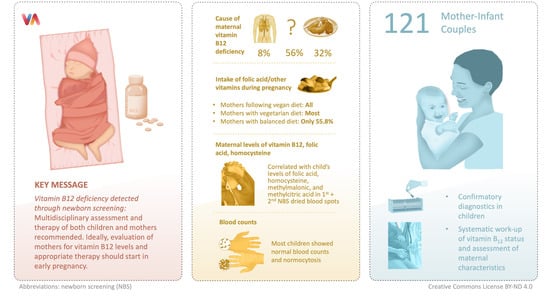
Maternal Vitamin B12 Deficiency Detected by Newborn Screening—Evaluation of Causes and Characteristics
Abstract
Share and Cite
Reischl-Hajiabadi, A.T.; Garbade, S.F.; Feyh, P.; Weiss, K.H.; Mütze, U.; Kölker, S.; Hoffmann, G.F.; Gramer, G. Maternal Vitamin B12 Deficiency Detected by Newborn Screening—Evaluation of Causes and Characteristics. Nutrients 2022, 14, 3767. https://doi.org/10.3390/nu14183767
Reischl-Hajiabadi AT, Garbade SF, Feyh P, Weiss KH, Mütze U, Kölker S, Hoffmann GF, Gramer G. Maternal Vitamin B12 Deficiency Detected by Newborn Screening—Evaluation of Causes and Characteristics. Nutrients. 2022; 14(18):3767. https://doi.org/10.3390/nu14183767
Chicago/Turabian StyleReischl-Hajiabadi, Anna T., Sven F. Garbade, Patrik Feyh, Karl Heinz Weiss, Ulrike Mütze, Stefan Kölker, Georg F. Hoffmann, and Gwendolyn Gramer. 2022. "Maternal Vitamin B12 Deficiency Detected by Newborn Screening—Evaluation of Causes and Characteristics" Nutrients 14, no. 18: 3767. https://doi.org/10.3390/nu14183767
APA StyleReischl-Hajiabadi, A. T., Garbade, S. F., Feyh, P., Weiss, K. H., Mütze, U., Kölker, S., Hoffmann, G. F., & Gramer, G. (2022). Maternal Vitamin B12 Deficiency Detected by Newborn Screening—Evaluation of Causes and Characteristics. Nutrients, 14(18), 3767. https://doi.org/10.3390/nu14183767