Abstract
Improving the intestinal microbiota using probiotics, prebiotics, and synbiotics has attracted attention as a method of disease prevention and treatment. This is the first study to discuss the effects of food intake on the intestinal microbiota using a large Japanese intestinal microbiota database. Here, as a case study, we determined changes in the intestinal microbiota caused by ingestion of a processed natto food containing B. subtilis var. natto SONOMONO spores, SONOMONO NATTO POWDER CAPSULESTM, by analyzing 16S rRNA sequence data generated using next-generation sequencing techniques. The results showed that the relative abundance of Bifidobacterium and Blautia as well as the relative abundance of Bifidobacterium were increased in males and females in the ingesting group, respectively. Additionally, the effects of SONOMONO NATTO POWDER CAPSULESTM intake on Bifidobacterium and Blautia abundance depended on the relative abundance of Bifidobacterium at baseline. Finally, analysis of a large Japanese intestinal microbiota database suggested that the bacterial genera that fluctuated with the ingestion of SONOMONO NATTO POWDER CAPSULESTM may be associated with lifestyle-related diseases such as diabetes.
1. Introduction
Research on the human intestinal microbiota has seen great advances, with improvements in bacterial isolation, culture techniques, and phylogenetic classification [1,2,3]. In recent years, 16S rRNA metagenomic analysis has been used to analyze the intestinal microbiota of patients with various diseases and to characterize the intestinal microbiota of healthy individuals. As a result, it has become clear that the diversity of the intestinal microbiota and abnormal bacterial species composition (dysbiosis) are involved in various diseases [4,5,6]. In addition, as 16S rRNA metagenomic analysis has identified multiple changes in the intestinal microbiota following food intake, improvement of intestinal microbiota using probiotics (microorganisms that have beneficial effects on the host), prebiotics (foods that selectively alter the growth and activity of specific bacteria in the colon and have beneficial effects on the host), and synbiotics (a combination of probiotics and prebiotics) is attracting attention as a method of disease prevention and treatment [7,8,9]. Furthermore, the characteristics of the intestinal microbiota are influenced by factors such as age, sex, and geographical location, and it has been reported that the characteristics of the intestinal microbiota in healthy people differ between countries [10,11]. Therefore, it is significant, as it enabled us to determine the effects of changes in the intestinal microbiota due to food intake on the health status of people by comparing them with large-scale data from people living in the same area.
Natto is a traditional Japanese fermented food made by fermenting steamed soybeans with Bacillus subtilis var. natto. The fermentation process produces a variety of functional molecules [12]. Natto is rich in soy-derived proteins and vitamin K, which is essential for the production of blood coagulation factors. Natto is also rich in dietary fiber, which is thought to have a beneficial effect on intestinal regulation. In particular, natto is expected to be an excellent probiotic because it contains B. subtilis var. natto itself [13]. A study investigating the effects of natto on the gut microbiota reported an increase in Bifidobacterium abundance following natto consumption [14]. There are also reports that natto intake can prevent diabetes [15] and dyslipidemia [16] by suppressing the increase in postprandial blood glucose levels.
This is the first study to make use of the large Japanese gut microbiota database containing 16S rRNA metagenomic data to discuss the effects of food intake on intestinal microbiota. In this case study, 16S rRNA metagenomic analysis was used to analyze the effects of ingesting natto processed food for 62 days on the intestinal microbiota. Since sex differences affect the intestinal microbiota, analyses were conducted separately for males and females.
2. Materials and Methods
2.1. Study Population
A total of 205 Kohoku residents in Saga Prefecture, Japan (100 males and 105 females), aged 20 to 89 years, who fully understood the purpose, maintenance, usage, and safety of the research, gave their consent for participation in the study. All participants gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Review Board of the sonomono Inc., Fukuoka, Japan (protocol no. 2020001; 2 October 2020). Those who met the following exclusion criteria were excluded from the study: (1) had a history of abnormal laboratory values or cardiopulmonary function and were judged to have problems participating in the study, (2) were at risk of developing a serious allergy associated with the study product, (3) had a disease requiring constant medication or a history of a serious disease requiring treatment, (4) were participating in other clinical trials at the start of this study, (5) were planning to become pregnant or were breastfeeding during the study period, and (6) were deemed inappropriate by the investigators.
Kohoku Town is located in the central part of Saga Prefecture, Japan. Its main industries are agriculture and livestock farming. Medical expenses associated with diabetes and dyslipidemia are increasing in Kohoku Town, and according to health checkup data, the percentage of Kohoku Town residents with a HbA1c level of 5.6% or higher (subject to guidance during metabolic syndrome health checkups) is above the national average for both males and females.
2.2. Study Design
The study was an open study in which participants of both sexes were randomly divided into two groups: one group ingested the test product (LNC group: Lyophilized natto capsules group), and the second group did not ingest the test product (control group). Participants assigned to the LNC group were asked to ingest three SONOMONO NATTO POWDER CAPSULESTM per day for 62 days (from November 2020 to January 2021).
Stool samples from individuals in the LNC group were collected at three time points: before intake (Day 0), 31 days after intake (Day 31), and 62 days after intake (Day 62). Stool samples from participants in the control group were collected at the same time points. Stool samples were collected by the participants themselves. Additionally, the participant’s background information was investigated using a self-reporting system. The background information collected included age, sex, height, weight, lifestyle, health status, and dietary habits. During the intake period, participants were asked to fill a checklist every day, including whether they ingested the test product, their body weight, whether and how often they defecated, the condition of their stool (quantity, shape, color, and odor), their physical condition, whether they took any drugs, and whether they ate other fermented foods, lactic acid bacteria beverages, or dietary supplements. They were instructed not to change their usual dietary habits during the study period.
2.3. Test Product
SONOMONO NATTO POWDER CAPSULESTM contain 380 mg of lyophilized natto powder made from 100% “Fukuyutaka” soybeans—grown in Kohoku Town without chemical pesticides or fertilizers—in a hard-shell capsule made of plant-derived hydroxypropyl methylcellulose (HPMC). Each gram of lyophilized natto powder contains 1.6 × 1010 colony forming units (CFUs) of Bacillus natto in the spore state. Bacillus subtilis var. natto SONOMONO was used as the Bacillus natto strain. The strain was selected by single colony isolation from commercially available natto strains and is characterized by lower production of odor and stickiness components and higher protease activity compared with common natto strains (unpublished data). The intake was three capsules per day (1140 mg of natto powder and 1.8 × 1010 CFUs of Bacillus subtilis var. natto SONOMONO).
2.4. Stool Sample Collection
Stool samples were collected by the participants using a stool collection kit (TechnoSuruga Laboratory, Co., Ltd., Shizuoka, Japan), suspended in guanidine thiocyanate (GTC) solution (100 mM Tris-HCL (pH 9.0), 40 mM Tris-EDTA (pH 8.0), 4 M guanidine thiocyanate, and 0.001% bromothymol blue and mailed at room temperature.
2.5. DNA Extraction
DNA was extracted from stool samples using a fully automated enterobacterial DNA extractor (DEX-I; PMT Corporation, Fukuoka, Japan). Briefly, 100 µL of lysozyme (10 mg/mL) and 400 mg of glass beads were added to 900 µL of GTC solution containing a stool sample the size of a grain of rice in suspension, stirred, and allowed to stand at 50 °C for 30 min. Then, 500 µL of TE-saturated phenol-chloroform-isoamyl alcohol solution (25:24:1) and 110 µL of 10% SDS solution were added to the sample. Samples were processed at 2500 rpm for 2 min in a bead crusher (PMT Corporation, Fukuoka, Japan) and then incubated at 70 °C for 10 min. Sample crushing and incubation were performed twice. The samples were cooled, centrifuged at 13,200× g for 10 min, and the supernatant aliquoted. Next, 700 µL of isopropyl alcohol and 70 µL of 3 M sodium acetate solution were added to the supernatant, the solution stirred and centrifuged at 13,200× g for 15 min, and the supernatant discarded. The precipitated DNA pellet was washed twice with 70% ethanol solution, dissolved in 200 µL of TE buffer, and then stored at −80 °C until DNA sequencing.
2.6. DNA Sequencing
Using 12.5 ng of DNA extracted from stool specimens, variable regions V1 to V3 of the 16S rRNA gene was amplified using 35F primer (5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTGGCTCAGGATGAACG-3′) [17] and 520R primer (5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGACCGCGGCTGCTGGC-3′). PCR amplifications were carried out in 25 µL solutions containing 0.2 mM dNTPs, 0.2 µM of each primer, 0.5 U Q5 Hot Start High-Fidelity DNA Polymerase (New England Biolabs, Ipswich, EX, USA), 1 × Q5 Reaction Buffer, and 12.5 ng of sample DNA. The following thermal cycling conditions were used: initial denaturation at 98 °C for 3 min followed by 20 cycles of denaturation (98 °C for 10 s), annealing (55 °C for 30 s), and extension (72 °C for 1 min), and a final extension at 72 °C for 7 min. PCR products were purified with 20 µL of AMPure XP (Beckman Coulter, Inc., Brea, CA, USA) and eluted into 50 µL of 10 mM Tris-HCl, pH 8.5.
To prepare a DNA library for Illumina MiSeq sequencing using Nextera XT Index Kit v2 primers (Illumina, San Diego, CA, USA), PCR reactions were performed in 50 µL solutions containing 0.2 mM dNTPs, 5 µL of each primer, 1 U Q5 Hot Start High-Fidelity DNA Polymerase, 1 × Q5 Reaction Buffer, and 5 µL of the eluted DNA. The following thermal cycling conditions were used: initial denaturation at 98 °C for 3 min followed by 8 cycles of denaturation (98 °C for 10 s), annealing (55 °C for 30 s), and extension (72 °C for 1 min), and a final extension at 72 °C for 7 min. PCR products were purified with 56 µL of Agencourt AMPure XP in accordance with the manufacturer’s protocol and eluted into 25 µL of 10 mM Tris-HCl, pH 8.5. The concentration of the index PCR product was measured using the QuantiFluor (R) dsDNA System (Promega, Madison, WI, USA) and then diluted to 4 nM. Five microliters of each sample was used to generate a sequencing library and denatured in 0.2 N sodium hydroxide solution to a final concentration of 8 pM. To this solution, 8 pM of denatured PhiX was added to obtain a final concentration of 25–40% (v/v). The adjusted solution was processed on a MiSeq system (Illumina, San Diego, CA, USA) using Reagent Kit v3 (Illumina, San Diego, CA, USA) for DNA sequencing. The DNA sequencing process consisted of two rounds of 300 cycle reactions.
2.7. 16S rRNA Data Analysis
Fastq files were created from the base call (bcl) MiSeq file outputs using the bcl2fastq software ver. 2.20.0.422 (Illumina, San Diego, CA, USA). The generated fastq files were processed using clsplitseq ver. 0.2.2019.05.10 (https://www.claident.org/, accessed on 1 January 2022) to remove primer sequences and create demultiplexed fastq files. The quality score in clsplitseq was set to 20. The generated overlapping and paired-end fastq files were processed using DADA2 ver.1.16 package in the R software ver. 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria) to create amplicon sequence variants (ASVs) [18]. The DADA2 package was executed using the DADA2 pipeline version 1.16 (https://benjjneb.github.io/dada2/tutorial.html, accessed on 1 January 2022). The filterAndTrim function arguments used were truncLen = c(0, 0), maxN = 0, maxEE = 5, truncQ = 4, rm.phix = TRUE, compress = TRUE, multithread = TRUE, verbose = TRUE, and minLen = 50. A rarefaction curve was generated for each sample based on the number of reads for each unique ASV sequence. For each rarefaction curve, the minimum number of unique ASV sequences with a slope of 0.002308 or less was examined, and the number of sequences plus 1 was used as the sampling depth for rarefying. Rarefaction was conducted using the vegan package (ver. 2.5.7) in the R software. Rdp_train_set_18 (https://zenodo.org/record/4310151#.Yg8oWOjP1PY, accessed on 1 January 2022) was used to assign a genus name to each unique ASV sequence.
2.8. Statistical Analysis
Continuous data are presented as mean ± standard deviation (SD), while categorical data are presented as frequencies and percentages. The Welch’s t test and the Wilcoxon rank-sum test were used for between-group data comparisons, depending on the distribution of the data. Within-group comparisons were conducted using the Friedman test, which was performed on a total of three observations. Observations that were significantly different in the Friedman test were further analyzed using paired t test or Wilcoxon signed-rank test, depending on the distribution of the data. p values were corrected for multiple testing using the Benjamini–Hochberg method. Welch’s t test and paired t test were performed using the t test function with paired = FALSE and paired = TRUE. Benjamini–Hochberg multiple test correction was performed using the p.adjust function with method = BH. Friedman test was performed using the friedman.test function.
Alpha diversity of the intestinal microbiota was assessed using the Simpson diversity index.
PerMANOVA analysis was performed to compare the structural similarity of intestinal microbiota. Adonis function in the vegan package (ver. 2.5.7) was used for PerMANOVA analysis, using 9999 permutations. PerMANOVA variance tests were performed using the betadisper function in the vegan package and ANOVA function in the stats package.
Genus-level comparisons of intestinal microbiota were performed using the ALDEx2 package ver. 1.26.0 (https://github.com/ggloor/ALDEx_bioc, accessed on 27 October 2021) in R software ver. 4.1.0. For this comparison, the microbiota abundance count data were subjected to a centered log-ratio (CLR) transformation using the ALDEx2 aldex.clr function, with mc.samples = 128 and denom = “all” as arguments. For between-group comparisons, the Wilcoxon rank sum test was performed using the ALDEx2 aldex.ttest function. For within-group comparisons, the Friedman test was performed using the friedman.test function, and the Wilcoxon signed rank test was performed using the aldex.ttest function in ALDEx2. Correction for multiple testing was conducted using the Benjamini–Hochberg method, using the p.adjust function and method = BH.
The threshold for statistical significance was set at a p value of 0.05.
2.9. Large Japanese Gut Microbiota Database
The large Japanese gut microbiota database used for the analysis was SymMAD (Symbiosis Microbiome Analysis Database, not available to the public due to privacy reasons), which contains 16S rRNA metagenomic data. This database contains two major datasets. The first is a dataset of intestinal bacterial DNA extracted from stool samples collected from a Japanese population by the former Benno Laboratory, RIKEN Baton Zone Program, RIKEN Cluster for Science, Technology and Innovation Hub (Saitama, Japan) and analyzed by the Japan Agricultural Frontier Development Organization (Tokyo, Japan), which is licensed by Symbiosis Solutions Inc. The second dataset was generated by Symbiosis Solutions Inc., which obtained consent for individual participation in the study. The two datasets have a combined total of 23,139 samples (as of March 2022). SymMAD contains data on intestinal microbiota as well as information from questionnaires on the participants’ disease status and lifestyle, including eating habits.
3. Results
3.1. Selection of Analysis Population
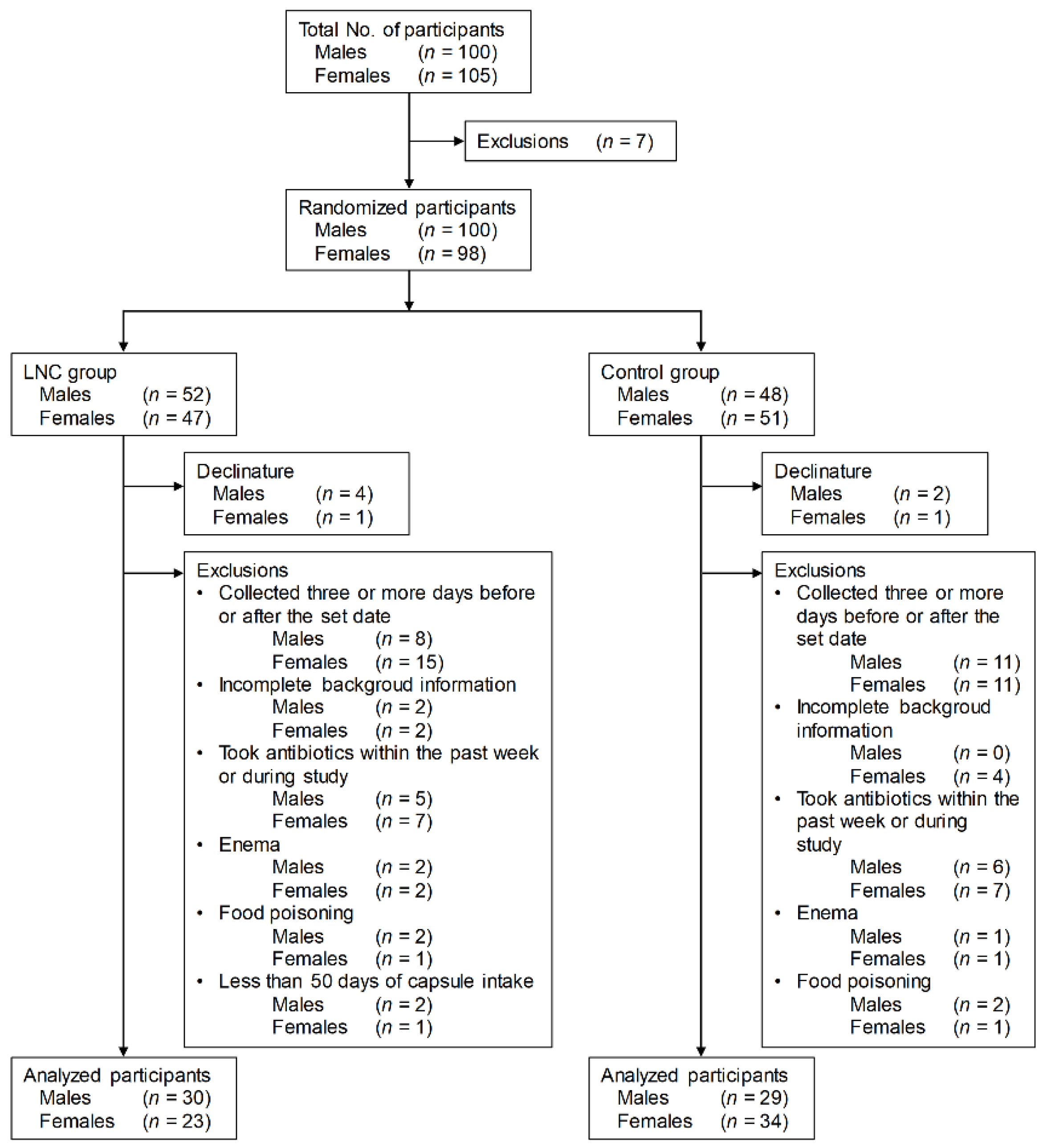
Males and females aged 20 to 89 years living in Kohoku, Saga Prefecture, Japan, were recruited into the study based on the screening criteria (Figure 1). Participants who met the exclusion criteria were excluded from the 205 townspeople monitors (100 males and 105 females) who gave consent to participate in the study. Participants were randomly divided into two groups. Participants who withdrew during the study, whose stool samples were collected three or more days before or after the set date, who did not respond to the questionnaire, who used antibiotics in the week before and during the study period, who had their stool collected by enema, and who suffered from food poisoning during the study period were also excluded. Also excluded from the LNC group were participants who ingested the test food for less than 50 days.
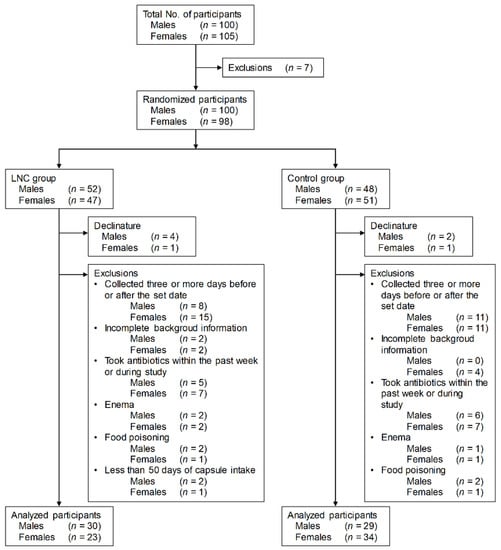
Figure 1.
Screening of participants for inclusion in the study. LNC, lyophilized natto capsules.
The final analysis included 30 males and 23 females in the LNC group and 29 males and 34 females in the control group.
There were no significant differences in age, weight, or BMI between participants of either sex in the LNC and control groups (Table 1). There were also no significant differences in the intake of fermented foods, including natto, between participants of either sex in the LNC and control groups.

Table 1.
Characteristics of the study population at the beginning of the study (Day 0).
3.2. Variations in Weight, BMI, and Defecation Status
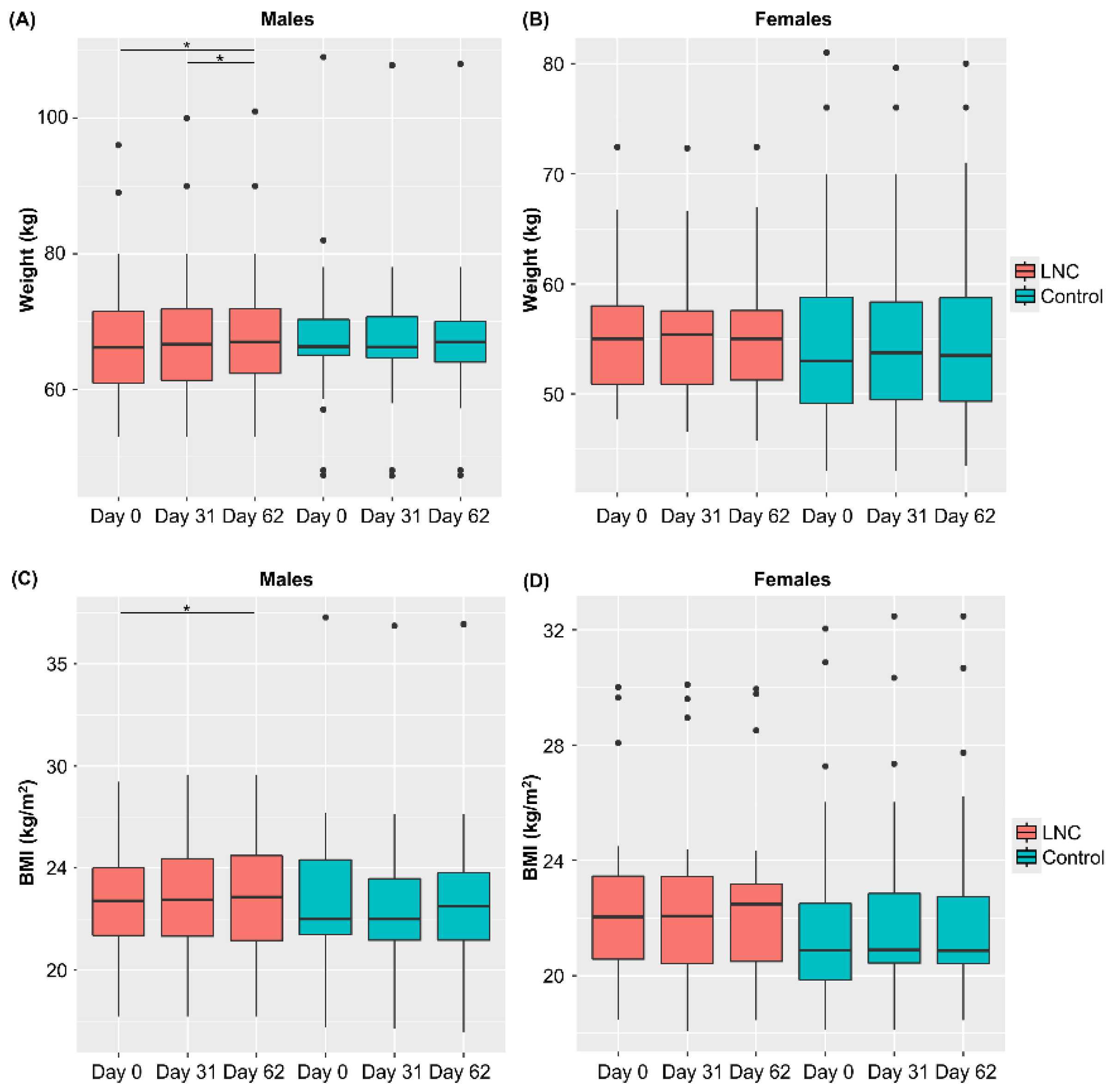
Changes in weight and BMI during the study period were analyzed (Figure 2). Weight and BMI increased significantly from Day 0 to 62 in males in the LNC group, but not in males in the control group. No significant changes in body weight or BMI were observed in females in the LNC and control groups. There were also no significant changes in the defecation status in individuals of either sex.
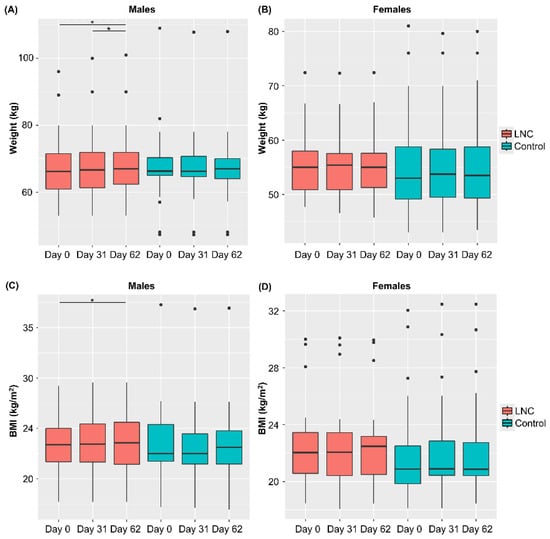
Figure 2.
Changes in body weight and BMI following ingestion of SONOMONO NATTO POWDER CAPSULESTM. Each dot represents outliers. (A) Wight of males, (B) weight of females, (C) BMI of males, (D) BMI of females. Significant increase in weight was observed between samples collected from males in the LNC (lyophilized natto capsules) group on Day 0 and 62 and on Day 31 and 62. Furthermore significant increase in BMI was observed between samples collected from males in the LNC group on Day 0 and 62. * p < 0.05.
3.3. Changes in Intestinal Microbiota
We analyzed the intestinal microbiota using 16S rRNA sequence data and generated microbiota composition data from the participants’ stool samples.
We used this intestinal microbiota composition data to analyze the diversity of each sample (α-diversity: Simpson index) and the distance of the intestinal microbiota between samples (β-diversity). There were no significant differences in α-diversity and β-diversity of the intestinal microbiota at the beginning of the study (Day 0) between males and females in the LNC and control groups (Table S1). Additionally, when genus-level CLR-transformed abundance was compared with the ALDEx2 pipeline, no significant differences in abundance were observed in the bacterial genus in participants of both sexes on Day 0 (Table S2).
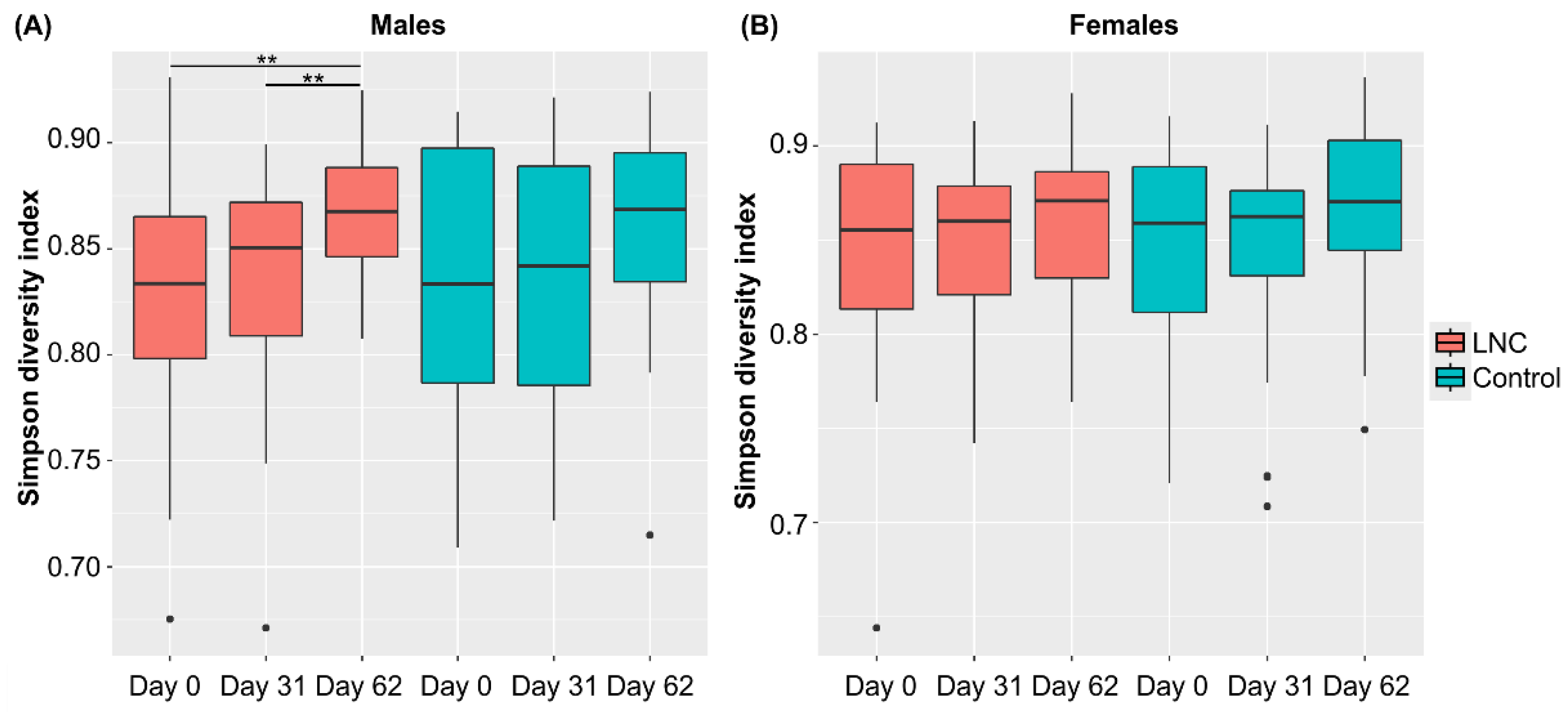
Analysis of changes in α-diversity during the study period revealed that α-diversity in males in the LNC group was significantly higher on Day 62 than on Day 0 and 31 (p < 0.01, Figure 3). No significant changes in α-diversity were observed in males in the LNC group and in females in the LNC and control groups. Significant differences in β-diversity were also detected between males and females in the LNC and control groups (Table S3).
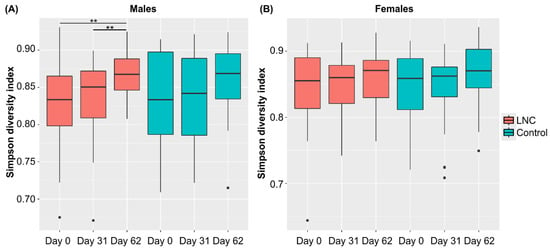
Figure 3.
Changes in the diversity of intestinal microbiota following ingestion of SONOMONO NATTO POWDER CAPSULESTM. Box plot of α-diversity calculated using the Simpson diversity index. Each dot represents outliers. (A) Males, (B) females. Significant increase in α-diversity was observed between samples collected from males in the LNC (lyophilized natto capsules) group on Day 0 and 62 and on Day 31 and 62. ** p < 0.01.
CLR-transformed abundances were compared at the genus level. The increase or decrease in the abundance of each genus was analyzed by calculating the mean relative abundance. Some genera showed significant differences in abundance between participants in the LNC group. The genera whose abundance changed significantly among males in the LNC group were Bacteroides, Bifidobacterium, Blautia, Collinsella, Phocaeicola, and unclassified bacteria (Table 2A). Bifidobacterium abundance differed significantly between males in the LNC and control groups, although the difference in abundance between Day 0 and 62 was significant only among males in the LNC group (Table 2A,B). The abundance of Bacteroides, Collinsella, Phocaeicola, and unclassified bacteria also differed significantly in males in the control group, and the increasing and decreasing trends were consistent between males in LNC and control groups.

Table 2.
Changes in the relative abundance of each genus.
Bifidobacterium, Faecalibacterium, Parabacteroides, and unclassified bacteria abundance changed significantly in females in the LNC group (Table 2C). Similar to males, Bifidobacterium abundance changed significantly among females in LNC and control groups, although the change between Day 0 and 62 was only significant in females in the LNC group (Table 2C,D). Faecalibacterium abundance changed only in females in the LNC group, with significant changes being observed between samples collected on Day 31 and 62. However, no significant differences were observed between samples collected on Day 0 and 62. The relative abundance of Parabacteroides in females in the LNC group increased significantly between Day 0 and 31 but decreased significantly between Day 31 and 62. Females in the LNC group had significantly lower Parabacteroides abundance on Day 62 than on Day 0. The abundance of unclassified bacteria also changed significantly in females in the control group, and the increasing and decreasing trends were consistent between females in the LNC and control groups.
3.4. Characteristics of Male Participants with a Significant Increase in Blautia Abundance in the LNC Group
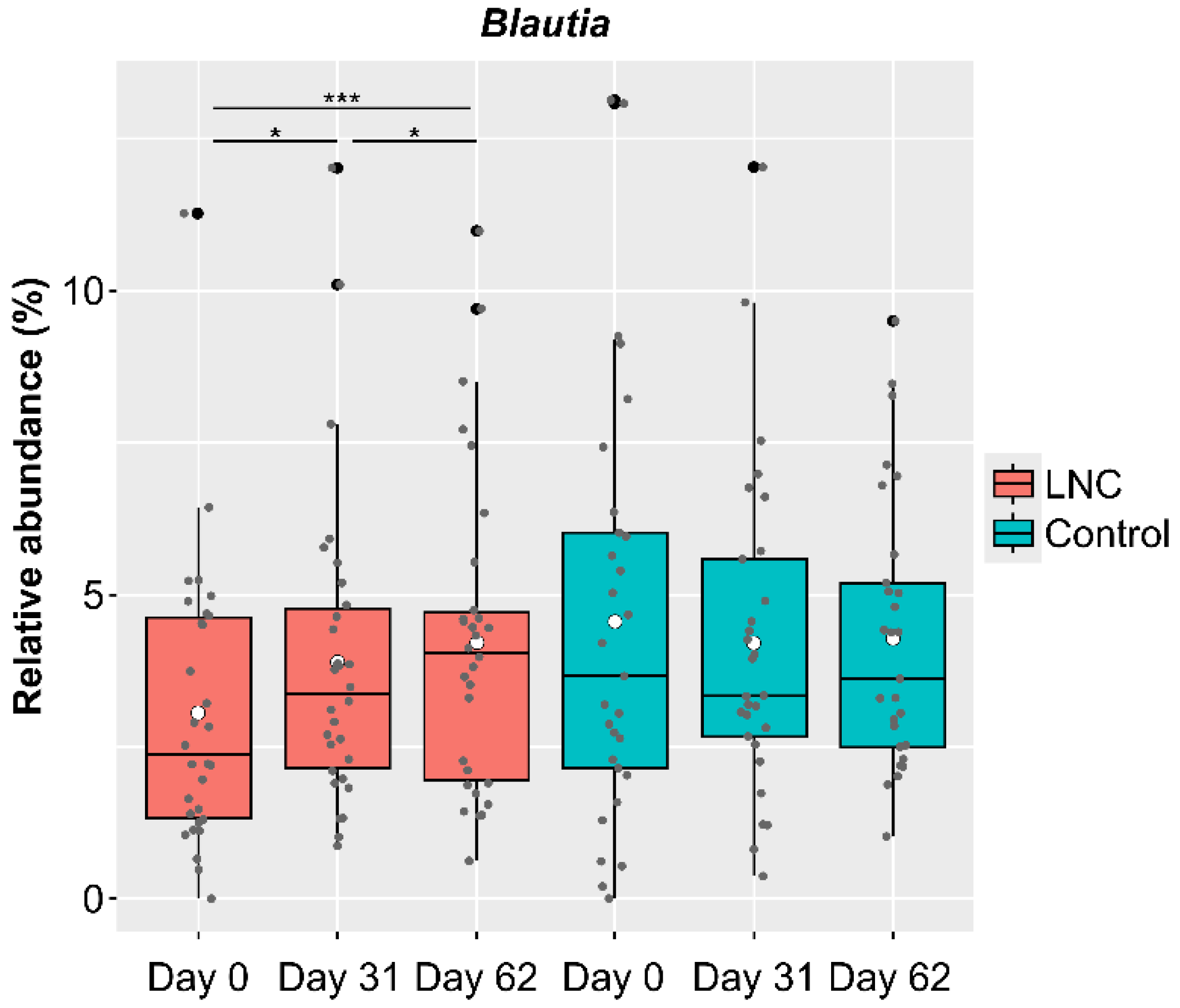
Genus-level analysis showed significant changes in CLR-transformed abundance of Blautia, with an increase in relative abundance in males in the LNC group. However, there were a certain number of participants whose Blautia abundance had not increased by the end of the study (Day 62) (Figure 4). To clarify the differences between participants whose Blautia abundance increased and those whose Blautia abundance did not increase, we compared the intestinal microbiota data with the responses to the background survey taken at the beginning of the study (Day 0). We selected participants whose relative Blautia abundance was below the mean (3.06%) on Day 0. Of these, participants whose relative Blautia abundance increased to the mean (4.22%) or above at the end of the study were defined as the IB group (increased Blautia group: n = 9), while those whose relative Blautia abundance remained below the mean were classified as the NB group (no change in Blautia group: n = 9).
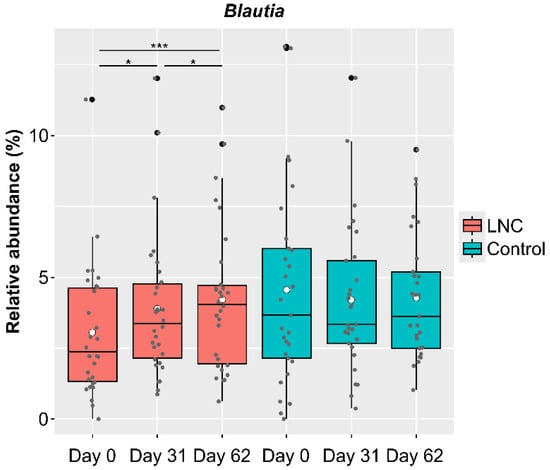
Figure 4.
Changes in the relative abundance of Blautia among male study participants. Black dots show outliers and gray dots show values for each participant. Comparison of CLR-transformed Blautia abundance showed significant differences between Day 0 and 31 and between Day 31 and 62. There were significant increases in Blautia abundance between Day 0 and 31, between Day 31 and 62, and between Day 0 and 62 among males in the LNC (lyophilized natto capsules) group. In contrast, no significant changes were observed in males in the control group. * p < 0.05; *** p < 0.001.
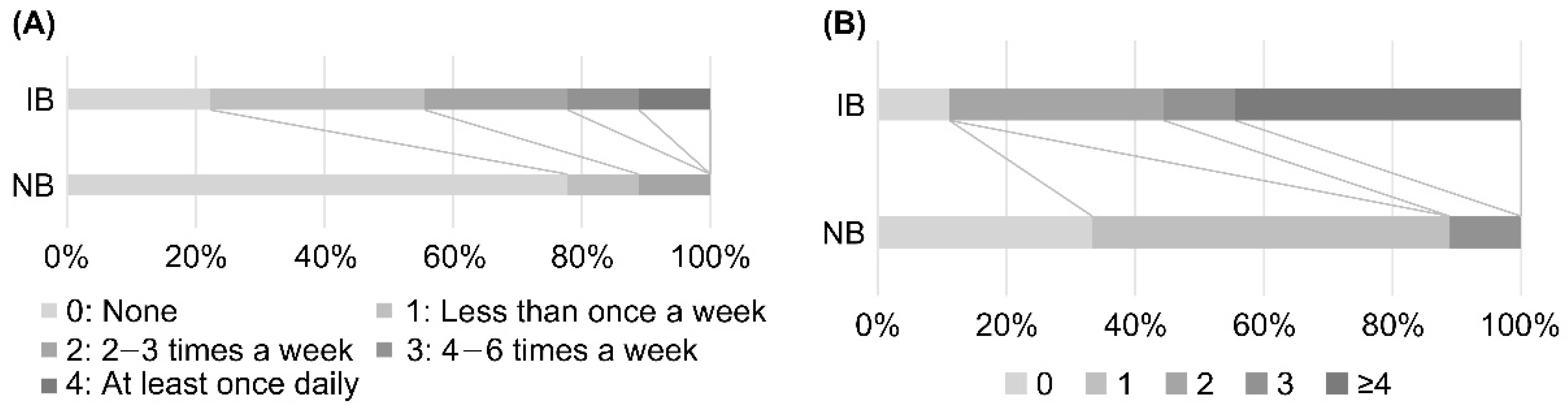
Comparison of the background survey responses of individuals in the IB and NB groups showed a significant difference in the frequency of natto intake in the previous month, with individuals in the IB group consuming natto more frequently than individuals in the NB group (p < 0.05, Figure 5A). Individuals in the IB group also consumed significantly more fermented foods (fermented pickles, cheese, miso soup, natto, koji food, sake lees food, vinegar, and others) in the previous month compared with individuals in the NB group (p < 0.01, Figure 5B). Analysis of the CLR-transformed abundance of each genus on Day 0 showed a significant difference in Bifidobacterium only, with higher relative abundance of Bifidobacterium in the IB group than in the NB group (Table S4).
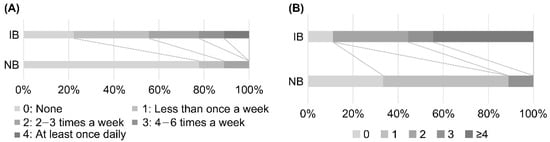
Figure 5.
Comparison of dietary habits of individuals in IB (increased Blautia) and NB (no change in Blautia) groups. (A) Comparison of the frequency of natto intake in the previous month. There was a significant difference (p < 0.05) between individuals in the IB and NB groups, with those in the IB group consuming natto more frequently than those in the NB group. (B) Comparison of the number of fermented foods consumed in the previous month. The following eight categories of fermented foods were considered: pickles, cheese, miso soup, natto, koji food, sake lees food, vinegar, and others. There was a significant difference (p < 0.01) between the IB and NB groups, with more fermented foods consumed in the IB group than in the NB group.
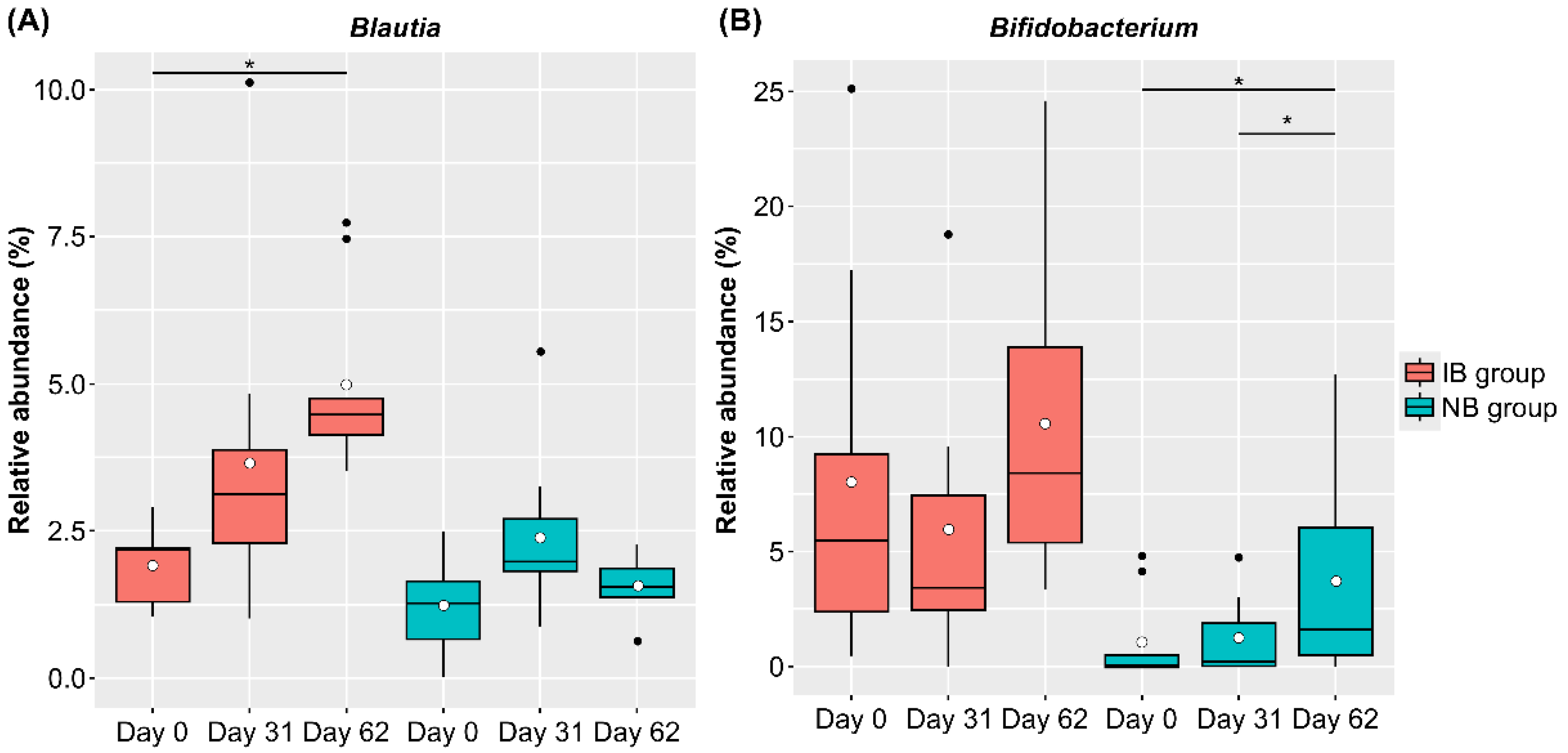
We also compared changes in the intestinal microbiota due to the intake of SONOMONO NATTO POWDER CAPSULESTM. Analysis of changes in CLR-transformed abundance at the genus level in each group showed a significant increase in Blautia in the IB group and a significant increase in Bifidobacterium in the NB group (Figure 6).
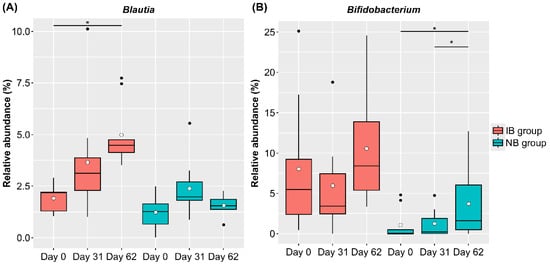
Figure 6.
Relative abundance of bacterial genera that changed significantly in the IB (increased Blautia) and NB (no change in Blautia) groups. Each dot represents outliers. (A) Blautia, (B) Bifidobacterium. Comparison of CLR-transformed abundance of each genus using the ALDEx2 pipeline showed that Blautia abundance in the IB group was significantly higher on Day 62 than on Day 0, while Bifidobacterium abundance in the NB group was significantly higher on Day 62 than on Day 0 and on Day 31. * p < 0.05.
There were no significant differences in defecation status between individual in the IB and NB groups. In addition, there was no significant difference in CLR-transformed abundance of Bifidobacterium between those who were in the habit of consuming fermented foods and those who were not in the habit.
3.5. Baseline-Specific Analysis of Bifidobacterium in Females in the LNC Group
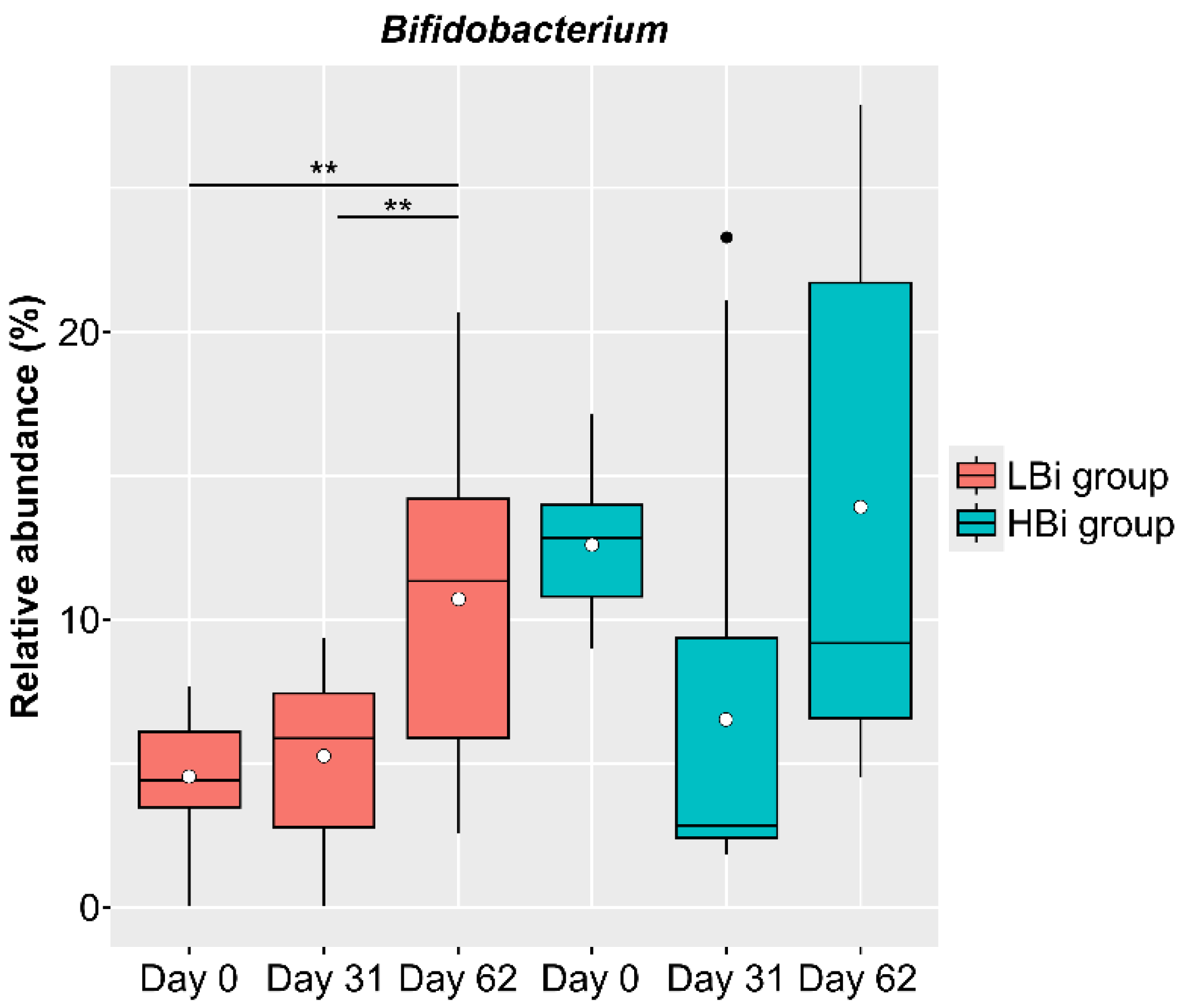
The baseline condition of the intestinal microbiota of females in the LNC group may also affect the changes observed in the intestinal microbiota following the ingestion of SONOMONO NATTO POWDER CAPSULESTM. Therefore, we compared the effects of ingesting SONOMONO NATTO POWDER CAPSULESTM on the intestinal microbiota by dividing the LNC group into two: one with high Bifidobacterium abundance on Day 0 and the other with low Bifidobacterium abundance on Day 0. The LBi (Low Bifidobacterium: n = 14) group included females whose relative Bifidobacterium abundance on Day 0 was below average, while the HBi (High Bifidobacterium: n = 9) group included females whose relative Bifidobacterium abundance was above average on Day 0. The results showed a significant increase in Bifidobacterium in the LBi group (Figure 7), but no significant change in the HBi group. Further analysis found no differences in the frequency of consumption of fermented foods between participants in the LBi and Hbi groups.
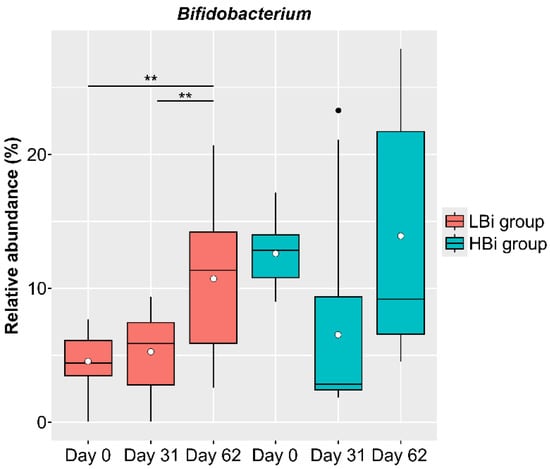
Figure 7.
Changes in relative abundance of Bifidobacterium in participants in LBi (low Bifidobacterium) and HBi (high Bifidobacterium) groups. Each dot represents outliers. Comparison of the abundance of each genus in the ALDEx2 pipeline showed a significant increase in abundance between Day 0 and 62 and between Day 31 and 62 in the LBi group. ** p < 0.01.
3.6. Effects of Ingesting SONOMONO NATTO POWDER CAPSULESTM Based on Analysis of the SymMAD Database
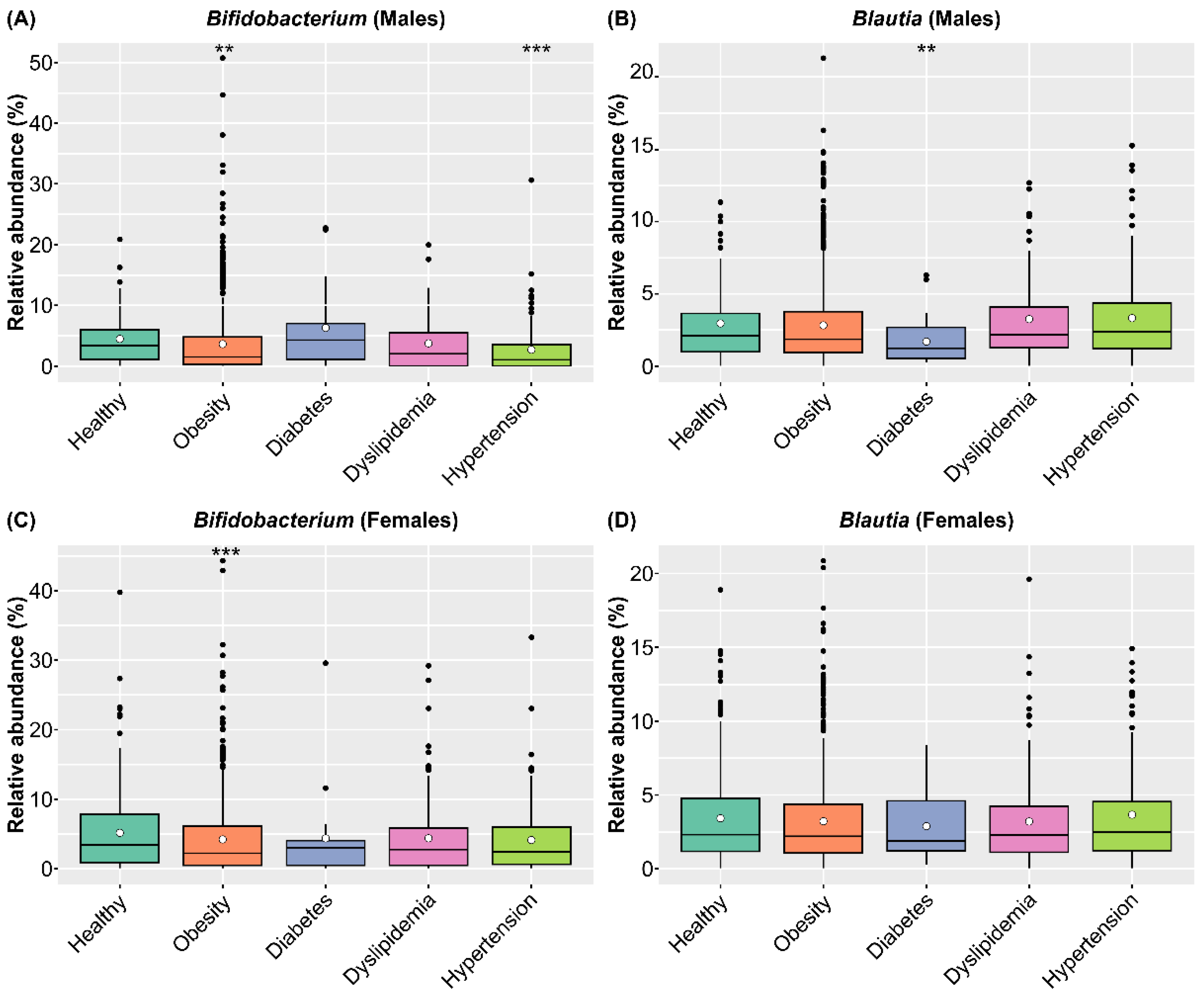
To clarify the medical effects of consuming SONOMONO NATTO POWDER CAPSULESTM, we compared the abundance of Bifidobacterium and Blautia in healthy and diseased participants in the SymMAD database. We included participants aged 40–79 years old with obesity (BMI ≥ 25), diabetes, dyslipidemia, and hypertension, which are major lifestyle-related diseases (Table S5). Comparison of intestinal microbiota of healthy and sick participants using the ALDEx2 pipeline showed that obese participants of both sexes had significantly lower abundance of Bifidobacterium compared with healthy participants. In males, individuals with hypertension had a significantly lower abundance of Bifidobacterium compared with healthy participants, while individuals with diabetes had significantly lower levels of Blautia compared with healthy participants (Figure 8 and Table S6).
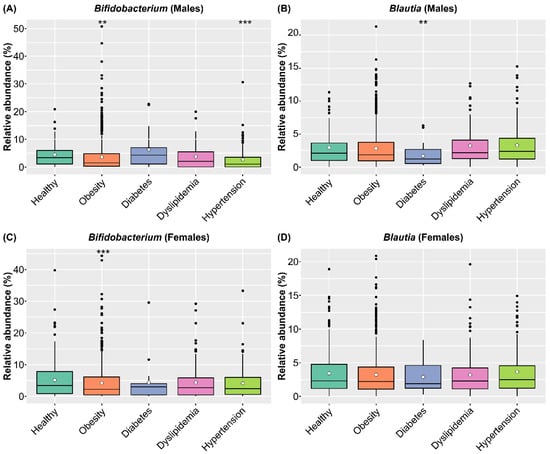
Figure 8.
Relative abundance of Bifidobacterium and Blautia in healthy participants and participants with different diseases in the SymMAD database. (A) Relative abundance of Bifidobacterium in males. (B) Relative abundance of Blautia in males. (C) Relative abundance of Bifidobacterium in females. (D) Relative abundance of Blautia in females. Each dot represents an outlier. The abundance of each genus in healthy and sick participants was compared using the ALDEx2 pipeline. Obese participants of both sexes had significantly lower abundance of Bifidobacterium compared with healthy participants. In males, participants with hypertension had significantly lower abundance of Bifidobacterium compared with healthy participants, while participants with diabetes had significantly lower levels of Blautia compared with healthy participants. ** p < 0.01 and *** p < 0.001.
4. Discussion
Males who ingested SONOMONO NATTO POWDER CAPSULESTM had a significant increase in body weight and BMI. However, the changes were within an appropriate range and did not affect the health status of the participants.
The α-diversity (Simpson diversity index) of the genus of the intestinal microbiota was significantly higher only in males in the LNC group, suggesting that SONOMONO NATTO POWDER CAPSULESTM may increase α-diversity in males. Analysis of β-diversity showed significant differences between both sexes in the LNC group. However, significant differences were also detected in the control group, and it is therefore not clear whether the change in β-diversity can be attributed to the SONOMONO NATTO POWDER CAPSULESTM.
Analysis of changes in the genera of the intestinal microbiota revealed that Bacteroides, Bifidobacterium, Blautia, Collinsella, Phocaeicola, and an unclassified genus were significantly altered in males in the LNC group, while Bifidobacterium, Faecalibacterium, Parabacteroides, and unclassified bacteria were significantly altered in females in the LNC group. Among these genera, we considered the genus that showed a significant difference in the only LNC group between Day 0 and 62 as the genus affected by ingestion. The genera whose abundances may have changed as a result of ingesting SONOMONO NATTO POWDER CAPSULESTM were Blautia and Bifidobacterium in males and Bifidobacterium in females, both of which showed an increase in relative abundance. Furthermore, Bacteroides, Collinsella, Phocaeicola, and unclassified bacteria in males and unclassified bacteria in females showed significant changes even in the control group, and the direction of increase or decrease was the same in the LNC and control groups; thus, the changes could not be attributed solely to the intake of SONOMONO NATTO POWDER CAPSULESTM. Although there were significant changes in the abundance of Faecalibacterium and Parabacteroides in females, the changes between Day 0 and 62 were not significant, and we could not conclude from this experiment alone that the changes in abundance were due to ingesting the capsules.
Bifidobacterium, which tended to increase in both sexes following the ingestion of SONOMONO NATTO POWDER CAPSULESTM, is a bacterium that breaks down sugar and produces acetic and lactic acids. Bifidobacterium abundance also increases with natto intake [14]. Soybeans, natto’s raw material, are rich in oligosaccharides such as stachyose and raffinose [19], which have been reported to increase Bifidobacterium abundance in human feces [20,21,22]. The ratio and content of soybean oligosaccharides change during the fermentation process of natto production, with stachyose and raffinose abundance decreasing and manninotriose abundance increasing [23,24]. Similar to stachyose and raffinose, manninotriose increases Bifidobacterium abundance in human feces [25]. Although the specific mechanism of action is largely unknown, it is believed that the probiotic action of Bacillus requires germination of Bacillus spores in the intestinal tract and metabolic activity of the cells [13,26]. Takemura et al. reported that B. subtilis MC1 spores reached the intestine in a live state following the ingestion of natto [27]. In addition, Hatanaka et al. used in vitro stomach and small intestine and colon models to show that B. subtilis C-3102 spores increase Bifidobacterium abundance in the stool, with 99% of the C-3102 spores being viable and 8% germinating [28]. Although no change was observed in Bacillus abundance in this study, it is possible that B. subtilis var. natto SONOMONO reached the intestine in a live state and contributed to the increase in Bifidobacterium abundance.
Comparing the dietary habits and intestinal microbiota of males with increased Blautia abundance (IB group) and no change in Blautia abundance (NB group) in the LNC group showed that individuals in the IB group consumed fermented foods, including natto, significantly more frequently and had significantly higher Bifidobacterium abundance at baseline. Relative Bifidobacterium abundance increased with the intake of natto [14] and amazake [29] containing koji and sake lees and kimchi [30]. Therefore, differences in the relative abundance of Bifidobacterium at baseline between participants in the IB and NB groups could be attributed to the frequency of consuming fermented foods, including natto.
The increase in Bifidobacterium abundance following the intake of natto is more pronounced in individuals with low baseline Bifidobacterium abundance [27]. Analysis of males in the LNC group whose Blautia abundance increased (IB group) and those whose Blautia abundance did not increase (NB group), and comparison of females in the LNC group who had high Bifidobacterium abundance (HBi group) and those who had low Bifidobacterium abundance (LBi group) at baseline revealed that the increase in Bifidobacterium due to SONOMONO NATTO POWDER CAPSULESTM intake was more pronounced in individuals with lower relative Bifidobacterium abundance at baseline.
Blautia, whose abundance was significantly higher in males following the ingestion of SONOMONO NATTO POWDER CAPSULESTM, consumes sugars and produces acetic, lactic, and succinic acids as metabolites [31,32,33]. This genus is relatively new, created in 2008 when certain species previously classified as Clostridium and Ruminococcus were reclassified into this genus [31]. Although omega-3 fatty acids [34], caffeine [35], and RS4-resistant starch [36] increase Blautia abundance, these components are not abundant in SONOMONO NATTO POWDER CAPSULESTM. Plichta et al. showed that the metabolic activity of Blautia hydrogenotrophica was activated in the presence of Bifidobacterium bifidum, suggesting the possibility of cross-feeding between B. hydrogenotrophica and Bi. Bifidum [37]. Although species-level analysis was not conducted in this study, the cross-feeding between Bifidobacterium and Blautia may be one of the reasons for the increase in Blautia in males with high Bifidobacterium abundance. In addition, although this study was conducted over a two-month period, when males with low relative Bifidobacterium abundance at baseline continuously ingested SONOMONO NATTO POWDER CAPSULESTM, Bifidobacterium abundance increased due to the effect of B. subtilis var. natto SONOMONO, leading to an increase in Blautia abundance due to the cross-feeding effect. Furthermore, there was no increase in Blautia abundance in females following ingestion of SONOMONO NATTO POWDER CAPSULESTM, indicating that the effects of ingestion differed between males and females. This may be attributed to the existence of sex differences in the intestinal microbiota [38].
Bifidobacterium support human health and protect against infection by producing antimicrobial peptides and intestinal pH-lowering acetic and lactic acids [39,40,41,42]. Moreover, they suppress pathogen growth [43], and induce immunostimulatory pathways via other molecular mechanisms [44,45]. Blautia is considered a useful bacteria because it is abundant in people with lower visceral fat area [46]. The relative abundance of Blautia is also lower in patients with colorectal cancer [47] and diabetes [48]. In addition, comparison of the intestinal microbiota of healthy people in 12 countries, including Japan, showed that Japanese people had the highest relative abundance of Blautia and Bifidobacterium [11]. Based on these observations, the increase in the relative abundance of Bifidobacterium and Blautia following the intake of SONOMONO NATTO POWDER CAPSULESTM is likely to improve the dysbiosis associated with decreased Bifidobacterium and Blautia, suggesting that it is useful as a probiotic product. In particular, it may be useful for improving the intestinal microbiota of people who consume fermented foods (including natto) infrequently in their daily diet and have low Bifidobacterium abundance. Furthermore, analysis of data from the large SymMAD database showed that obese participants of both sexes had significantly lower Bifidobacterium abundance compared with healthy participants. Among the males, participants with hypertension had significantly lower abundance of Bifidobacterium compared with healthy participants, while participants with diabetes had significantly lower levels of Blautia compared with healthy participants. This suggests that changes in the intestinal microbiota caused by the ingestion of SONOMONO NATTO POWDER CAPSULESTM may be effective at preventing and treating obesity, hypertension, and diabetes in Japanese populations.
In this study, we observed the changes in relative abundance of the intestinal microbiota by 16S rRNA metagenomic analysis. However, an increase or decrease in relative abundance does not necessarily correspond to an increase or decrease in the actual number of intestinal bacteria. Even if the relative abundance is increasing, the actual number of cells may remain unchanged or even decrease. Unfortunately, this study did not measure the number of cells in each intestinal bacterium and could not determine the actual changes in the number of intestinal bacteria. Future studies measuring not only the relative abundance but also the cell count of intestinal bacteria are needed for more detailed analysis of changes in the intestinal microbiota.
This study revealed that ingesting SONOMONO NATTO POWDER CAPSULESTM, which contains approximately 1.8 × 1010 CFUs of B. subtilis var. natto SONOMONO spores, increased the relative abundance of Bifidobacterium and Blautia in males and Bifidobacterium in females. Using the large-scale SymMAD database, we showed that changes in the intestinal microbiota caused by consuming SONOMONO NATTO POWDER CAPSULESTM may prevent or treat lifestyle-related diseases, including diabetes, whose incidence is on the rise among the residents of Kohoku Town. Additionally, the ability of SONOMONO NATTO POWDER CAPSULESTM to increase the abundance of Bifidobacterium and Blautia was dependent on the relative baseline abundance of Bifidobacterium in both males and females. This suggests that it is important to understand the intestinal microbiota prior to consuming food for the purpose of improving the intestinal microbiota, and to select food based on the contents of the intestinal microbiota.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14183839/s1, Table S1: Comparison of diversity (genus level) on Day 0; Table S2: Comparison of genus-level intestinal microbiota on Day 0; Table S3: Changes in β-diversity at the genus level; Table S4: Genera whose abundance differed significantly between IB and NB groups. Table S5: Sample size for each group; Table S6: Comparison of genus-level intestinal microbiota in each disease and healthy group.
Author Contributions
Conceptualization, K.S.; Data curation, A.E., K.O. (Kana Okuma), A.O., E.H. and K.S.; Formal Analysis and Visualization, K.K. and A.E.; Methodology, Y.M. and S.K.; Project administration, E.H., S.K. and S.M.; Resources, E.H.; Software, H.T.; Supervision, T.M. and K.S.; Writing—original draft, K.K., K.O. (Kazuya Ogasawara) and M.T.; Writing—review and editing, Y.M., K.O. (Kazuya Ogasawara), H.M., M.T. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Review Board of the sonomono Inc., Fukuoka, Japan (protocol no. 2020001; 2 October 2020).
Informed Consent Statement
Informed consent was obtained from all individuals involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
Acknowledgments
We would like to express our sincere gratitude to the following people for their help with this research: Kyosuke Yamada (Kohoku Town Mayor), all staff of Kohoku Town Hall, and the Kohoku Town Monitor who helped with our study. Yasuaki Kitahara (Chairman of the Organic Research Association of Kohoku Town) and Ryota Kitahara, who also assisted with our study. Takehiko Fujino and Mitsuo Koga who provided valuable advice on the research. We gratefully acknowledge the members of the former Benno Laboratory, RIKEN Cluster for Science, Technology and Innovation Hub, the Japan Agricultural Frontier Development Organization.
Conflicts of Interest
E.H. is the President of sonomono Inc. (Fukuoka, Japan) K.O. (Kazuya Ogasawara) is a part-time employee of sonomono Inc. H.M. is the President of Symbiosis Solutions Inc. (Tokyo, Japan). K.K., A.E., K.O. (Kana Okuma), H.T., and A.O. are employees of Symbiosis Solutions Inc.
References
- Moore, W.E.; Holdeman, L.V. Human Fecal Flora: The Normal Flora of 20 Japanese-Hawaiians. Appl. Microbiol. 1974, 27, 961–979. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Sakamoto, M.; Benno, Y. Phylogenetic Analysis of the Human Gut Microbiota Using 16S RDNA Clone Libraries and Strictly Anaerobic Culture-Based Methods. Microbiol. Immunol. 2002, 46, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; de Vos, W.M. The First 1000 Cultured Species of the Human Gastrointestinal Microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Zhu, S.; Wang, B.; Duan, L. Alterations of Gut Microbiota in Patients With Irritable Bowel Syndrome Based on 16S RRNA-Targeted Sequencing: A Systematic Review. Clin. Transl. Gastroenterol. 2019, 10, e00012. [Google Scholar] [CrossRef]
- Petrov, V.A.; Saltykova, I.V.; Zhukova, I.A.; Alifirova, V.M.; Zhukova, N.G.; Dorofeeva, Y.B.; Tyakht, A.V.; Kovarsky, B.A.; Alekseev, D.G.; Kostryukova, E.S.; et al. Analysis of Gut Microbiota in Patients with Parkinson’s Disease. Bull. Exp. Biol. Med. 2017, 162, 734–737. [Google Scholar] [CrossRef]
- Frémont, M.; Coomans, D.; Massart, S.; De Meirleir, K. High-Throughput 16S RRNA Gene Sequencing Reveals Alterations of Intestinal Microbiota in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Anaerobe 2013, 22, 50–56. [Google Scholar] [CrossRef]
- Kim, Y.A.; Keogh, J.B.; Clifton, P.M. Probiotics, Prebiotics, Synbiotics and Insulin Sensitivity. Nutr. Res. Rev. 2018, 31, 35–51. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Li, H.-Y.; Zhou, D.-D.; Gan, R.-Y.; Huang, S.-Y.; Zhao, C.-N.; Shang, A.; Xu, X.-Y.; Li, H.-B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Oki, K.; Toyama, M.; Banno, T.; Chonan, O.; Benno, Y.; Watanabe, K. Comprehensive Analysis of the Fecal Microbiota of Healthy Japanese Adults Reveals a New Bacterial Lineage Associated with a Phenotype Characterized by a High Frequency of Bowel Movements and a Lean Body Type. BMC Microbiol. 2016, 16, 284. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.-W.; Hirose, Y.; Morita, H.; Hattori, M. The Gut Microbiome of Healthy Japanese and Its Microbial and Functional Uniqueness. DNA Res. 2016, 23, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.-H.; Green-Johnson, J.M.; Buckley, N.D.; Lin, Q.-Y. Bioactivity of Soy-Based Fermented Foods: A Review. Biotechnol. Adv. 2019, 37, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, T. Probiotic Effects of Bacillus Subtilis (Natto). J. Brew. Soc. Japan. 2003, 98, 830–839. [Google Scholar] [CrossRef][Green Version]
- Terada, A.; Yamamoto, M.; Yoshimura, E. Effect of the Fermented Soybean Product “Natto” on the Composition and Metabolic Activity of the Human Fecal Flora. Jpn. J. Food Microbiol. 1999, 16, 221–230. [Google Scholar] [CrossRef][Green Version]
- Ishikawa, A.; Kishi, M.; Yamagami, K. Effect of Intake of Natto and Soybeans on Postprandial Blood Glucose Levels in Healthy Adults. SEIKATSU EISEI (J. Urban Living Health Assoc.) 2009, 53, 257–260. [Google Scholar] [CrossRef]
- Kokubo, Y.; Hurukawa, Y.; Banno, M. Prospective Study of the Preventive Effect of Intensive Dietary Soy Intake on Atherosclerosis in an Urban General Population(Part 2). Soy Protein Res. Japan 2012, 15, 6–12. [Google Scholar]
- Hayashi, H.; Sakamoto, M.; Benno, Y. Evaluation of Three Different Forward Primers by Terminal Restriction Fragment Length Polymorphism Analysis for Determination of Fecal Bifidobacterium Spp. in Healthy Subjects. Microbiol. Immunol. 2004, 48, 1–6. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Holmes, S.P. Exact Sequence Variants Should Replace Operational Taxonomic Units in Marker-Gene Data Analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef] [PubMed]
- Švejstil, R.; Musilová, Š.; Rada, V. Raffinose-Series Oligosaccharides in Soybean Products. Sci. Agric. Bohem. 2015, 46, 73–77. [Google Scholar] [CrossRef]
- Benno, Y.; Endo, K.; Shiragami, N.; Sayama, K.; Mitsuokai, T. Effects of Raffinose Intake on Human Fecal Microflora. Bifidobact. Microflora 1987, 6, 59–63. [Google Scholar] [CrossRef][Green Version]
- Hayakawa, K.; Mizutani, J.; Wada, K.; Masai, T.; Yoshihara, I.; Mitsuoka, T. Effects of Soybean Oligosaccharides on Human Faecal Flora. Microb. Ecol. Health Dis. 1990, 3, 293–303. [Google Scholar] [CrossRef]
- Bouhnik, Y.; Raskine, L.; Simoneau, G.; Vicaut, E.; Neut, C.; Flourié, B.; Brouns, F.; Bornet, F.R. The Capacity of Nondigestible Carbohydrates to Stimulate Fecal Bifidobacteria in Healthy Humans: A Double-Blind, Randomized, Placebo-Controlled, Parallel-Group, Dose-Response Relation Study. Am. J. Clin. Nutr 2004, 80, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Takamatsu, H.; Takano, N.; Akimoto, T. Change of Saccharides in Soybeans during Manufacturing of Natto. J. Jpn. Soc. Food Sci. Technol. 1982, 29, 105–110. [Google Scholar] [CrossRef]
- Ishikawa, C.; Kodama, S.; Takagaki, R.; Morimitsu, Y. Factors Affecting Manninotriose Concentration in Fermented Soybean Foods. J. Jpn. Soc. Food Sci. Technol. 2020, 67, 58–66. [Google Scholar] [CrossRef]
- Wada, K.; Mizutani, J.; Suzuki, H.; Hayakawa, K. Effect of Manninotriose on Human Fecal Microflora. JSNFS 1991, 44, 171–176. [Google Scholar] [CrossRef][Green Version]
- Hosoi, T.; Ametani, A.; Kiuchi, K.; Kaminogawa, S. Changes in Fecal Microflora Induced by Intubation of Mice with Bacillus Subtilis (Natto) Spores Are Dependent upon Dietary Components. Can. J. Microbiol. 1999, 45, 59–66. [Google Scholar] [CrossRef]
- Takemura, H.; Shioya, N.; Komori, Y.; Tho, Y. Effect of Fermented Soybeans Containing Bacillus Subtilis MC1 on Defecation, Fecal Properties and Fecal Microflora of Healthy Female Volunteers. SEIKATSU EISEI (J. Urban Living Health Assoc.) 2009, 53, 11–18. [Google Scholar] [CrossRef]
- Hatanaka, M.; Nakamura, Y.; Maathuis, A.; Venema, K.; Murota, I.; Yamamoto, N. Influence of Bacillus Subtilis C-3102 on Microbiota in a Dynamic in Vitro Model of the Gastrointestinal Tract Simulating Human Conditions. Benef. Microbes 2012, 3, 229–236. [Google Scholar] [CrossRef]
- Sadao, M.; Minoru, M.; Tsuneo, M. Effect of Oral Intake of Amazake Containing Sake Lees and Rice Koji on the Human Intestinal Microbiota of Amazake Using-The Randomized Placebo-Controlled Crossover Comparison Study. JPT 2020, 48, 1187–1193. [Google Scholar]
- Han, K.; Bose, S.; Wang, J.-H.; Kim, B.-S.; Kim, M.; Kim, E.; Kim, H. Contrasting Effects of Fresh and Fermented Kimchi Consumption on Gut Microbiota Composition and Gene Expression Related to Metabolic Syndrome in Obese Korean Women. Mol. Nutr. Food Res. 2015, 59, 1004–1008. [Google Scholar] [CrossRef]
- Liu, C.; Finegold, S.M.; Song, Y.; Lawson, P.A.Y. Reclassification of Clostridium Coccoides, Ruminococcus Hansenii, Ruminococcus Hydrogenotrophicus, Ruminococcus Luti, Ruminococcus Productus and Ruminococcus Schinkii as Blautia Coccoides Gen. Nov., Comb. Nov., Blautia Hansenii Comb. Nov., Blautia Hydrogenotrophica Comb. Nov., Blautia Luti Comb. Nov., Blautia Producta Comb. Nov., Blautia Schinkii Comb. Nov. and Description of Blautia Wexlerae Sp. Nov., Isolated from Human Faeces. Int. J. Syst. Evol. Microbiol. 2008, 58, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Ezaki, T.; Li, N.; Kawamura, Y. The Anaerobic Gram-Positive Cocci. In The Prokaryotes: Volume 4: Bacteria: Firmicutes, Cyanobacteria; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 795–808. ISBN 978-0-387-30744-2. [Google Scholar]
- Simmering, R.; Taras, D.; Schwiertz, A.; Le Blay, G.; Gruhl, B.; Lawson, P.A.; Collins, M.D.; Blaut, M. Ruminococcus Luti Sp. Nov., Isolated from a Human Faecal Sample1 1The GenBank/EMBL/DDBJ Accession Number for the 16S RRNA Gene Sequence of Ruminococcus Luti Strain DSM 14534T Is AJ133124. Syst. Appl. Microbiol. 2002, 25, 189–193. [Google Scholar] [CrossRef]
- Noriega, B.S.; Sanchez-Gonzalez, M.A.; Salyakina, D.; Coffman, J. Understanding the Impact of Omega-3 Rich Diet on the Gut Microbiota. Case Rep. Med. 2016, 2016, e3089303. [Google Scholar] [CrossRef] [PubMed]
- Gurwara, S.; Dai, A.; Ajami, N.; El-Serag, H.B.; Graham, D.Y.; Jiao, L. 196 Caffeine Consumption and the Colonic Mucosa-Associated Gut Microbiota. Am. J. Gastroenterol. 2019, 114, S119. [Google Scholar] [CrossRef]
- Upadhyaya, B.; McCormack, L.; Fardin-Kia, A.R.; Juenemann, R.; Nichenametla, S.; Clapper, J.; Specker, B.; Dey, M. Impact of Dietary Resistant Starch Type 4 on Human Gut Microbiota and Immunometabolic Functions. Sci. Rep. 2016, 6, 28797. [Google Scholar] [CrossRef]
- Plichta, D.R.; Metagenomics of the Human Intestinal Tract (MetaHIT) Consortium; Juncker, A.S.; Bertalan, M.; Rettedal, E.; Gautier, L.; Varela, E.; Manichanh, C.; Fouqueray, C.; Levenez, F.; et al. Transcriptional Interactions Suggest Niche Segregation among Microorganisms in the Human Gut. Nat. Microbiol. 2016, 1, 16152. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Unno, T.; Kim, B.-Y.; Park, M.-S. Sex Differences in Gut Microbiota. World J. Men’s Health 2019, 38, 48–60. [Google Scholar] [CrossRef]
- Lim, H.J.; Shin, H.S. Antimicrobial and Immunomodulatory Effects of Bifidobacterium Strains: A Review. J. Microbiol. Biotechnol. 2020, 30, 1793–1800. [Google Scholar] [CrossRef]
- Natividad, J.M.M.; Hayes, C.L.; Motta, J.-P.; Jury, J.; Galipeau, H.J.; Philip, V.; Garcia-Rodenas, C.L.; Kiyama, H.; Bercik, P.; Verdu, E.F. Differential Induction of Antimicrobial REGIII by the Intestinal Microbiota and Bifidobacterium Breve NCC2950. Appl. Environ. Microbiol. 2013, 79, 7745–7754. [Google Scholar] [CrossRef]
- Moreno Muñoz, J.A.; Chenoll, E.; Casinos, B.; Bataller, E.; Ramón, D.; Genovés, S.; Montava, R.; Ribes, J.M.; Buesa, J.; Fàbrega, J.; et al. Novel Probiotic Bifidobacterium Longum Subsp. Infantis CECT 7210 Strain Active against Rotavirus Infections. Appl. Environ. Microbiol. 2011, 77, 8775–8783. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria Can Protect from Enteropathogenic Infection through Production of Acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Fanning, S.; Hall, L.J.; Cronin, M.; Zomer, A.; MacSharry, J.; Goulding, D.; O’Connell Motherway, M.; Shanahan, F.; Nally, K.; Dougan, G.; et al. Bifidobacterial Surface-Exopolysaccharide Facilitates Commensal-Host Interaction through Immune Modulation and Pathogen Protection. Proc. Natl. Acad. Sci. USA 2012, 109, 2108–2113. [Google Scholar] [CrossRef]
- López, P.; González-Rodríguez, I.; Sánchez, B.; Gueimonde, M.; Margolles, A.; Suárez, A. Treg-Inducing Membrane Vesicles from Bifidobacterium Bifidum LMG13195 as Potential Adjuvants in Immunotherapy. Vaccine 2012, 30, 825–829. [Google Scholar] [CrossRef] [PubMed]
- López, P.; de Paz, B.; Rodríguez-Carrio, J.; Hevia, A.; Sánchez, B.; Margolles, A.; Suárez, A. Th17 Responses and Natural IgM Antibodies Are Related to Gut Microbiota Composition in Systemic Lupus Erythematosus Patients. Sci. Rep. 2016, 6, 24072. [Google Scholar] [CrossRef] [PubMed]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Kakuta, M.; Imoto, S.; Ihara, K.; et al. Blautia Genus Associated with Visceral Fat Accumulation in Adults 20–76 Years of Age. NPJ Biofilms Microbiomes 2019, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human Intestinal Lumen and Mucosa-Associated Microbiota in Patients with Colorectal Cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).