VDR)-Mediated Actions of 1α, 25 (OH) 2vitamin D3: Genomic and Non-Genomic Mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Karlic, H.; Varga, F. Impact of Vitamin D Metabolism on Clinical Epigenetics. Clin. Epigenet. 2011, 2, 55–61. [Google Scholar] [CrossRef]
- Carlberg, C.; Molnár, F. Transcription Factors. In Mechanisms of Gene Regulation; Springer: Berlin/Heidelberg, Germany, 2016; pp. 57–73. [Google Scholar]
- Vaquerizas, J.M.; Kummerfeld, S.K.; Teichmann, S.A.; Luscombe, N.M. A Census of Human Transcription Factors: Function, Expression and Evolution. Nat. Rev. Genet. 2009, 10, 252–263. [Google Scholar] [CrossRef]
- Català-Moll, F.; Ferreté-Bonastre, A.G.; Godoy-Tena, G.; Morante-Palacios, O.; Ciudad, L.; Barberà, L.; Fondelli, F.; Martínez-Cáceres, E.M.; Rodríguez-Ubreva, J.; Li, T.; et al. Vitamin D Receptor, STAT3, and TET2 Cooperate to Establish Tolerogenesis. Cell Rep. 2022, 38, 110244. [Google Scholar] [CrossRef]
- Doig, C.L.; Singh, P.K.; Dhiman, V.K.; Thorne, J.L.; Battaglia, S.; Sobolewski, M.; Maguire, O.; O’Neill, L.P.; Turner, B.M.; McCabe, C.J. Recruitment of NCOR1 to VDR Target Genes Is Enhanced in Prostate Cancer Cells and Associates with Altered DNA Methylation Patterns. Carcinogenesis 2013, 34, 248–256. [Google Scholar] [CrossRef]
- Vetter, V.M.; Sommerer, Y.; Kalies, C.H.; Spira, D.; Bertram, L.; Demuth, I. Vitamin D Supplementation Is Associated with Slower Epigenetic Aging. GeroScience 2022, 44, 1847–1859. [Google Scholar] [CrossRef]
- Herdick, M.; Carlberg, C. Agonist-Triggered Modulation of the Activated and Silent State of the Vitamin D(3) Receptor by Interaction with Co-Repressors and Co-Activators. J. Mol. Biol. 2000, 304, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Lutz, W.H.; Kumar, R.; Jameson, J.L. The Interaction of the Vitamin D Receptor with Nuclear Receptor Corepressors and Coactivators. Biochem. Biophys. Res. Commun. 1998, 253, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, H.; Fujiki, R.; Yoshimura, K.; Mezaki, Y.; Uematsu, Y.; Matsui, D.; Ogawa, S.; Unno, K.; Okubo, M.; Tokita, A.; et al. RETRACTED: The Chromatin-Remodeling Complex WINAC Targets a Nuclear Receptor to Promoters and Is Impaired in Williams Syndrome. Cell 2003, 113, 905–917. [Google Scholar] [CrossRef] [Green Version]
- Baudino, T.A.; Kraichely, D.M.; Jefcoat, S.C.; Winchester, S.K.; Partridge, N.C.; MacDonald, P.N. Isolation and Characterization of a Novel Coactivator Protein, NCoA-62, Involved in Vitamin D-Mediated Transcription. J. Biol. Chem. 1998, 273, 16434–16441. [Google Scholar] [CrossRef]
- Kinuta, K.; Tanaka, H.; Moriwake, T.; Aya, K.; Kato, S.; Seino, Y. Vitamin D Is an Important Factor in Estrogen Biosynthesis of Both Female and Male Gonads. Endocrinology 2000, 141, 1317–1324. [Google Scholar] [CrossRef]
- Panda, D.K.; Miao, D.; Tremblay, M.L.; Sirois, J.; Farookhi, R.; Hendy, G.N.; Goltzman, D. Targeted Ablation of the 25-Hydroxyvitamin D 1α-Hydroxylase Enzyme: Evidence for Skeletal, Reproductive, and Immune Dysfunction. Proc. Natl. Acad. Sci. USA 2001, 98, 7498–7503. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hollis, B.W. The extraordinary metabolism of vitamin D. Elife 2022, 11, e77539. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzeczka, A.; Graczyk, S.; Skowronska, A.; Skowronski, M.T.; Kordowitzki, P. Relevance of Vitamin D and Its Deficiency for the Ovarian Follicle and the Oocyte: An Update. Nutrients 2022, 14, 3712. https://doi.org/10.3390/nu14183712
Grzeczka A, Graczyk S, Skowronska A, Skowronski MT, Kordowitzki P. Relevance of Vitamin D and Its Deficiency for the Ovarian Follicle and the Oocyte: An Update. Nutrients. 2022; 14(18):3712. https://doi.org/10.3390/nu14183712
Chicago/Turabian StyleGrzeczka, Arkadiusz, Szymon Graczyk, Agnieszka Skowronska, Mariusz T. Skowronski, and Paweł Kordowitzki. 2022. "Relevance of Vitamin D and Its Deficiency for the Ovarian Follicle and the Oocyte: An Update" Nutrients 14, no. 18: 3712. https://doi.org/10.3390/nu14183712





