The Use of Post-Natal Skeleton Development as Sensitive Preclinical Model to Test the Quality of Alternative Protein Sources in the Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diet Preparation and Composition
2.3. Histological Staining of Growth-Plate (GP) Sections
2.4. Imaging and Measurement of GPs
2.5. Bone Microarchitecture
2.6. Three-Point Bending for Bone Mechanical Analysis
2.7. Serum Biochemistry, Diets’ Macronutrients, and Amino Acids Analyses
2.8. Heat Mapping
2.9. Statistical Analysis
3. Results
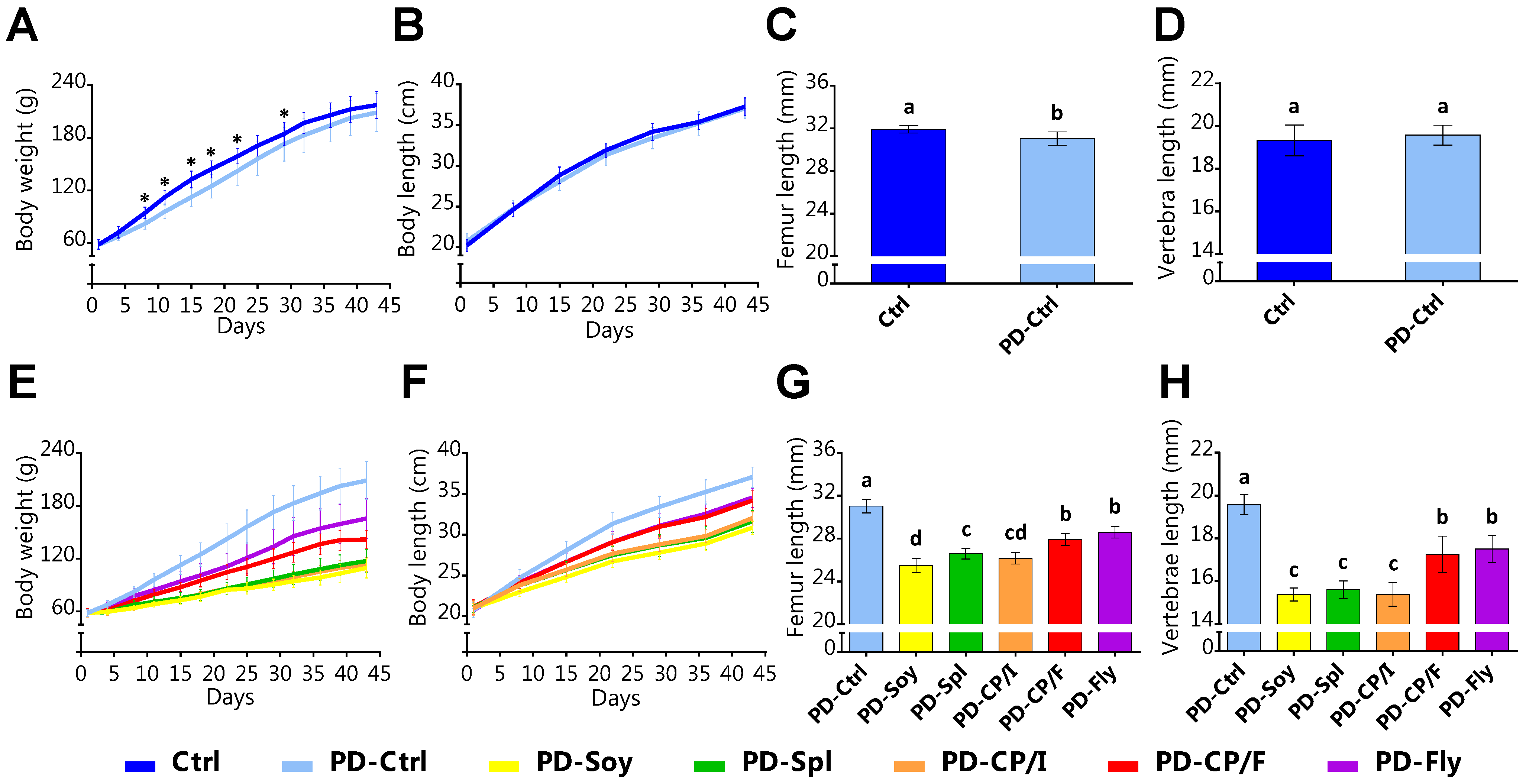
3.1. The Effect of the Alternative Protein Sources on Growth Patterns
3.2. The Effect of the Alternative Protein Sources on Food Intake and Its Utilization for Growth
3.3. The Effect of the Alternative Protein Sources on Serum Biochemistry Parameters
3.4. The Effect of the Alternative Protein Sources on Bone Quality
3.5. The Amino Acid Analysis of the Tested Diets
3.6. Data Integration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition; WHO: Geneva, Switzerland, 2002.
- Cao, J.J. High dietary protein intake and protein-related acid load on bone health. Curr. Osteoporos. Rep. 2017, 15, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Tsagari, A. Dietary protein intake and bone health. J. Frailty Sarcopenia Falls 2020, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Abrams, S.; Dawson-Hughes, B.; Looker, A.; Marcus, R.; Matkovic, V.; Weaver, C. Peak bone mass. Osteoporos. Int. 2000, 11, 985–1009. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Clark, M. Global diets link environmental sustainability and human health. Nature 2014, 515, 518–522. [Google Scholar] [CrossRef] [PubMed]
- van Zanten, H.H.E.; Mollenhorst, H.; Klootwijk, C.W.; van Middelaar, C.E.; de Boer, I.J.M. Global food supply: Land use efficiency of livestock systems. Int. J. Life Cycle Assess. 2016, 21, 747–758. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Oomah, B.D. Minor components of pulses and their potential impact on human health. Food Res. Int. 2010, 43, 461–482. [Google Scholar] [CrossRef]
- Maphosa, Y.; Jideani, V.A. The role of legumes in human nutrition. In Functional Food Maria Chavarri; IntechOpen: London, UK, 2017; Volume 13, Available online: https://www.intechopen.com/books/functional-food-improve-health-through-adequate-food/the-role-of-legumes-in-human-nutrition (accessed on 7 September 2022). [CrossRef]
- Hughes, G.J.; Ryan, D.J.; Mukherjea, R.; Schasteen, C.S. Protein digestibility-corrected amino acid scores (PDCAAS) for soy protein isolates and concentrate: Criteria for evaluation. J. Agric. Food Chem. 2011, 59, 12707–12712. [Google Scholar] [CrossRef]
- Torre-Villalvazo, I.; Tovar, A.R.; Ramos-Barragán, V.E.; Cerbón-Cervantes, M.A.; Torres, N. Soy protein ameliorates metabolic abnormalities in liver and adipose tissue of rats fed a high fat diet. J. Nutr. 2008, 138, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Lavigne, C.; Marette, A.; Jacques, H. Cod and soy proteins compared with casein improve glucose tolerance and insulin sensitivity in rats. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E491–E500. [Google Scholar] [CrossRef]
- Wang, X.; Gao, W.; Zhang, J.; Zhang, H.; Li, J.; He, X.; Ma, H. Subunit, amino acid composition and in vitro digestibility of protein isolates from Chinese kabuli and desi chickpea (Cicer arietinum L.) cultivars. Food Res. Int. 2010, 43, 567–572. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef] [PubMed]
- Bawa, S. The significance of soy protein and soy bioactive compounds in the prophylaxis and treatment of osteoporosis. J. Osteoporos. 2010, 2010, 1–8. [Google Scholar] [CrossRef]
- Haidong, L.; Yu, F.; Yuan, B.; Zhao, Z.N.; Tong, C.G.; Liu, X.H.; Wu, S. Effect of dietary soy isoflavones on bone loss in ovariectomized rats. Trop. J. Pharm. Res. 2018, 17, 91–96. [Google Scholar] [CrossRef]
- Ma, H.R.; Wang, J.; Qi, H.; Gao, Y.; Pang, L.; Yang, Y.; Wang, Z.; Duan, M.; Chen, H.; Cao, X.; et al. Assessment of the estrogenic activities of chickpea (Cicer arietinum L) sprout isoflavone extract in ovariectomized rats. Acta Pharmacol. Sin. 2013, 34, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.R.; Soliman, A.M.; Sayed, A.A.; Marzouk, M. Possible antiosteoporotic mechanism of cicer arietinum extract in ovariectomized rats. Int. J. Clin. Exp. Pathol. 2015, 8, 3477–3490. [Google Scholar] [PubMed]
- Zeng, J.; Feng, Y.; Feng, J.; Chen, X. The effect of soy intervention on insulin-like growth factor 1 levels: A meta-analysis of clinical trials. Phyther. Res. 2020, 34, 1570–1577. [Google Scholar] [CrossRef]
- Sayed, A.A.; Elfiky, A.A. In silico estrogen-like activity and in vivo osteoclastogenesis inhibitory effect of Cicer arietinum extract. Cell. Mol. Biol. 2018, 64, 29–39. [Google Scholar] [CrossRef]
- van der Spiegel, M.; Noordam, M.Y.; van der Fels-Klerx, H.J. Safety of novel protein sources (insects, microalgae, seaweed, duckweed, and rapeseed) and legislative aspects for their application in food and feed production. Compr. Rev. Food Sci. Food Saf. 2013, 12, 662–678. [Google Scholar] [CrossRef]
- Tarantino, L.M. Agency Response Letter GRAS Notice No. GRN000127 FDA Home Page 2003. Available online: https://www.algbiotek.com/spirulina-sertifikalar/spirulina-gras-2012.pdf (accessed on 7 September 2022).
- Kay, R.A. Microalgae as food and supplement. Crit. Rev. Food Sci. Nutr. 1991, 30, 555–573. [Google Scholar] [CrossRef]
- Ciferri, O. Spirulina, the edible microorganism. Microbiol. Rev. 1983, 47, 551–578. [Google Scholar] [CrossRef] [PubMed]
- Romay, C.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-Phycocyanin: A biliprotein with antioxidant, anti-Inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2005, 4, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Vonshak, A. Spirulina Platensis (Arthrospira): Physiology, Cell-Biology, and Biotechnology; Ben-Gurion University of the Negev: Be’er Sheva, Israel, 1996. [Google Scholar]
- Sides, R.; Griess-Fishheimer, S.; Zaretsky, J.; Shitrit, A.; Kalev-Altman, R.; Rozner, R.; Beresh, O.; Dumont, M.; Penn, S.; Shahar, R.; et al. The Use of Mushrooms and Spirulina Algae as Supplements to Prevent Growth Inhibition in a Pre-Clinical Model for an Unbalanced Diet. Nutrients 2021, 13, 4316. [Google Scholar] [CrossRef] [PubMed]
- Food and Argriculture Organization of the United Nations. Edible insects. In Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; Volume 171. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition Ad of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Griess-Fishheimer, S.; Zaretsky, J.; Travinsky-Shmul, T.; Zaretsky, I.; Penn, S.; Shahar, R.; Monsonego-Ornan, E. Nutritional Approaches as a Treatment for Impaired Bone Growth and Quality Following the Consumption of Ultra-Processed Food. Int. J. Mol. Sci. 2022, 23, 841. [Google Scholar] [CrossRef]
- Travinsky-Shmul, T.; Beresh, O.; Zaretsky, J.; Griess-Fishheimer, S.; Rozner, R.; Kalev-Altman, R.; Penn, S.; Shahar, R.; Monsonego-Ornan, E. Ultra-Processed Food Impairs Bone Quality, Increases Marrow Adiposity and Alters Gut Microbiome in Mice. Foods 2021, 10, 3107. [Google Scholar] [CrossRef]
- Simsa-Maziel, S.; Zaretsky, J.; Reich, A.; Koren, Y.; Shahar, R.; Monso-nego-Ornan, E. IL-1RI participates in normal growth plate development andbone modeling. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E15–E21. [Google Scholar] [CrossRef]
- Idelevich, A.; Kerschnitzki, M.; Shahar, R.; Monsonego-Ornan, E. 1,25(OH)2D3 Alters Growth Plate Maturation and Bone Architecture in Young Rats with Normal Renal Function. PLoS ONE 2011, 6, e20772. [Google Scholar] [CrossRef]
- Zaretsky, J.; Griess-Fishheimer, S.; Carmi, A.; Travinsky-Shmul, T.; Ofer, L.; Sinai, T.; Penn, S.; Shahar, R. Ultra-processed food targets bone quality via endochondral ossification. Bone Res. 2021, 9, 1–13. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.L.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Rozner, R.; Vernikov, J.; Griess-Fishhimer, S.; Travinsky, T.; Penn, S.; Schwarts, B.; Mesilati-Stahy, R.; Argov-Argaman, N.; Shahar, R.; Monsonego-Ornan, E. The role of omega-3 polyunsaturated fatty acids from different sources in bone development. Nutrients 2020, 12, 3494. [Google Scholar] [CrossRef] [PubMed]
- Solomon, G.; Atkins, A.; Shahar, R.; Gertler, A.; Monsonego-Ornan, E. Effect of peripherally administered leptin antagonist on whole bodymetabolism and bone microarchitecture and biomechanical properties inthe mouse. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E14–E27. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.D. The impact of age on the female reproductive system: A pathologist’s perspective. Toxicol. Pathol. 2017, 45, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, H.M. Developmental regulation of the growth plat. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.H.; van der Meulen, M.C.H. Whole bone mechanics and bone quality. Clin. Orthop. Relat. Res. 2011, 469, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Madani, S.; Prost, J.; Belleville, J. Dietary protein level and origin (casein and highly purified soybean protein) affect hepatic storage, plasma lipid transport, and antioxidative defense status in the rat. Nutrition 2000, 16, 368–375. [Google Scholar] [CrossRef]
- Zheng, X.; Lee, S.K.; Chun, O.K. Soy isoflavones and osteoporotic bone loss: A review with an emphasis on modulation of bone remodeling. J. Med. Food 2016, 19, 1–14. [Google Scholar] [CrossRef]
- Ahn, H.; Park, Y.K. Soy isoflavone supplementation improves longitudinal bone growth and bone quality in growing female rats. Nutrition 2017, 37, 68–73. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.; Van Horn, L.; Harris, W.; Kris-Etherton, P.; Winston, M. Soy protein, isoflavones, and cardiovascular health: An American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation 2006, 113, 1034–1044. [Google Scholar] [CrossRef] [Green Version]
- Sukalingam, K.; Ganesan, K.; Das, S.; Thent, Z.C. Review an insight into the harmful effects of soy protein: A review. Clin. Ther. 2015, 166, 131–139. [Google Scholar] [CrossRef]
- Liener, I.E. Implications of antinutritional components in soybean foods. Crit. Rev. Food Sci. Nutr. 1994, 34, 31–67. [Google Scholar] [CrossRef] [PubMed]
- Muys, M.; Sui, Y.; Schwaiger, B.; Lesueur, C.; Vandenheuvel, D.; Vermeir, P.; Vlaeminck, S.E. High variability in nutritional value and safety of commercially available chlorella and spirulina biomass indicates the need for smart production strategies. Bioresour. Technol. 2019, 275, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Fournier, C.; Rizzoli, R.; Bouzakri, K.; Ammann, P. Selective protein depletion impairs bone growth and causes liver fatty infiltration in female rats: Prevention by Spirulina alga. Osteoporos. Int. 2016, 27, 3365–3376. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.A.; Baek, S.Y.; Cheong, S.H.; Kim, M.R. Spirulina enhances bone modeling in growing male rats by regulating growth-related hormones. Nutrients 2020, 12, 1187. [Google Scholar] [CrossRef]
- Aouir, A.; Amiali, M.; Bitam, A.; Benchabane, A. Comparison of the biochemical composition of different Arthrospira platensis strains from Algeria, Chad and the USA. J. Food Meas. Charact. 2017, 11, 913–923. [Google Scholar] [CrossRef]
- Ogbonda, K.H.; Aminigo, R.E.; Abu, G.O. Influence of temperature and pH on biomass production and protein biosynthesis in a putative Spirulina sp. Bioresour. Technol. 2007, 98, 2207–2211. [Google Scholar] [CrossRef]
- Rubio, L.A.; Grant, G.; Dewey, P.; Brown, D.; Annand, M.; Bardocz, S.; Pusztai, A. Nutritional utilization by rats of chickpea (Cicer arietinum) meal and its isolated globulin proteins is poorer than that of defatted soybean or lactalbumin. J. Nutr. 1998, 128, 1042–1047. [Google Scholar] [CrossRef]
- Tavano, O.L.; Da Silva, S.I.; Démonte, A.; Neves, V.A. Nutritional responses of rats to diets based on chickpea (Cicer arietinum L.) seed meal or its protein fractions. J. Agric. Food Chem. 2008, 56, 11006–11010. [Google Scholar] [CrossRef]
- Gessner, D.K.; Schwarz, A.; Meyer, S.; Wen, G.; Most, E.; Zorn, H.; Ringseis, R.; Eder, K. Insect meal as alternative protein source exerts pronounced lipid-lowering effects in hyperlipidemic obese Zucker rats. J. Nutr. 2019, 149, 566–577. [Google Scholar] [CrossRef]
- Poelaert, C.; Francis, F.; Alabi, T.; Caparros Megido, R.; Crahay, B.; Bindelle, J.; Beckers, Y. Protein value of two insects, subjected to various heat treatments, using growing rats and the protein digestibility-corrected amino acid score. J. Insects Food Feed 2018, 4, 77–87. [Google Scholar] [CrossRef]
- Finke, M.D. Estimate of chitin in raw whole insects. Zoo Biol. 2007, 26, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Nosworthy, M.G.; House, J.D. Factors influencing the quality of dietary proteins: Implications for pulses. Can. Cent. Agri. Food Res. Health Med. 2017, 94, 49–57. [Google Scholar] [CrossRef]
- WRAP. Food Futures: From Business as Usual to Business Unusual. Available online: http://www.wrap.org.uk/content/food-futures (accessed on 7 September 2022).




| Ctrl | PD-Ctrl | PD-Soy | PD-Spl | PD-CP/I | PD-CP/F | PD-Fly | |
|---|---|---|---|---|---|---|---|
| Diet Composition | g/kg | g/950 g | g/956 g | g/923 g | g/949 g | g/978 g | g/962 g |
| Cornstarch | 397 | 406 | 406 | 375 | 401 | 235 | 397 |
| Dextrinized cornstarch | 132 | 135 | 135 | 125 | 133 | 78 | 132 |
| Sucrose | 100 | 102 | 102 | 94 | 101 | 59 | 100 |
| Soybean oil | 70 | 110 | 111 | 107 | 111 | 84 | 97 |
| Cellulose fibers | 50 | 50 | 50 | 19 | 50 | 0 | 50 |
| Casein (≥85% protein) | 200 | 98 | |||||
| L-Cystine | 3 | 1 | |||||
| Soy protein isolate | 103 | ||||||
| Spirulina powder | 156 | ||||||
| Chickpea protein isolate | 104 | ||||||
| Chickpea flour | 475 | ||||||
| Fly larvae protein powder | 139 | ||||||
| Mineral mix (AIN-93G-MX) | 35 | 35 | 35 | 35 | 35 | 35 | 35 |
| Vitamin mix (AIN-93G-MX) | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Tert-Butylhydroquinone | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 |
| Nutritional level | |||||||
| Energy, kcal/g | 3.10 | 3.48 | 3.42 | 3.62 | 3.60 | 3.46 | 3.40 |
| Protein, % | 21 | 10 | 10 | 10 | 10 | 10 | 10 |
| Carbohydrate, % | 61 | 62 | 62 | 62 | 62 | 62 | 62 |
| Fat, % | 18 | 28 | 28 | 28 | 28 | 28 | 28 |
| Fibers, g/kg | 50 | 52.6 | 52.4 | 54.1 | 52.7 | 55.2 | 51.9 |
| 100 gr Protein Source | Caseinate | Soy Isolate | Spirulina Powder | Chickpea Isolate | Chickpea Flour | Fly Larvae Powder |
|---|---|---|---|---|---|---|
| Energy (kcal) | 375 | 365 | 380 | 364 | 356 | 319 |
| Protein (g) | 91 | 90 | 60 | 90 | 20 | 67 |
| Carbohydrate (g) | 0.1 | 0 | 28 | 7 | 50 | 10 |
| Fat (g) | 0.8 | 0.5 | 3 | 0 | 6 | 10 |
| Fibers (g) | 0 | 0 | 20 | 0 | 11.5 | 0 |
| Blood Parameter | Normal Range | Ctrl | PD-Ctrl | PD-Soy | PD-Spl | PD-CP/I | PD-CP/F | PD-Fly | |
|---|---|---|---|---|---|---|---|---|---|
| Liver function | ALT (U/L) | 30–82 | 23 ± 5 b | 23 ± 4 b | 44 ± 11 a | 49 ± 8 a | 43 ± 6 a | 46 ± 9 a | 47 ± 12 a |
| AST (U/L) | 70–178 | 101 ± 32 ab | 96 ± 14 b | 117 ± 24 ab | 129 ± 14 a | 102 ± 13 ab | 106 ± 10 ab | 117 ± 25 ab | |
| Bilirubin (mg/dL) | 0.04–0.21 | 0.01 ± 0.01 c | 0.03 ± 0.00 bc | 0.03 ± 0.02 bc | 0.05 ± 0.03 ab | 0.06 ± 0.02 a | 0.04 ± 0.03 ab | 0.01 ± 0.02 bc | |
| Renal function | Urea (mg/dL) | 28.8–61.3 | 36.3 ± 3.0 b | 37.5 ± 8.0 b | 50.8 ± 6.2 a | 30.3 ± 6.8 b | 53.0 ± 10.8 a | 30.8 ± 1.7 b | 37.4 ± 6.7 b |
| Creatinine (mg/dL) | 0.26–0.65 | 0.37 ± 0.07 ab | 0.46 ± 0.05 a | 0.37 ± 0.05 ab | 0.46 ± 0.09 a | 0.36 ± 0.06 ab | 0.35 ± 0.05 b | 0.39 ± 0.06 ab | |
| Nutrtional status | Albumin (g/dL) | 3.5–5.1 | 5.1 ± 0.4 ab | 5.5 ± 0.5 a | 4.3 ± 0.6 c | 4.9 ± 0.5 abc | 4.3 ± 0.3 c | 4.6 ± 0.6 bc | 4.6 ± 0.4 bc |
| Total protein (g/dL) | 6–7.3 | 6.1 ± 0.8 ab | 6.5 ± 0.8 a | 5.2 ± 0.7 b | 5.6 ± 0.7 ab | 5.2 ± 0.5 b | 5.3 ± 0.6 b | 5.3 ± 0.6 b | |
| MetabolicParameters | Glucose (mg/dL) | 113–185 | 193 ± 32 a | 217 ± 30 a | 180 ± 27 a | 202 ± 28 a | 196 ± 29 a | 206 ± 19 a | 209 ± 34 a |
| Cholesterol (mg/dL) | 71–148 | 77 ± 12 c | 83 ± 11 bc | 105 ± 19 abc | 127 ± 22 a | 106 ± 15 ab | 115 ± 23 a | 101 ± 17 abc | |
| Triglycerides (mg/dL) | 16–77 | 46 ± 16 bc | 41 ± 14 c | 74 ± 21 ab | 77 ± 25 a | 53 ± 20 abc | 48 ± 7 bc | 43 ± 11 c | |
| Bone parameters | Calcium (mg/dL) | 10.1–12 | 10.9 ± 1.4 a | 11.6 ± 1.4 a | 11.0 ± 1.2 a | 12.3 ± 1.2 a | 11.0 ± 1.1 a | 11.0 ± 1.5 a | 10.4 ± 0.9 a |
| Phosphorus (mg/dL) | 7.1–11.6 | 7.4 ± 0.9 ab | 7.6 ± 0.5 ab | 6.3 ± 1.2 bc | 5.0 ± 0.7 c | 8.0 ± 1.1 ab | 8.3 ± 1.4 a | 8.0 ± 0.8 ab | |
| Ca/P ratio | - | 1.5 ± 0.2 bc | 1.4 ± 0.1 bc | 1.7 ± 0.1 b | 2.4 ± 0.3 a | 1.4 ± 0.2 bc | 1.3 ± 0.1 c | 1.3 ± 0.1 c | |
| ALP (U/L) | 161.2–258 | 201.6 ± 34.6 d | 302.7 ± 56.7 bc | 371.8 ± 52.3 ab | 448.2 ± 97.5 a | 339.5 ± 33.3 b | 247.2 ± 41.0 cd | 220.8 ± 57.5 cd |
| Ctrl | PD-Ctrl | PD-Soy | PD-Spl | PD-CP/I | PD-CP/F | PD-Fly | |
|---|---|---|---|---|---|---|---|
| Trabecular analysis of the femur | |||||||
| BV/TV (%) | 33.98 ± 2.21 bc | 36.52 ± 2.24 b | 30.48 ± 3.08 c | 33.50 ± 3.95 bc | 34.78 ± 4.93 bc | 42.58 ± 3.24 a | 38.11 ± 4.27 ab |
| Tb.N (1/mm) | 3.10 ± 0.15 bc | 3.28 ± 0.17 abc | 2.87 ± 0.25 c | 3.21 ± 0.37 bc | 3.16 ± 0.41 bc | 3.72 ± 0.30 a | 3.48 ± 0.29 ab |
| Tb.Sp (mm) | 0.24 ± 0.01 ab | 0.22 ± 0.01 bc | 0.28 ± 0.03 a | 0.23 ± 0.03 bc | 0.23 ± 0.03 bc | 0.19 ± 0.03 c | 0.21 ± 0.02 bc |
| Tb.Th (mm) | 0.109 ± 0.004 abc | 0.111 ± 0.002 ab | 0.105 ± 0.003 bc | 0.104 ± 0.003 c | 0.109 ± 0.003 abc | 0.114 ± 0.005 a | 0.109 ± 0.005 abc |
| Trabecular analysis of the vertebra | |||||||
| BV/TV (%) | 29.05 ± 1.86 ab | 31.17 ± 1.44 ab | 28.85 ± 1.63 b | 32.19 ± 2.18 a | 28.28 ± 2.30 b | 28.44 ± 1.99 b | 28.16 ± 2.95 b |
| Tb.N (1/mm) | 2.44 ± 0.12 b | 2.48 ± 0.08 b | 2.51 ± 0.12 b | 2.79 ± 0.15 a | 2.45 ± 0.18 b | 2.44 ± 0.16 b | 2.39 ± 0.16 b |
| Tb.Sp (mm) | 0.306 ± 0.015 a | 0.311 ± 0.017 a | 0.301 ± 0.015 ab | 0.276 ± 0.017 b | 0.314 ± 0.024 a | 0.302 ± 0.019 ab | 0.310 ± 0.022 a |
| Tb.Th (mm) | 0.118 ± 0.003 b | 0.125 ± 0.003 a | 0.114 ± 0.003 b | 0.115 ± 0.002 b | 0.115 ± 0.002 b | 0.116 ± 0.001 b | 0.117 ± 0.005 b |
| Cortical analysis of the femur | |||||||
| Tt.Ar (mm2) | 9.78 ± 0.68 a | 9.91 ± 0.91 a | 7.01 ± 0.41 cd | 6.82 ± 0.41 d | 6.86 ± 0.40 d | 7.83 ± 0.32 bc | 8.61 ± 0.49 b |
| Ct.Ar (mm2) | 4.93 ± 0.25 a | 5.11 ± 0.30 a | 3.44 ± 0.25 c | 3.21 ± 0.15 c | 3.41 ± 0.16 c | 3.94 ± 0.14 b | 4.26 ± 0.20 b |
| Ct.Ar/Tt.Ar(%) | 50.60 ± 2.52 a | 51.71 ± 2.90 a | 49.01 ± 1.12 ab | 47.16 ± 1.70 b | 49.74 ± 1.60 ab | 50.31 ± 1.70 ab | 49.52 ± 2.26 ab |
| Ct.Th (mm) | 0.47 ± 0.02 a | 0.49 ± 0.02 a | 0.39 ± 0.01 c | 0.36 ± 0.01 c | 0.39 ± 0.01 c | 0.42 ± 0.01 b | 0.43 ± 0.01 b |
| Ma.Ar (mm2) | 4.84 ± 0.53 a | 4.80 ± 0.68 a | 3.57 ± 0.17 c | 3.60 ± 0.30 c | 3.45 ± 0.28 c | 3.89 ± 0.25 bc | 4.35 ± 0.40 ab |
| BMD (g/cm3) | 1.34 ± 0.03 abc | 1.36 ± 0.02 ab | 1.32 ± 0.02 bcd | 1.27 ± 0.04 d | 1.37 ± 0.03 a | 1.31 ± 0.029 bcd | 1.308 ± 0.018 cd |
| Mechanical properties of the left femora | |||||||
| Slope (N/mm) | 278.92 ± 44.5 a | 296.62 ± 40.4 a | 211.07 ± 71.4 b | 153.88 ± 17.4 c | 199.76 ± 25.8 bc | 215.19 ± 35.4 b | 222.18 ± 26.2 b |
| Young’s modulus (N/mm2) | 1161.79 ± 203.4 c | 1194.03 ± 129.6 c | 1727.27 ± 113.4 a | 1368.23 ± 225.2 c | 1693.95 ± 232.8 ab | 1430.46 ± 141.4 bc | 1283.57 ± 224.9 c |
| Yield load (N) | 37.19 ± 4.7 bc | 45.70 ± 3.0 a | 42.03 ± 3.6 ab | 36.04 ± 2.0 c | 43.92 ± 4.3 a | 45.01 ± 3.8 a | 44.41 ± 4.3 a |
| Max load (N) | 73.69 ± 6.4 b | 92.86 ± 8.7 a | 63.43 ± 4.8 c | 53.86 ± 3.0 d | 62.17 ± 3.4 c | 69.25 ± 3.8 bc | 71.41 ± 3.5 b |
| Fracture load (N) | 49.33 ± 14.6 bc | 77.95 ± 9.0 a | 47.57 ± 15.6 bc | 41.91 ± 10.6 c | 54.47 ± 10.5 bc | 59.63 ± 5.0 b | 57.98 ± 6.0 bc |
| E to F (N×mm) | 72.87 ± 12.6 a | 57.22 ± 12.5 ab | 35.13 ± 10.2 c | 41.23 ± 20.5 bc | 28.53 ± 7.1 c | 37.69 ± 9.7 c | 47.57 ± 11.6 bc |
| PD-Ctrl Diet | PD-Soy Diet | PD-Spl Diet | PD-CP/I Diet | PD-CP/F Diet | PD-Fly Diet | |
|---|---|---|---|---|---|---|
| Histidine | 100% | 94% | 67% | 103% | 190% | 109% |
| Isoleucine | 100% | 87% | 109% | 104% | 113% | 120% |
| Leucine | 100% | 84% | 84% | 94% | 108% | 98% |
| Lysine | 100% | 81% | 54% | 89% | 123% | 92% |
| Methionine + Cysteine | 100% | 43% | 62% | 46% | 73% | 60% |
| Phenylalanine + Tyrosine | 100% | 82% | 76% | 94% | 181% | 122% |
| Threonine | 100% | 92% | 111% | 86% | 119% | 124% |
| Valine | 100% | 65% | 77% | 79% | 89% | 97% |
| Tryptophan | 100% | 96% | 128% | 75% | 67% | 148% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shitrit-Tovli, A.; Sides, R.; Kalev-Altman, R.; Meilich, D.; Becker, G.; Penn, S.; Shahar, R.; Ornan, E.M. The Use of Post-Natal Skeleton Development as Sensitive Preclinical Model to Test the Quality of Alternative Protein Sources in the Diet. Nutrients 2022, 14, 3769. https://doi.org/10.3390/nu14183769
Shitrit-Tovli A, Sides R, Kalev-Altman R, Meilich D, Becker G, Penn S, Shahar R, Ornan EM. The Use of Post-Natal Skeleton Development as Sensitive Preclinical Model to Test the Quality of Alternative Protein Sources in the Diet. Nutrients. 2022; 14(18):3769. https://doi.org/10.3390/nu14183769
Chicago/Turabian StyleShitrit-Tovli, Astar, Roni Sides, Rotem Kalev-Altman, Dana Meilich, Gal Becker, Svetlana Penn, Ron Shahar, and Efrat Monsonego Ornan. 2022. "The Use of Post-Natal Skeleton Development as Sensitive Preclinical Model to Test the Quality of Alternative Protein Sources in the Diet" Nutrients 14, no. 18: 3769. https://doi.org/10.3390/nu14183769





