Abstract
Due to the rate of occurrence of neonatal hypoxia-ischemia, its neuronal sequelae, and the lack of effective therapies, the development of new neuroprotective strategies is required. Polyphenols (including resveratrol) are molecules whose anti-apoptotic, anti-inflammatory, and anti-oxidative properties could be effective against the damage induced by neonatal hypoxia-ischemia. In this review article, very recent data concerning the neuroprotective role of polyphenols and the mechanisms at play are detailed, including a boost in brain energy metabolism. The results obtained with innovative approaches, such as maternal supplementation at nutritional doses, suggest that polyphenols could be a promising prophylactic treatment for neonatal hypoxia-ischemia.
1. Introduction
Neonatal hypoxia-ischemia (HI) is a major public health problem, both in terms of its occurrence (in 1% to 6% of births in developed countries) and its subsequent adverse effects, such as lethality, cognitive disorders, and/or motor disabilities. It usually results from a prolapse of the umbilical cord, a uterine rupture, or maternal hypotension. The disruption of a newborn’s cerebral blood flow is the primary cause of brain injury. The pathophysiology of neonatal HI is characterized by a succession of stages (Figure 1). At the cellular level, HI induces energy failure in two steps, separated by a latency phase. The primary step is an immediate reaction to the reduction in blood flow, and therefore the supply of O2 and energy substrates to the brain. This process induces a drastic drop in the production of adenosine triphosphate (ATP), which leads to a failure of the sodium/potassium ATP-dependent pump (Na+/K+ATPase) [1] followed by a depolarization of the cell. In turn, this induces the release of excitatory amino acids, mainly glutamate. Massively released glutamate binds to its receptors, causing a huge influx of Ca2+ and Na+ into the cell [2].
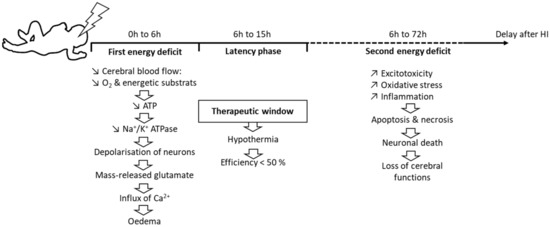
Figure 1.
Dynamic events during neonatal HI.
This Ca2+ entry is deleterious and leads to cerebral edema and microvascular damage, resulting in apoptosis or necrosis, depending on the severity of the HI episode. Necrosis occurs in very severe conditions of neonatal HI [3]. It causes cell swelling and membrane rupture, leading to cell death. When membranes break down, the cell content is released, causing further inflammation. This inflammation is responsible for an influx of microglial cells that release pro-inflammatory factors [4]. In turn, these factors can damage the white matter and lead to the formation of scar tissue.
If HI is less severe, cells will undergo apoptosis, with preservation of the membrane and without additional inflammation [2]. Reperfusion is followed by a latency period of 1 h to 6 h, during which the impairment of oxidative metabolism can be resorbed before the irreversible failure of mitochondrial function [3]. The duration of this phase depends on the severity of the HI episode: the more severe the HI, the shorter the phase [5]. This latency phase corresponds to the therapeutic window [6]. It is during this therapeutic window that moderate hypothermia is established—only for newborns at over 35 weeks of gestation—by lowering the core temperature (either at the head level only or throughout the whole body) to 33 °C to 34 °C for 72 h, then slowly and gradually warming the newborn for 6 h to 12 h.
After the latency phase, a second energy deficit occurs. It is mainly characterized by oxidative stress and inflammation. The exacerbated production of free radicals is responsible for oxidative stress, which causes damage to the membranes of neurons and leads to necrosis or apoptosis. This oxidative stress is particularly deleterious for a newborn’s brain [7], due to the low concentration of antioxidants at this age and the high consumption of O2 that is induced by the transition from fetal to neonatal life [8]. Newborns also have a high concentration of unsaturated fatty acids that are precursors of free radicals [9]. In addition, protein-bound iron is released, as Fe2+, and reacts with peroxides to form free radicals, increasing neuronal tissue damage [7].
Excitotoxicity is also characteristic of this step, due to an excess of extracellular neurotransmitters, in particular glutamate, just as during the first energy deficit. Glutamate overstimulates its receptors, inducing, for the second time, a massive influx of Ca2+ and Na+. The necrosis and apoptosis resulting from these two successive energy deficits lead to neuronal death, which is responsible for sensorimotor and cognitive deficits.
Treatment of neonatal HI is primarily symptomatic and is essentially based on maintaining fluids, electrolytes, and respiratory homeostasis. The only interventional therapy that is currently applied is moderate therapeutic hypothermia [10]. However, 44% to 53% of neonates do not respond to such therapy [11]. Confronted by this situation, any opportunity for developing new therapeutic avenues is a priority. Among the molecules with high neuroprotective potential, resveratrol has drawn particular attention, because numerous studies have demonstrated its anti-inflammatory and antioxidant properties, as well as its capacity to modulate the intracellular effectors that are involved in neuronal cell metabolism and survival/death. Resveratrol and its derivatives are promising, as the latest studies have demonstrated a neuroprotective transgenerational and nutritional application in the context of neonatal HI. Here, we review the in vivo elements that favor a nutraceutical or therapeutic development of the use of these compounds to counteract the deleterious effects of HI. We develop very recent in vivo innovative research on the use of these molecules.
2. Methods
In writing this review, the PubMed database was screened with the following terms: “neonatal hypoxia-ischemia” (5305 results) and “neuroprotection” (coupled with NHI: 1478 results). To identify articles referring specifically to the topic of our review, we used the term “resveratrol” (coupled with NHI: 15 results) and the term “polyphenols” (coupled with NHI: 19 results). Among the articles identified in the last two searches, only the studies carried out in vivo were considered (n = 17).
3. Resveratrol and Derivatives
Resveratrol (3,5,4′-trihydroxystilbene, RSV) is a natural non-flavonoid polyphenol belonging to the stilbene family. Stilbenes are involved in plant defense responses and demonstrate protective properties against plant fungal pathogens and nematodes. RSV is formed from phenylalanine through the action of a key enzyme, the stilbene synthase, which couples 4-hydroxycoumaryl-CoA and three molecules of malonyl-CoA. The resulting RSV molecule consists of two aromatic rings that are connected by a methylenic bridge (Figure 2). It exists as two isomers: trans-RSV and cis-RSV. Trans-RSV has greater stability and biological activity. The cis-RSV form occurs due to the isomerization that follows the breakdown of the RSV molecule, which is caused by UV light or under high-pH conditions [12]. The bioavailability of RSV, which is defined as the digested, absorbed, and metabolized fraction that reaches the bloodstream and targets tissues to exert biological activity, is very low (less than 1%). Indeed, if RSV is well-absorbed in the intestine (i.e., 70% or more), it is present in a very low concentration (nanomolar to micromolar) in the bloodstream, because it is rapidly metabolized by glucuronidation and sulfation on the hydroxyl groups. [13].
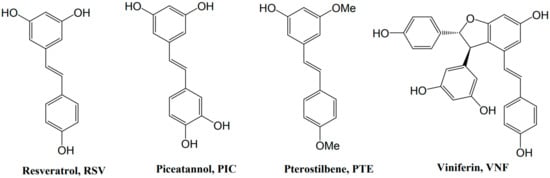
Figure 2.
Formulas of stilbenes whose neuroprotection has been shown in HI.
Although RSV is the most studied of the polyphenols, a scientific interest in some of its derivatives, such as pterostilbene (trans-3,5-dimethoxy-4′-hydroxystilbene, PTE) and ε-viniferin (VNF), is emerging, due to their potentially better bioavailability. Surprisingly, however, few previous studies focused on the effects of piceatannol (trans-3′,4′,3,5-tetrahydroxy-stilbene, PIC), whose greater bioavailability that of RSV has been proven [14]. Structurally, PTE, a dimethylether analog of RSV, differs by the presence of two methoxy groups, instead of the hydroxyl groups. These additional methoxy groups confer to PTE better lipophilicity, absorption, cellular uptake, and bioavailability, compared with RSV [15,16]. VNF is a dehydrodimer of RSV; this difference in structure could improve its properties, compared with those of RSV, as well as reduce its metabolism [17].
The neuroprotection of RSV and some of its derivatives (Figure 2) has been shown in the context of neonatal HI [18,19,20].
4. Biological Activity
4.1. Anti-Apoptotic Properties
The anti-apoptotic properties of RSV require the activation of the silent mating-type information regulation 2 homolog (SIRT1), a nicotine adenine dinucleotide (NAD+)-dependent deacetylase, which would induce an inhibition of the transcription factor nuclear factor-kappa B (NF-κB) via a negative regulation of the signaling pathway involving the extracellular signal-regulated kinase 1 and 2/mitogen-activated protein kinase (Erk1/2 MAPK) complex. Through this pathway, RSV (pre-treatment and post-treatment) causes an overexpression of the anti-apoptotic gene B-cell lymphoma 2 (Bcl2) and a decrease in the expression of the pro-apoptotic gene Bcl-2–associated X (Bax), as well a decrease in the metalloproteases [18,21,22,23,24]. In addition, the activation of SIRT1, which leads to inactivation of the transcription factor p53 by deacetylation of a lysine residue (Lys382) [25], leads to an increase in the production of anti-apoptotic molecules and a decrease in the production of pro-apoptotic molecules, such as caspase 3 [24,26]. A decrease in caspase 3 expression was also measured 12 h after neonatal HI when RSV was injected (intraperitoneally) 10 min before the insult [27]. The HI-induced rise in intracellular Ca2+ levels is responsible for an increase in calpain activities, resulting in the degradation of spectrin, microtubule, and neurofilament proteins. Their high activity disrupts intracellular axonal transport and membrane functions that are involved in the stability of dendritic and axonal processes [28]. Their involvement in excitotoxic neuronal damage and necrosis has been widely demonstrated [29,30]. Moreover, in the context of HI, calpains would activate caspase 3. However, it has been shown, in a model of middle cerebral artery occlusion in rats, that RSV neuroprotection involves the inhibition of calpain activities [31].
4.2. Antioxidant Properties
During the reperfusion period after a neonatal HI insult, the abrupt reestablishment of the O2 supply leads to an overproduction of reactive oxygen species (ROS), which are involved in the oxidative damage of macromolecules, such as nucleic acids, proteins, and lipids, which can lead to cell death [32]. This oxidative stress and subsequent inflammatory reaction due to an HI event are the major contributors to brain damage [33,34,35]. RSV exhibits antioxidant properties that involve the regulation of several signaling pathways (Figure 3). In the context of neonatal HI, it was demonstrated that maternal supplementation with RSV (0.15 mg/kg/d) induces an increase in superoxide dismutase (SOD2) and SIRT1 [18]. These results are in line with the data of Shin et al., which showed that RSV is neuroprotective in the context of stroke via a stimulation of the SIRT1-PGC-1α (peroxisome proliferator-activated receptor-gamma coactivator 1α) signaling pathway [36]. PGC-1α is a regulator of mitochondrial biogenesis and oxidative stress [37]. Its activation leads to an antioxidant effect by the induction of SOD2 and uncoupling protein 2 (UCP2).
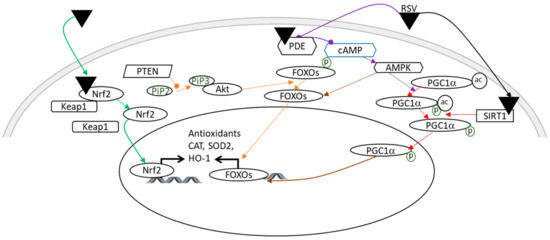
Figure 3.
Illustration of the main antioxidant effects of RSV, which may be involved in its neuroprotection in a context of HI. A color code linking the signaling pathways to their bibliographic references is used: green arrows [38]; orange arrows [43,44]; purple arrows [45,46]; red arrows [36]; and a brown arrow [37]. Pointed arrows: stimulation; rounded arrows: inhibition. Ac, acetylation; Akt, Akt kinase; AMPK, AMP-activated protein kinase; cAMP, cyclic adenosine monophosphate; CAT, catalase; FOXO, forkhead Box O3; HO-1, heme oxygenase; Keap1, kelch-like ECH associated protein; Nrf2, nuclear factor erythroid 2 related factor 2; P, phosphorylation; PDE, phosphodiesterase; PGC1α, peroxisome proliferator-activated receptor-gamma coactivator 1α; PTEN, phosphatase and tensin homolog; RSV, resveratrol; SIRT1, silent mating type information regulation 2 homolog; SOD2, super-oxide dismutase.
In another study, using P14 rats as a model of neonatal HI, the administration of RSV (20 mg/kg or 40 mg/kg for seven consecutive days prior to the induction of HI) reduced oxidative stress, in part by increasing the expression of glutathione peroxidase (GPx), SOD2, and catalase (CAT) via the nuclear factor erythroid 2 related factor 2/heme oxygenase 1 (Nrf2/HO-1) pathway just after the HI accident [38]. SOD2 allows the conversion of superoxide (O2−) to hydrogen peroxide (H2O2), while GPx and CAT catalyze the reduction of H2O2 to H2O [39]. The direct antioxidant effect of RSV is attributed to the ability to remove free radicals by scavenging ROS. This property is due to the presence of three hydroxyl groups in positions 3, 4, and 5, the presence of two aromatic rings, and a double bond in the molecules [40,41,42]. These data are in agreement with the decrease in ROS production, measured after injection of RSV (intraperitoneally, 20 mg/kg) 10 min before the neonatal HI event [33].
4.3. Anti-Inflammatory Properties
Inflammation is an important contributor to brain damage that is induced by HI. The anti-inflammatory properties of RSV involve several signaling pathways (Figure 4), including the SIRT1 pathway, which induces a negative regulation of the transcription factor NF-κB via the inhibition of the Erk1/2 MAPK complex [47]. In addition to direct allosteric activation, RSV can activate SIRT1 by inhibiting the activity of phosphodiesterase (PDE), which leads to an increase in 3′, 5′- cyclic adenosine monophosphate (cAMP) [48]. The increase in the cAMP level activates protein kinase A (PKA), which in turn phosphorylates and activates SIRT1 [46]. Moreover, the RSV-dependent activation of SIRT1 leads to anti-inflammatory effects through the negative regulation of the NOD-like receptor family, pyrin domain containing 3 (NLRP3) expression, involving inhibition of the NF-κB pathway by desacetylation of p65 Lys310 [38,49]. This inhibition of activated NLRP3 inflammasomes prevents the catalytic activation of caspase 1 and the release of the pro-inflammatory cytokines IL-1β and IL-18 [50]. In addition, RSV attenuates inflammation by inducing autophagy through the activation of the transcriptional factor, f transcription factor EB (TFEB) [51]. The anti-inflammatory effect also depends on the estrogen receptor ERα, on which RSV can bind allosterically, leading to the inhibition of the NF-κB pathway [52]. Coupled with its antioxidant properties, Nrf2 plays a pivotal role in inflammation by inducing the HO-1 gene, resulting in the inhibition of the NF-κB signaling pathway [53], and by inhibiting the production of pro-inflammatory cytokines, including IL-1β and IL-6 [38,54]. The expression of tumor necrosis factor alpha (TNFα), a pivotal actor in inflammatory processes, is also decreased by the administration of RSV (intraperitoneally, 20 mg/kg, 10 min before HI or 20 mg/kg/d to 40 mg/kg/d during the 7 days preceding the insult) [27,38].
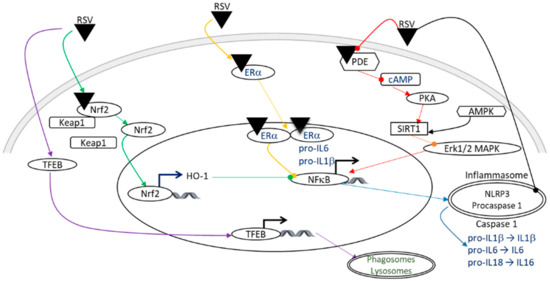
Figure 4.
Illustration of the anti-inflammatory effects of RSV, which may be involved in neuroprotection in the context of HI. A color code linking the signaling pathways to their bibliographic references is used: purple arrows [51]; green arrows [38,53]; yellow arrows [52]; red arrows [46,48]; an orange arrow [47]; and blue arrows [38,49,50]. Pointed arrows: stimulation; rounded arrows: inhibition. AMPK, AMP-activated protein kinase; cAMP, cyclic adenosine monophosphate; ER, estrogen receptor; Erk1/2, extracellular signal-regulated kinase 1 and 2; HO-1, heme oxygenase; IL, interleukin; Keap1, kelch-like ECH associated protein; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor-kappa B; NLRP3, NOD-like receptor family, pyrin domain containing 3; Nrf2, nuclear factor erythroid 2 related factor 2; PDE, phosphodiesterase; PKA, protein kinase A; RSV, resveratrol; SIRT1, silent mating type information regulation 2 homolog; TFEB, f transcription factor EB.
5. Direct RSV Administration for Neuroprotection
Oxidative stress [55,56] and inflammation [57,58] are two critical mechanisms that are involved in neonatal HI brain injury. The neuroprotective role of RSV, especially via the activation of the SIRT1 signaling pathways, has been well described in the context of hypoxia, involving antioxidant, anti-apoptotic, and anti-inflammatory properties [36,59,60,61] (Figure 5). Due to these neuroprotective properties, RSV has generated growing interest in research in order to develop strategies to counteract HI-induced brain damage. Most of these studies evaluated the effect of intraperitoneal (i.p.) administration of RSV. Two kinds of approaches have been considered, in equal proportion: (i) a preventive approach, with an administration of RSV pre-HI, and (ii) a curative approach, with an administration of RSV post-HI.
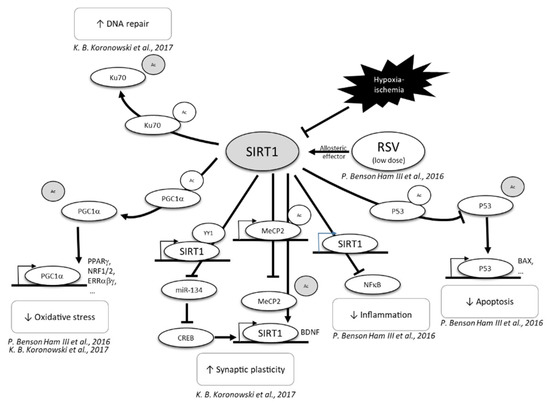
Figure 5.
Neuroprotective effect of RSV, through SIRT1 activation [59,61]. CREB, C-AMP response element-binding protein; MECP2, methyl CpG binding protein 2; NF-κB, nuclear factor-kappa B; P53, tumor; PGC1α, peroxisome proliferator-activated receptor-gamma coactivator 1α; PPP, pentose phosphate pathway; RSV, resveratrol; SIRT1, sirtuin 1.
5.1. Preventive Strategies
Different studies have evaluated the neuroprotective effect of RSV through a preventive strategy: e.g., RSV being administrated to pups before the HI event. All of these studies are summarized in Table 1. The administrated doses of RSV ranged from 200 µg/kg to 40 mg/kg; the most frequent dose was 20 mg/kg. For all of the considered studies, the mode of administration was an intraperitoneal (i.p.) injection and the treatment window covered a wide period ranging from 7 d to only 10 min before the HI event. Although the hypoxic levels (% O2) were quite similar, the duration of hypoxia varied from 45 min to 2.5 h. In all these studies, RSV administration was neuroprotective, showing a decrease in oxidative stress [33,38,62], a decrease in inflammation [27,33,38], and a limitation of neuronal death [26,27], leading to a decrease in cerebral injury [27,33,38]. Interestingly, Toader et al. combined RSV pre-treatment (during the 7 d before HI, 20 mg/kg, i.p.) with hypothermia (rectal temperature 33 °C to 34 °C for 3 h), which is the gold standard in human newborn care [62]. Data showed a synergistic neuroprotection involving a decrease in oxidative stress. Only one study evaluated this RSV neuroprotection using behavioral tests [33]. At P90, Artega et al. measured the ability of RSV administration to counteract the deleterious effects of HI on the anxiety, neophobia, and visual episodic memory of rats which had undergone an HI event at P7. All of tested cognitive functions were preserved by pre-treatment with RSV. In the same study, the efficiency of pre-treatment with RSV was compared with that of post-treatment with RSV. In the study’s conditions (RSV: 20 mg/kg, i.p. injection just after HI), the authors could not highlight any effect of RSV post-treatment. This lack of efficiency could be attributed to the dose used or the time window of administration. In fact, some other studies were able to demonstrate the neuroprotective role of a post-HI administration of RSV.

Table 1.
In vivo evaluation of the neuroprotective effects of pre-treatment with RSV, in the context of neonatal HI.
5.2. Curative Strategies
The curative neuroprotective effect of RSV has been tested in five different studies (Table 2). Greater experimental condition variabilities were noted in these studies, compared with the conditions used for preventive treatment. The doses used ranged from 10 mg/kg/d to 100 mg/kg/d. The time of hypoxia was from 45 min to 2.5 h. The time window of the treatment was from 0 h (just after the HI) to 14 d. While RSV was mostly administered intraperitoneally, in one study the authors used oral gavage [63]. That study also used the lowest doses of RSV, in order to mimic what would be an acceptable dose for humans. However, the similarities between the times of hypoxia and the experimental tests, in some studies, allowed for some comparisons. Pan et al. and Bian et al. used a 2.5 h duration for hypoxia and administrated 100 mg/kg of RSV (i.p., 0 h, 8 h, 18 h, 48 h, and 72 h and 0 h, 8 h, 18 h post-HI, respectively) [23,24]. Both studies investigated the modulation of Bax expression in their HI model. In these two studies, the administration of RSV after the HI event led to a decrease in Bax expression and a decrease in the associated apoptosis. In three of the studies, pups underwent a hypoxic event at 8% O2/92% N2 [64,65], or 10% O2/90% N2 (which are close values), during 1 h. In all three studies, memory was evaluated using the Morris water maze. All data showed that RSV reversed HI-induced learning and memory impairments. Moreover, even if the Morris water maze was not performed at the same age (from P28 to P33), mice that were treated with RSV during 14 d (P14 to P28; RSV: 40 mg/kg/d; oral gavage) performed as well as mice treated at 0 h, 24 h, and 48 h after HI (P7 to P9; RSV: 100 mg/kg/d; i.p. injection). Those results highlighted that a lower dose administered during a longer period can be as neuroprotective as a higher dose administered during a shorter time. Moreover, as similar neuroprotection by RSV is provided by i.p. injection and force-feeding, force-feeding should be preferred.

Table 2.
In vivo evaluation of the neuroprotective effects of post-treatment with RSV, in the context of neonatal HI.
Altogether, these data suggest that RSV can be a promising therapeutic drug to counteract brain damage that is linked to neonatal HI injury, in both preventive and curative administration. Although these previous studies elegantly demonstrated a neuroprotective role of RSV in the context of neonatal HI, two major limitations precluded an eventual transfer to human newborns: (i) the high doses used, and (ii) the mode of administration, which was mainly by i.p. injection. In order to promote a clinical translation of RSV treatment to offer neuroprotection in a context of HI, the neuroprotective effect of RSV was tested at a very low dose (0.15 mg/kg/d) through maternal supplementation.
6. Maternal Supplementation for Neuroprotection
6.1. Maternal Supplementation with RSV
Isac et al. were the first to test the neuroprotective effect of maternal supplementation with RSV [66]. They did not used a neonatal HI model, but rather an asphyxia model: pups were exposed to a mixture of gas composed of 9% O2, 20% CO2, and 71% N2, for 90 min. For maternal supplementation, female Wistar rats received an RSV-enriched diet at a dose of 50 mg/kg/d, beginning at P30. Rats were mated at an age between P90 and P100 and the enriched diet was maintained until the offspring were 7 d old. Taking into consideration the intergenerational aspect, the doses used in that study were much lower than the doses used in previous studies with oral gavage or direct i.p. injection. The RSV-enriched maternal diet led to hippocampal neuroprotection of the offspring in the context of asphyxia. The maternal RSV-enriched diet decreased neuronal inflammation in the offspring after asphyxia by decreasing IL-1β and TNFα production. RSV also decreased protein S-100B expression, an increase that can be considered as a marker of brain injury [67].
Moreover, the RSV-enriched diet prevented hippocampal damage by acting at the epigenetic level through a decrease in miR-15a expression, which led to accelerated neuronal growth and maturation. Even if the doses used in the study by Isac et al. were lower than those that were usually used, they were still relatively high.
An innovative approach was recently developed to counteract the deleterious effect of neonatal HI. The neuroprotection of a maternal supplementation with RSV was evaluated, as it was in the study by Isac et al. The innovative aspect of this approach was the nutritional consideration, with extremely low doses in the order of what would be found in a standard diet. In that study, dams were supplemented via drinking water with 0.15 mg/kg/d of RSV during different time windows, either before or after the HI event (before HI: during the last week of gestation or the first week of breastfeeding, or both; after HI: during the week after the HI event) [18]. If the curative supply of RSV was neuroprotective, the best neuroprotection was expected preventatively, over the longest period (i.e., during the last week of gestation and the first week of breastfeeding). This neuroprotection was characterized by (i) a decrease in brain lesion volume, (ii) a decrease in the severity of edema, and (iii) a preservation of the cognitive-sensori-motor abilities of the pups. The anti-apoptotic pathway via the increase in SIRT1 and Bcl2, as well as the antioxidative pathway via the overexpression of SOD, were involved, despite the low doses used. Moreover, the neuroprotective effects of the maternal supplementation involved the stimulation of brain energy metabolism in the pups (Figure 6). These results were the first to show a stimulation of cerebral energy metabolism by maternal supplementation with resveratrol, in the context of neonatal HI. Interestingly, the results can be related to the neuroprotection offered by post-HI lactate administration [68]. In particular, RSV maternal supplementation induced an increase in the expression of the main components of the astrocyte–neuron lactate shuttle (ANLS)—the monocarboxylate transporter 2, MCT2; the lactate dehydrogenases a and b, LDHa, LDHb; the glutamate–aspartate transporter, GLAST; the glutamate transporter, GLT1; and the Na+/K+ ATPase). This stimulation of the ANLS may spare glucose, benefiting the pentose phosphate pathway and the neuronal red/ox balance. These studies, which involved maternal supplementation, are summarized in Table 3.
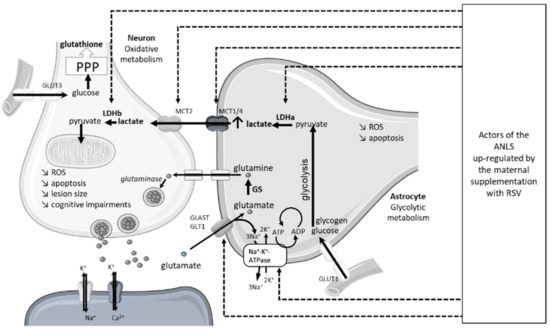
Figure 6.
Molecular targets in offspring ANLS upregulated by maternal RSV supplementation. ANLS, astrocyte-neuron lactate shuttle; GLAST, glutamate aspartate transporter 1; GLT1, astrocytic glutamate transporter 1; GLUT, glucose transporter; GS, glutamine synthetase; LDH, lactate dehydrogenase; MCT, monocarboxylate transporter; PPP, pentose phosphate pathway; ROS, reactive oxygen species; RSV, resveratrol.

Table 3.
In vivo evaluation of the neuroprotective effects of maternal supplementation with RSV, in the context of neonatal asphyxia or neonatal HI.
Although accumulating preclinical studies show a neuroprotective effect of RSV in numerous pathologies—obesity, diabetes, metabolic syndrome, cancer, cardiovascular diseases, and neuronal pathologies including HI—the therapeutic application in humans remains limited due to the short half-life of RSV, its rapid metabolism (into glucuronide and sulfate or methoxy derivatives) and high urinary elimination. The dichotomy between data from preclinical studies and clinical studies has been reviewed previously [69,70]. Despite very promising results in preclinical studies following the administration of high, or even low, doses of RSV, data from clinical studies are less convincing. However, it should be emphasized that clinical studies have, for the most part, been conducted on small cohorts with different protocols, a wide range of resveratrol doses, and short periods of time. Moreover, the best results with RSV were obtained when RSV was administrated as part of polyphenol-rich extracts. These results suggested that beneficial effects may be attributed to a synergistic action between RSV and other phenolic compounds, rather than RSV alone. An increase in bioavailability would result in an increase in bio-efficacy. Two approaches have been envisaged in order to increase bio-efficacy: the use of an RSV derivative that has a higher bioavailability, or the use of a polyphenolic cocktail that exerts synergistic effects. Both strategies were tested.
6.2. Maternal Supplementation with PIC
To increase the bio-efficacy and neuroprotection through maternal supplementation, the effect of PIC, which is four times more bioavailable than RSV, was tested in rats [19] (Table 4). The PIC neuroprotective properties were compared with those of RSV in a more deleterious context when neonatal HI was combined with maternal alcoholism [71]. The data showed a strong neuroprotection that was obtained by maternal supplementation with PIC (0.15 mg/kg/d, in the drinking water), characterized by (i) a decrease in brain lesion volume, (ii) a decrease in the severity of edema, (iii) a long-term rearrangement of the white matter, and (iv) a preservation of the cognitive-sensory-motor abilities of the pups. The most convincing result was with respect to the incidence of brain lesions. Indeed, the incidence of brain lesions was 80% for pups whose dam had been supplemented with RSV (0.15 mg/kg/d, during the last week of gestation and the first week of breastfeeding), whereas it was 55% for pups whose dam had been supplemented with PIC (same dose, same time window for maternal supplementation). These data demonstrate that better bioavailability is linked to better bio-efficacy.

Table 4.
In vivo evaluation of the neuroprotective effects of maternal supplementation with PIC, in the context of neonatal HI.
6.3. Maternal Supplementation with Multiple Polyphenols
Another strategy to increase bio-efficacy is to take advantage of putative synergistic effects of a combination of different polyphenols (Table 5). An assembly of polyphenols or a polyphenol associated with its food source could show stronger beneficial effects than an isolated polyphenol, in terms of complementarity and synergy. Feng et al. showed that i.p. administration of grape seed extract, which is rich in polyphenols, including RSV (5.89 μg/g dry weight [72]), was neuroprotective [73]. Injections of 50 mg/kg of grape seed extract, before or after the neonatal HI event, led to a decrease in apoptosis and brain lesions, as well as a decrease in lipid peroxidation [73,74]. However, as in most studies, polyphenols were administered by i.p. injections, which is not realistic for translation to human neonates. To increase the bio-efficacy of RSV, polyphenols extracted by green chemistry were administered at nutritional doses, via a supplementation of pregnant and/or breastfeeding rat females (RSV, 0.15 mg/kg/d; PTE, 0.15 mg/kg/d and ε-VNF, 0.30 mg/kg/d) [20]. In this study, a neuroprotective effect of maternal supplementation with eco-sustainably extracted grape polyphenols was demonstrated for the first time in the context of neonatal HI. Compared with the maternal supplementation with RSV alone, the polyphenolic cocktail induced a better striatal neuroprotection.

Table 5.
In vivo evaluation of the neuroprotective effects of maternal supplementation with grape seed extract or a polyphenolic cocktail in the context of neonatal HI.
Polyphenols are produced as needed by plants, but they cannot be synthesized by the human body; the intake of these micronutrients is therefore from the diet. In order to compare and determine an order of magnitude of the doses tested in the studies mentioned above, we evaluated the quantities of elements rich in resveratrol, commonly found in a diet, which should be consumed by a pregnant woman to match the amount administered in animal experiments. Quantities are given for a pregnant woman weighing 70 kg (Table 6). In contrast to the classically high doses used in animal experiments, 0.15 mg/kg/d is a nutritional dose that is realistically achievable through a natural food supply. Moreover, RSV can cross the blood–brain barrier (RSV: i.p., 30 mg/kg, [75]), as well as the placental barrier. Its consumption during pregnancy did not show any deleterious effects in animals [76], nor in pregnant women [77]. However, the non-toxic character of RSV is controversial, as some deleterious effects have been reported in a few studies in vitro and in vivo (non-pregnant condition). This duality was recently very well discussed by Shaito et al. [78]. In that review article, the authors highlighted the importance of the doses of RSV used to observe either beneficial (at low doses) or deleterious (at high doses) effects [79,80], a phenomenon called hormesis. Indeed, most of the toxic effects of RSV, in particular the pro-oxidant effect, were observed at high doses [81,82,83], indicating the use of low doses in preclinical studies in order to permit further translation to the clinic by precluding any possible toxic effect.

Table 6.
Examples of food intake necessary for a 70 kg pregnant woman to match the doses of resveratrol used in preclinical studies.
7. Conclusions and Future Perspectives
Due to its anti-apoptotic, antioxidant, anti-inflammatory properties, and its ability to stimulate cerebral energy metabolism, RSV is a very good candidate to counteract the deleterious effects of neonatal HI. However, its low bioavailability makes the translation from preclinical studies to human neonates difficult. An increase in polyphenolic bioavailability, in correlation with an increase in bio-efficacy, would be a very interesting avenue for transposing the benefits of RSV to humans. In addition, from the perspective of clinical application, some points should be considered for future studies: (i) the doses used should be low and of the nutritional order; and (ii) the mode of intraperitoneal administration should be abandoned in favor of maternal supplementation. The experimental conditions (doses, hypoxia time, and administration window) should be standardized in order to be able to compare the different conditions among the studies. The overall molecular mechanisms remain to be elucidated. The behavioral assessments of the beneficial effects of preventive administration of polyphenols could be substantiated in order to better understand the mode of action of polyphenolic neuroprotection. More studies on the neuroprotective effects of maternal supplementation with polyphenolic cocktails or plant extracts rich in RSV-derived polyphenols could open new, promising, and innovative avenues for counteracting the deleterious effects of neonatal HI.
Author Contributions
Conceptualization, H.R.; writing—original draft preparation, H.R.; writing—review and editing, A.-K.B.-S., L.P. and P.G.; funding acquisition, A.-K.B.-S., L.P. and H.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was conducted in the context of the framework of the University of Bordeaux’s IdEx “Investments for the Future” program/RRI “IMPACT”. This work was supported by grants (2018/01) from the Fondation de Recherche en Alcoologie (FRA) from the French State, in the context of the “Investments for the future” Programme IdEx (ANR-10-IDEX) and the LabEx TRAIL (ANR-10-LABX-57), and by a French-Swiss ANR-FNS grant (ANR-15-CE37-0012). This work is original, not previously published, and was not submitted for publication or consideration elsewhere.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Exinnov Company for the generous gift of the polyphenols and the Fytexia Company for the financing of the Master 2 internship of P.G.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hanrahan, J.D.; Sargentoni, J.; Azzopardi, D.; Manji, K.; Cowan, F.M.; Rutherford, M.A.; Cox, I.J.; Bell, J.D.; Bryant, D.J.; Edwards, A.D. Cerebral metabolism within 18 h of birth asphyxia: A proton magnetic resonance spectroscopy study. Pediatr. Res. 1996, 39, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.V.; Ishida, A.; Ishida, W.N.; Matsushita, H.B.; Nishimura, A.; Tsuji, M. Plasticity and injury in the developing brain. Brain Dev. 2009, 31, 1–10. [Google Scholar] [CrossRef]
- Drury, P.P.; Bennet, L.; Gunn, A.J. Mechanisms of hypothermic neuroprotection. Clin. Perinatol. 2014, 41, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Diaz, A.; Hilario, E.; De Cerio, F.G.; Valls-i-Soler, A.; Alvarez-Diaz, F.J. Hypoxic-ischemic injury in the immature brain—Key vascular and cellular players. Neonatology 2007, 92, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Iwata, O.; Iwata, S.; Thornton, J.S.; De Vita, E.; Bainbridge, A.; Herbert, L.; Scaravilli, F.; Peebles, D.; Wyatt, J.S.; Cady, E.B.; et al. “Therapeutic time window” duration decreases with increasing severity of cerebral hypoxia-ischaemia under normothermia and delayed hypothermia in newborn piglets. Brain Res. 2007, 1154, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Cotten, C.M.; Shankaran, S. Hypothermia for hypoxic-ischemic encephalopathy. Expert Rev. Obstet. Gynecol. 2010, 5, 227–239. [Google Scholar] [CrossRef]
- Buonocore, G.; Groenendaal, F. Anti-oxidant strategies. Semin. Fetal Neonatal Med. 2007, 12, 287–295. [Google Scholar] [CrossRef]
- Ferriero, D.M. Neonatal brain injury. N. Engl. J. Med. 2004, 351, 1985–1995. [Google Scholar] [CrossRef]
- Shalak, L.; Perlman, J.M. Hypoxic-ischemic brain injury in the term infant-current concepts. Early Hum. Dev. 2004, 80, 125–141. [Google Scholar] [CrossRef]
- Saliba, E.; Debillon, T. Hypothermia for hypoxic-ischemic encephalopathy in fullterm newborns. Arch. Pediatr. 2010, 17 (Suppl. 3), S67–S77. [Google Scholar] [CrossRef]
- Rogers, E.E.; Bonifacio, S.L.; Glass, H.C.; Juul, S.E.; Chang, T.; Mayock, D.E.; Durand, D.J.; Song, D.; Barkovich, A.J.; Ballard, R.A.; et al. Erythropoietin and hypothermia for hypoxic-ischemic encephalopathy. Pediatr. Neurol. 2014, 51, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Tokusoglu, O.; Unal, M.K.; Yemis, F. Determination of the phytoalexin resveratrol (3,5,4′-trihydroxystilbene) in peanuts and pistachios by high-performance liquid chromatographic diode array (HPLC-DAD) and gas chromatography-mass spectrometry (GC-MS). J. Agric. Food Chem. 2005, 53, 5003–5009. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, A.; Wadhwa, N.; Tiwari, A. Therapeutic role of resveratrol and piceatannol in disease prevention. J. Blood Disord. Trans. 2014, 5, 9. [Google Scholar] [CrossRef]
- Lin, H.S.; Yue, B.D.; Ho, P.C. Determination of pterostilbene in rat plasma by a simple HPLC-UV method and its application in pre-clinical pharmacokinetic study. Biomed. Chromatogr. 2009, 23, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef]
- Caillaud, M.; Guillard, J.; Richard, D.; Milin, S.; Chassaing, D.; Paccalin, M.; Page, G.; Bilan, A.R. Trans epsilon viniferin decreases amyloid deposits and inflammation in a mouse transgenic Alzheimer model. PLoS ONE 2019, 14, e0212663. [Google Scholar] [CrossRef]
- Dumont, U.; Sanchez, S.; Repond, C.; Beauvieux, M.C.; Chateil, J.F.; Pellerin, L.; Bouzier-Sore, A.K.; Roumes, H. Neuroprotective Effect of Maternal Resveratrol Supplementation in a Rat Model of Neonatal Hypoxia-Ischemia. Front. Neurosci. 2020, 14, 616824. [Google Scholar] [CrossRef] [PubMed]
- Dumont, U.; Sanchez, S.; Olivier, B.; Chateil, J.F.; Pellerin, L.; Beauvieux, M.C.; Bouzier-Sore, A.K.; Roumes, H. Maternal consumption of piceatannol: A nutritional neuroprotective strategy against hypoxia-ischemia in rat neonates. Brain Res. 2019, 1717, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Roumes, H.; Sanchez, S.; Benkhaled, I.; Fernandez, V.; Goudeneche, P.; Perrin, F.; Pellerin, L.; Guillard, J.; Bouzier-Sore, A.K. Neuroprotective Effect of Eco-Sustainably Extracted Grape Polyphenols in Neonatal Hypoxia-Ischemia. Nutrients 2022, 14, 773. [Google Scholar] [CrossRef]
- Matsushita, T.; Sasaki, H.; Takayama, K.; Ishida, K.; Matsumoto, T.; Kubo, S.; Matsuzaki, T.; Nishida, K.; Kurosaka, M.; Kuroda, R. The overexpression of SIRT1 inhibited osteoarthritic gene expression changes induced by interleukin-1beta in human chondrocytes. J. Orthop. Res. 2013, 31, 531–537. [Google Scholar] [CrossRef]
- He, D.S.; Hu, X.J.; Yan, Y.Q.; Liu, H. Underlying mechanism of Sirt1 on apoptosis and extracellular matrix degradation of osteoarthritis chondrocytes. Mol. Med. Rep. 2017, 16, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Shan, H.; Chen, T. Resveratrol ameliorates hypoxia/ischemia-induced brain injury in the neonatal rat via the miR-96/Bax axis. Childs Nerv. Syst. 2017, 33, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Li, S.; Hu, Y.; Zhang, H.; Liu, Y.; Jiang, H.; Fang, M.; Li, Z.; Xu, K.; Zhang, H.; et al. Resveratrol post-treatment protects against neonatal brain injury after hypoxia-ischemia. Oncotarget 2016, 7, 79247–79261. [Google Scholar] [CrossRef]
- Vaziri, H.; Dessain, S.K.; Eaton, E.N.; Imai, S.I.; Frye, R.A.; Pandita, T.K.; Guarente, L.; Weinberg, R.A. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001, 107, 149–159. [Google Scholar] [CrossRef]
- West, T.; Atzeva, M.; Holtzman, D.M. Pomegranate polyphenols and resveratrol protect the neonatal brain against hypoxic-ischemic injury. Dev. Neurosci. 2007, 29, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Revuelta, M.; Arteaga, O.; Alvarez, A.; Martinez-Ibargüen, A.; Hilario, E. Characterization of Gene Expression in the Rat Brainstem After Neonatal Hypoxic-Ischemic Injury and Antioxidant Treatment. Mol. Neurobiol. 2017, 54, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Siman, R.; Noszek, J.C. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron 1988, 1, 279–287. [Google Scholar] [CrossRef]
- Bednarski, E.; Vanderklish, P.; Gall, C.; Saido, T.C.; Bahr, B.A.; Lynch, G. Translational suppression of calpain I reduces NMDA-induced spectrin proteolysis and pathophysiology in cultured hippocampal slices. Brain Res. 1995, 694, 147–157. [Google Scholar] [CrossRef]
- Blomgren, K.; Zhu, C.; Wang, X.; Karlsson, J.O.; Leverin, A.L.; Bahr, B.A.; Mallard, C.; Hagberg, H. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: A mechanism of “pathological apoptosis”? J. Biol. Chem. 2001, 276, 10191–10198. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, F.; Zhang, J.; Wang, T.; Wei, X.; Wu, J.; Feng, Y.; Dai, Z.; Wu, Q. Neuroprotective effect of resveratrol on ischemia/reperfusion injury in rats through TRPC6/CREB pathways. J. Mol. Neurosci. 2013, 50, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab. 2001, 21, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, O.; Revuelta, M.; Urigüen, L.; Alvarez, A.; Montalvo, H.; Hilario, E. Pretreatment with Resveratrol Prevents Neuronal Injury and Cognitive Deficits Induced by Perinatal Hypoxia-Ischemia in Rats. PLoS ONE 2015, 10, e0142424. [Google Scholar] [CrossRef]
- Lu, Q.; Wainwright, M.S.; Harris, V.A.; Aggarwal, S.; Hou, Y.; Rau, T.; Poulsen, D.J.; Black, S.M. Increased NADPH oxidase-derived superoxide is involved in the neuronal cell death induced by hypoxia-ischemia in neonatal hippocampal slice cultures. Free Radic. Biol. Med. 2012, 53, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bonilla, L.; Benakis, C.; Moore, J.; Iadecola, C.; Anrather, J. Immune mechanisms in cerebral ischemic tolerance. Front. Neurosci. 2014, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-E.; Kim, H.-S.; Park, E.-M. Acute resveratrol treatment modulates multiple signaling pathways in the ischemic brain. Neurochem. Res. 2012, 37, 2686–2696. [Google Scholar]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef]
- Gao, Y.; Fu, R.; Wang, J.; Yang, X.; Wen, L.; Feng, J. Resveratrol mitigates the oxidative stress mediated by hypoxic-ischemic brain injury in neonatal rats via Nrf2/HO-1 pathway. Pharm. Biol. 2018, 56, 440–449. [Google Scholar] [CrossRef]
- Kamesh, V.; Sumathi, T. Nephroprotective potential of Bacopa monniera on hypercholesterolemia induced nephropathy via the NO signaling pathway. Pharm. Biol. 2014, 52, 1327–1334. [Google Scholar] [CrossRef]
- Yousuf, S.; Atif, F.; Ahmad, M.; Hoda, N.; Ishrat, T.; Khan, B.; Islam, F. Resveratrol exerts its neuroprotective effect by modulating mitochondrial dysfunctions and associated cell death during cerebral ischemia. Brain Res. 2009, 1250, 242–253. [Google Scholar] [CrossRef]
- Leonard, S.S.; Xia, C.; Jiang, B.-H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Iuga, C.; Alvarez-Idaboy, J.R.; Russo, N. Antioxidant activity of trans-resveratrol toward hydroxyl and hydroperoxyl radicals: A quantum chemical and computational kinetics study. J. Org. Chem. 2012, 77, 3868–3877. [Google Scholar] [CrossRef] [PubMed]
- Inglés, M.; Gambini, J.; Miguel, M.G.; Bonet-Costa, V.; Abdelaziz, K.M.; El Alami, M.; Viña, J.; Borrás, C. PTEN mediates the antioxidant effect of resveratrol at nutritionally relevant concentrations. Biomed. Res. Int. 2014, 2014, 580852. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Qu, Y.; Mao, M.; Zhang, X.; Li, J.; Ferriero, D.; Mu, D. Involvement of the PTEN-AKT-FOXO3a pathway in neuronal apoptosis in developing rat brain after hypoxia-ischemia. J. Cereb. Blood Flow Metab. 2009, 29, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Zhou, Y.; Wang, K.; Hou, Y.; Hou, R.; Ye, X. Resveratrol provides neuroprotection by inhibiting phosphodiesterases and regulating the cAMP/AMPK/SIRT1 pathway after stroke in rats. Brain Res. Bull. 2016, 121, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Gerszon, J.; Rodacka, A.; Puchala, M. Antioxidant Properties of Resveratrol and its Protective Effects in Neurodegenerative Diseases. Adv. Cell Biol. 2014, 4, 97–117. [Google Scholar] [CrossRef]
- Deng, Z.Y.; Hu, M.M.; Xin, Y.F.; Gang, C. Resveratrol alleviates vascular inflammatory injury by inhibiting inflammasome activation in rats with hypercholesterolemia and vitamin D2 treatment. Inflamm. Res. 2015, 64, 321–332. [Google Scholar] [CrossRef]
- Park, S.-J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Du, L.; Xu, C.; Cao, J.; Fan, T.; Liu, J.; Su, X.; Fan, S.; Liu, Q.; et al. Resveratrol inhibits ionising irradiation-induced inflammation in MSCs by activating SIRT1 and limiting NLRP-3 inflammasome activation. Int. J. Mol. Sci. 2013, 14, 14105–14118. [Google Scholar] [CrossRef]
- Agostini, L.; Martinon, F.; Burns, K.; McDermott, M.F.; Hawkins, P.N.; Tschopp, J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 2004, 20, 319–325. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Zhou, M.; Zhang, Y.; Liu, Y.; Hou, P.; Zeng, X.; Yi, L.; Mi, M. Resveratrol attenuates endothelial oxidative injury by inducing autophagy via the activation of transcription factor EB. Nutr. Metab. 2019, 16, 42. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, J.C.; Srinivasan, S.; Bruno, N.E.; Parent, A.A.; Hughes, T.; Pollock, J.A.; Gjyshi, O.; Cavett, V.; Nowak, J.; Garcia-Ordonez, R.D.; et al. Resveratrol modulates the inflammatory response via an estrogen receptor-signal integration network. eLife 2014, 3, e02057. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Yao, W.; Xia, H.; Jin, Y.; Li, X.; Cai, J.; Hei, Z. Elevation of HO-1 Expression Mitigates Intestinal Ischemia-Reperfusion Injury and Restores Tight Junction Function in a Rat Liver Transplantation Model. Oxid. Med. Cell. Longev. 2015, 2015, 986075. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, H.J.; Ditelberg, J.S.; Chen, S.F.; Sarco, D.P.; Chan, P.H.; Epstein, C.J.; Ferriero, D.M. Copper/zinc superoxide dismutase transgenic brain accumulates hydrogen peroxide after perinatal hypoxia ischemia. Ann. Neurol. 1998, 44, 357–364. [Google Scholar] [CrossRef]
- Weis, S.N.; Schunck, R.V.; Pettenuzzo, L.F.; Krolow, R.; Matté, C.; Manfredini, V.; Peralba, M.D.C.R.; Vargas, C.R.; Dalmaz, C.; Wyse, A.T.; et al. Early biochemical effects after unilateral hypoxia-ischemia in the immature rat brain. Int. J. Dev. Neurosci. 2011, 29, 115–120. [Google Scholar] [CrossRef]
- Volpe, J.J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef]
- Silbereis, J.C.; Huang, E.; Back, S.A.; Rowitch, D.H. Towards improved animal models of neonatal white matter injury associated with cerebral palsy. Dis. Model Mech. 2010, 3, 678–688. [Google Scholar] [CrossRef]
- Ham, P.B., 3rd; Raju, R. Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog. Neurobiol. 2016, 157, 92–116. [Google Scholar] [CrossRef]
- Chang, C.-C. Effect of resveratrol on oxidative and inflammatory stress in liver and spleen of streptozotocin-induced type 1 diabetic rats. Chin. J. Physiol. 2012, 55, 192–201. [Google Scholar] [CrossRef]
- Koronowski, K.B.; Khoury, N.; Saul, I.; Loris, Z.B.; Cohan, C.H.; Stradecki-Cohan, H.M.; Dave, K.R.; Young, J.I.; Perez-Pinzon, M.A. Neuronal SIRT1 (Silent Information Regulator 2 Homologue 1) Regulates Glycolysis and Mediates Resveratrol-Induced Ischemic Tolerance. Stroke 2017, 48, 3117–3125. [Google Scholar] [CrossRef]
- Toader, A.-M.; Filip, A.; Decea, N.; Muresan, A. Neuroprotective strategy in an experimental newborn rat model of brain ischemia and hypoxia: Effects of Resveratrol and hypothermia. Clujul Med. 2013, 86, 203–207. [Google Scholar] [PubMed]
- Li, H.; Li, X.; Liu, Z.; Wu, S.; Guo, J.; Shi, R.; Sun, Y.; Wang, Y.; Yin, H. Resveratrol reserved hypoxia-ischemia induced childhood hippocampal dysfunction and neurogenesis via improving mitochondrial dynamics. Neurosci. Res. 2020, 161, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, J.; Peng, J.; Jiang, H.; Le, K. Resveratrol Improves Synaptic Plasticity in Hypoxic-Ischemic Brain Injury in Neonatal Mice via Alleviating SIRT1/NF-kappaB Signaling-Mediated Neuroinflammation. J. Mol. Neurosci. 2022, 72, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Karalis, F.; Soubasi, V.; Georgiou, T.; Nakas, C.T.; Simeonidou, C.; Guiba-Tziampiri, O.; Spandou, E. Resveratrol ameliorates hypoxia/ischemia-induced behavioral deficits and brain injury in the neonatal rat brain. Brain Res. 2011, 1425, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Isac, S.; Panaitescu, A.M.; Spataru, A.; Iesanu, M.; Totan, A.; Udriste, A.; Cucu, N.; Peltecu, G.; Zagrean, L.; Zagrean, A.-M. Trans-resveratrol enriched maternal diet protects the immature hippocampus from perinatal asphyxia in rats. Neurosci. Lett. 2017, 653, 308–313. [Google Scholar] [CrossRef]
- Korfias, S.; Stranjalis, G.; Papadimitriou, A.; Psachoulia, C.; Daskalakis, G.; Antsaklis, A.; Sakas, D. Serum S-100B protein as a biochemical marker of brain injury: A review of current concepts. Curr. Med. Chem. 2006, 13, 3719–3731. [Google Scholar] [CrossRef]
- Roumes, H.; Dumont, U.; Sanchez, S.; Mazuel, L.; Blanc, J.; Raffard, G.; Chateil, J.-F.; Pellerin, L.; Bouzier-Sore, A.-K. Neuroprotective role of lactate in rat neonatal hypoxia-ischemia. J. Cereb. Blood Flow Metab. 2021, 41, 342–358. [Google Scholar] [CrossRef]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta 2015, 1852, 1155–1177. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Dumont, U.; Sanchez, S.; Olivier, B.; Chateil, J.-F.; Deffieux, D.; Quideau, S.; Pellerin, L.; Beauvieux, M.-C.; Bouzier-Sore, A.-K.; Roumes, H. Maternal alcoholism and neonatal hypoxia-ischemia: Neuroprotection by stilbenoid polyphenols. Brain Res. 2020, 1738, 146798. [Google Scholar] [CrossRef] [PubMed]
- Shrikanta, A.; Kumar, A.; Govindaswamy, V. Resveratrol content and antioxidant properties of underutilized fruits. J. Food Sci. Technol. 2015, 52, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, Y.-M.; Fratkins, J.D.; LeBlanc, M.H. Grape seed extract suppresses lipid peroxidation and reduces hypoxic ischemic brain injury in neonatal rats. Brain Res. Bull. 2005, 66, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, Y.-M.; Leblanc, M.H.; Bhatt, A.J.; Rhodes, P.G. Grape seed extract given three hours after injury suppresses lipid peroxidation and reduces hypoxic-ischemic brain injury in neonatal rats. Pediatr. Res. 2007, 61, 295–300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Q.; Xu, J.; Rottinghaus, G.E.; Simonyi, A.; Lubahn, D.; Sun, G.Y.; Sun, A.Y. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002, 958, 439–447. [Google Scholar] [CrossRef]
- Williams, L.D.; Burdock, G.A.; Edwards, J.A.; Beck, M.; Bausch, J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem. Toxicol. 2009, 47, 2170–2182. [Google Scholar] [CrossRef]
- Malvasi, A.; Kosmas, I.; Mynbaev, O.A.; Sparic, R.; Gustapane, S.; Guido, M.; Tinelli, A. Can trans resveratrol plus d-chiro-inositol and myo-inositol improve maternal metabolic profile in overweight pregnant patients? Clin. 2017, 168, e240–e247. [Google Scholar]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Mankowski, R.T.; You, L.; Buford, T.W.; Leeuwenburgh, C.; Manini, T.M.; Schneider, S.; Qiu, P.; Anton, S.D. Higher dose of resveratrol elevated cardiovascular disease risk biomarker levels in overweight older adults—A pilot study. Exp. Gerontol. 2020, 131, 110821. [Google Scholar] [CrossRef]
- Gadacha, W.; Ben-Attia, M.; Bonnefont-Rousselot, D.; Aouani, E.; Ghanem-Boughanmi, N.; Touitou, Y. Resveratrol opposite effects on rat tissue lipoperoxidation: Pro-oxidant during day-time and antioxidant at night. Redox Rep. 2009, 14, 154–158. [Google Scholar] [CrossRef]
- Guha, P.; Dey, A.; Chatterjee, A.; Chattopadhyay, S.; Bandyopadhyay, S. Pro-ulcer effects of resveratrol in mice with indomethacin-induced gastric ulcers are reversed by L-arginine. Br. J. Pharmacol. 2010, 159, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Rocha, K.; Souza, G.; Ebaid, G.; Seiva, F.; Cataneo, A.; Novelli, E. Resveratrol toxicity: Effects on risk factors for atherosclerosis and hepatic oxidative stress in standard and high-fat diets. Food Chem. Toxicol. 2009, 47, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Guha, P.; Chattopadhyay, S.; Bandyopadhyay, S.K. Biphasic activity of resveratrol on indomethacin-induced gastric ulcers. Biochem. Biophys. Res. Commun. 2009, 381, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Dudley, J.I.; Das, D.K. Dose-dependency of resveratrol in providing health benefits. Dose Response 2010, 8, 478–500. [Google Scholar] [CrossRef]
- Prasad, K. Resveratrol, wine, and atherosclerosis. Int. J. Angiol. 2012, 21, 7–18. [Google Scholar] [CrossRef]
- Lee, S.S.; Lee, S.M.; Kim, M.; Chun, J.; Cheong, Y.K.; Lee, J. Analysis of trans-resveratrol in peanuts and peanut butters consumed in Korea. Food Res. Int. 2004, 37, 247–251. [Google Scholar] [CrossRef]
- Rege, S.; Egeetha, T.; Griffin, G.D.; Broderick, T.L.; Babu, J.R. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front. Aging Neurosci. 2014, 6, 218. [Google Scholar] [CrossRef]
- Troian, S.A.; Vicenzi, K.; Alves, M.K. Content of resveratrol and total polyphenols in whole grape, reconstituted and sweetened juice sold in southern Brazil. Braz. J. Food Res. 2016, 7, 58–67. [Google Scholar] [CrossRef]
- Hurst, W.J.; Glinski, J.A.; Miller, K.B.; Apgar, J.; Davey, M.H.; Stuart, D.A. Survey of the trans-resveratrol and trans-piceid content of cocoa-containing and chocolate products. J. Agric. Food Chem. 2008, 56, 8374–8378. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).