Ketogenic Diet in the Treatment of Gliomas and Glioblastomas
Abstract
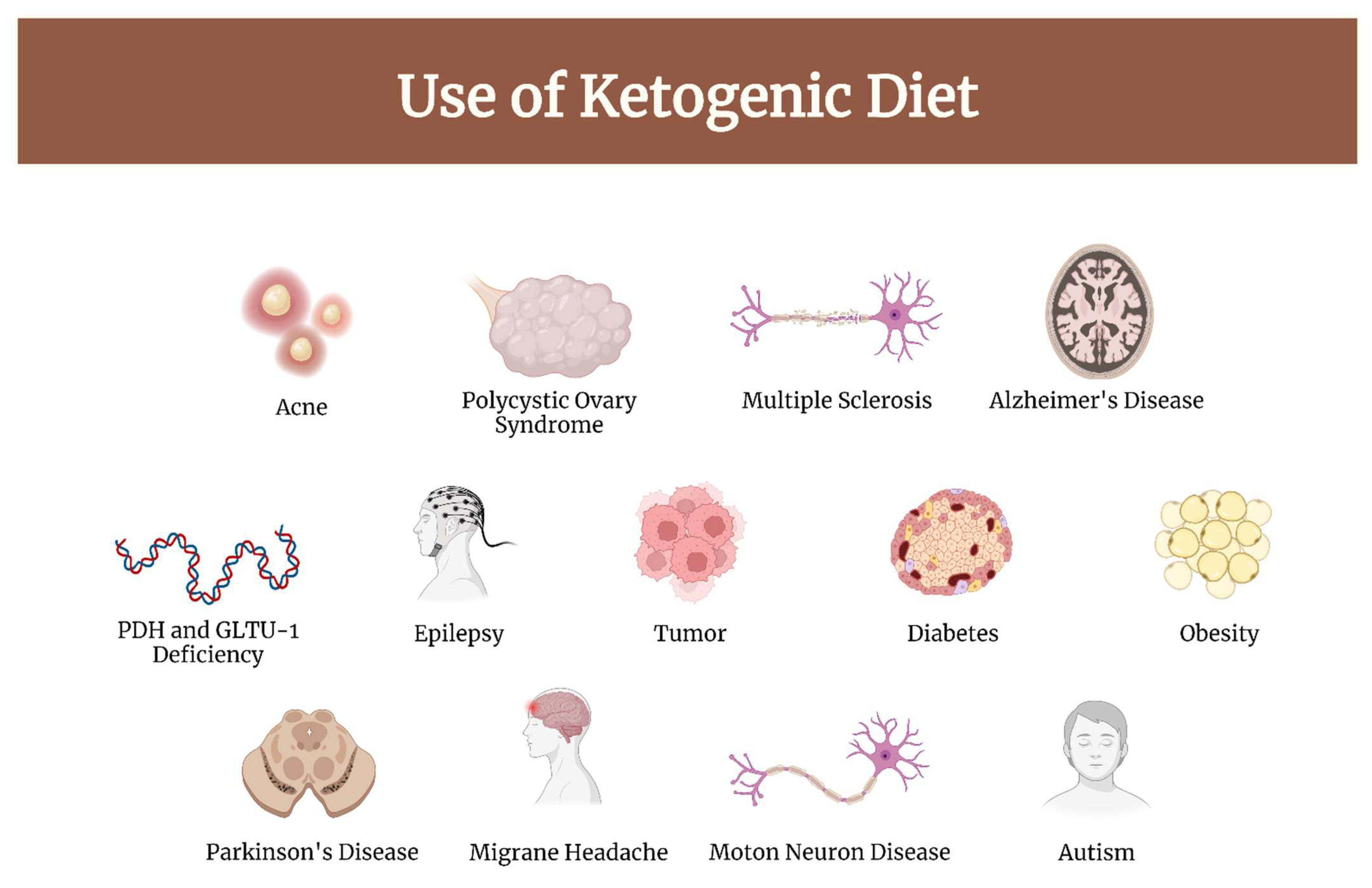
1. Introduction
- The Classic Ketogenic Diet (CKD), in which the ratio between fats and non-fats (carbohydrates + proteins) must be calculated; generally, this ratio is 3:1 or 4:1 (i.e., the intake in grams of fats is three or four times that of non-fats). This protocol is characterized by the higher content of fats compared to the protein portion (slightly reduced or normal) and carbohydrates (greatly reduced) [12];
- Modified Atkins Diet (MAD): the Atkins diet was created in the 1970s as a means to combat obesity. This diet allows more protein, and calories are not closely monitored. You can also start it without a fast. Calorie intake is 60% from fat, 30% from protein, and 10% from carbohydrates [12];
- Medium-Chain Triglyceride (MCT): Dr. Peter Huttenlocher and his group replaced some of the long-chain fat in the classic ketogenic diet—that is, fat from foods such as butter, oils, cream, and mayonnaise—with an alternative fat source with a shorter carbon chain length. This medium-chain fat, otherwise known as medium-chain triglyceride (MCT), is absorbed more efficiently than long-chain fat, is carried directly to the liver in the portal blood, and does not require carnitine to facilitate transport into cell mitochondria for oxidation. Because of these metabolic effects, MCT will yield more ketones per kilocalorie of energy than its long-chain counterparts. This increased ketogenic potential means less total fat is needed in the MCT diet. Whereas the classical 4:1 ratio ketogenic diet provides 90% of energy from fat, the MCT ketogenic diet typically provides 70% to 75% of energy from fat (both MCT and long-chain), allowing more protein and carbohydrate food to be included [5];
- The Very Low-Calorie Ketogenic Diet (VLCKD), an extremely restrictive nutritional protocol (600–800 kcal), limited in time (up to 12 weeks), characterized by a minimum protein content (≥75 g/day), a very limited carbohydrate content (30–50 g/day), a fixed amount of fat (20 g/day, mainly from olive oil and omega-3), and micronutrients to meet the Dietary Reference Intake (DRI), in accordance with the European Food Safety Authority (EFSA) [13].
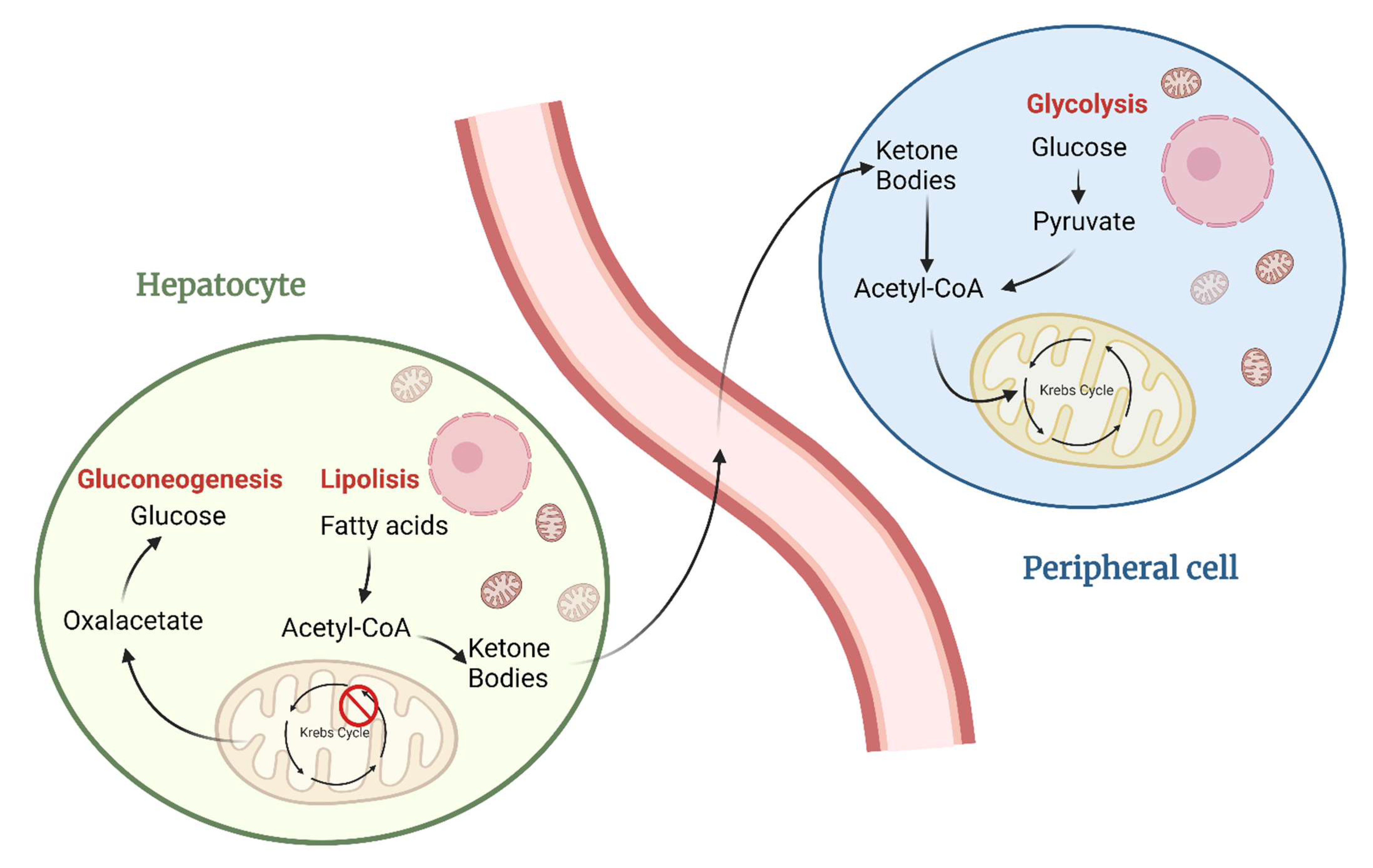
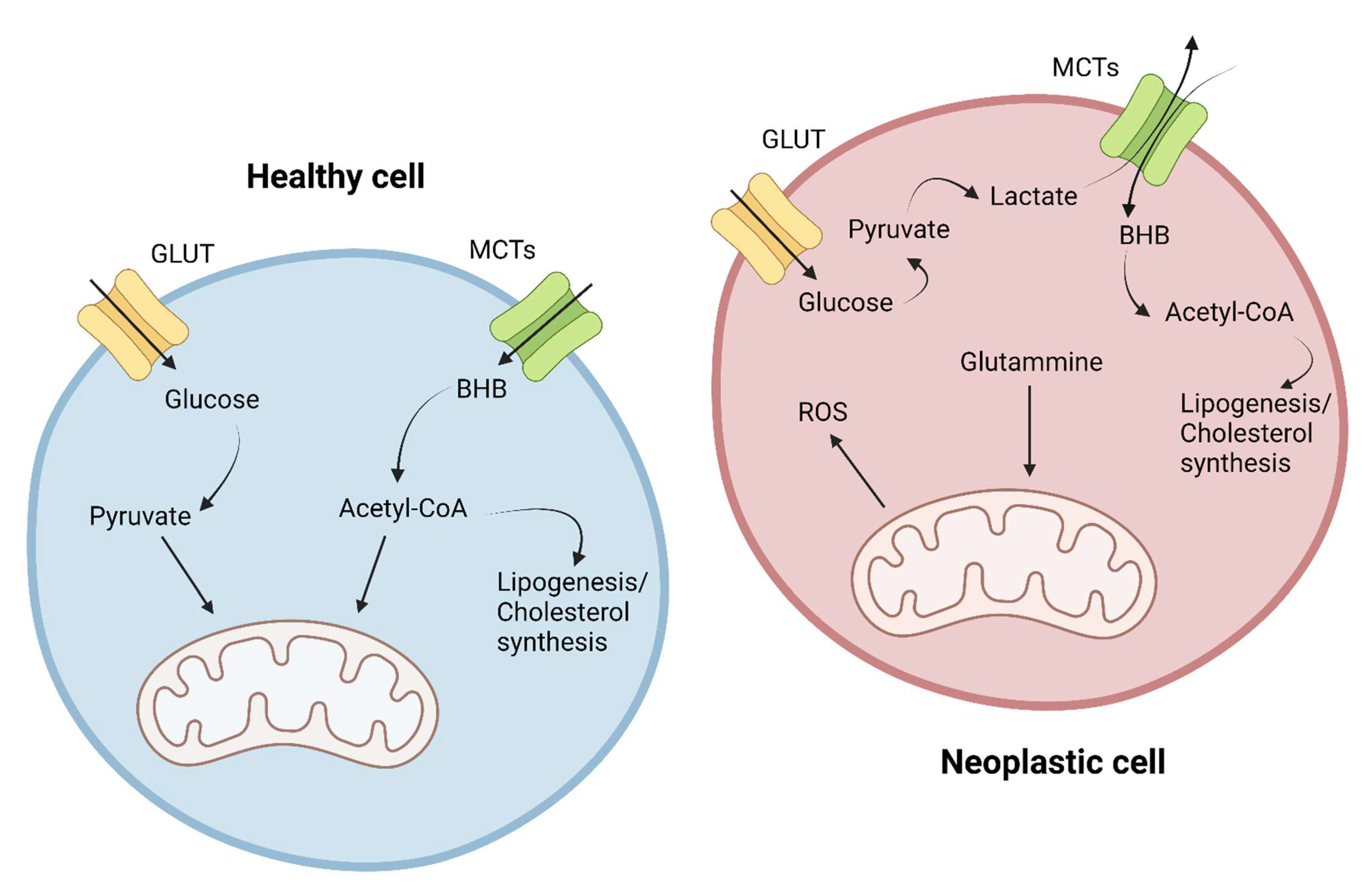
2. Metabolic Differences between Healthy and Cancer Cells
- Glutamine: a crucial amino acid used as a mitochondrial energy source for the synthesis of various molecules and for its antioxidant power through the synthesis of glutathione. Glutamate produced from glutamine can also be used as a starting substrate for the synthesis of other nonessential amino acids such as aspartate, alanine, arginine, and proline. In addition, by reductive carboxylation, glutamine is converted to citrate and is furthermore used for lipid synthesis. Glutamine is also a source of nitrogen for the biosynthesis of glycosylated molecules and nucleotides [14,19];
- Leucine: essential amino acid crucial for protein synthesis [21];
- Aspartate: amino acid fundamental for cellular protein synthesis and nucleotide biosynthesis, which is crucial especially in cells with a high proliferative index; the increase in its synthesis appears to be related to mitochondrial dysregulation [14].
3. Ketogenic Diet Targets
3.1. Metabolic Targets
3.2. Inflammation
3.3. Oncogenes and Tumor Suppressors
3.4. ROS
3.5. Epigenetic Modulation
4. Pre-Clinical Studies
5. Clinical Studies
6. Ketogenic Diet and Steroids
7. The Anti-Epileptic Effect of the Ketogenic Diet
8. Positive Effects, Negative Effects, Side Effects, and Doubts of KD
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMP kinase |
| AQP-4 | Aquaporin-4 |
| BHB | β-hydroxybutyrate |
| CD86 | Cluster of Differentiation 86 |
| CKD | Classic ketogenic diet |
| CNS | Central nervous system |
| COX-2 | Prostaglandin-endoperoxide synthase 2 |
| CR | Caloric restriction |
| CT | Chemotherapy |
| DON | 6-diazo-5-oxo-1-norleucine |
| DVT | Deep vein thrombosis |
| EFSA | European Food Safety Authority |
| EGF | Epidermal growth factor |
| EPO | Erythropoietin |
| GABA | Gamma-aminobutyric acid |
| GBM | Glioblastoma multiforme |
| GHS | Global health status |
| GLUT | Glucose transporter |
| HIF-1 | Hypoxia-inducible factor 1 |
| IDH | Isocitrate dehydrogenase |
| IGF-1 | Insulin-like growth factor 1 |
| KB | Ketone bodies |
| KD | Ketogenic diet |
| LKB1 | Serine/threonine kinase 11 (STK11), also known as liver kinase B1 (LKB1) |
| MAD | Modified Atkins diet |
| MCT | Medium-chain triglyceride |
| MCTs | Monocarboxylate transporter |
| miRNAs | microRNAs |
| MKD | Modified ketogenic diet |
| MMP | Matrix metalloproteinases |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NLR family pyrin domain containing 3 |
| NOS | Nitric oxide synthase |
| NSAID | Non-steroidal anti-inflammatory drugs |
| PD-L1 | Programmed death-ligand 1 |
| PDGFR | Platelet-derived growth factor receptors |
| PHD | Pyruvate dehydrogenase complex |
| PFS | Progression-free survival |
| PI3K | Phosphoinositide 3-kinases |
| ROS | Reactive oxygen species |
| RT | Radiotherapy |
| SD | Standard diet |
| TNF-alpha | Tumor necrosis factor alpha |
| UCP-2 | Mitochondrial uncoupling protein |
| VEGF | Vascular-endothelial growth factor |
| VLCKD | Very low-calorie ketogenic diet |
References
- Ostrom, Q.T.; Francis, S.S.; Barnholtz-Sloan, J.S. Epidemiology of Brain and Other CNS Tumors. Curr. Neurol. Neurosci. Rep. 2021, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Debinski, W. Gliomas; Exon publications: Brisbane, Australia, 2021. [Google Scholar]
- Sargaço, B.; Oliveira, P.A.; Antunes, M.L.; Moreira, A.C. Effects of the Ketogenic Diet in the Treatment of Gliomas: A Systematic Review. Nutrients 2022, 14, 1007. [Google Scholar] [CrossRef] [PubMed]
- Kossoff, E.H. More fat and fewer seizures: Dietary therapies for epilepsy. Lancet Neurol. 2004, 3, 415–420. [Google Scholar] [CrossRef]
- McDonald, T.J.W.; Cervenka, M.C. Ketogenic Diets for adult Neurological Disorders. Neurotherapeutics 2018, 15, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Vidali, S.; Aminzadeh, S.; Lambert, B.; Rutherford, T.; Sperl, W.; Kofler, B.; Feichtinger, R.G. Mitochondria: The ketogenic diet—A metabolism-based therapy. Int. J. Biochem. Cell Biol. 2015, 63, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.; Garbo, R.; Filippi, F.; Antonutti, A.; Ceccarini, V.; Tereshko, Y.; Di Lorenzo, C.; Gigli, G.L. Migraine Prevention through Ketogenic Diet: More than Body Mass Composition Changes. J. Clin. Med. Artic. 2022, 11, 4946. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Ballerini, G.; Barbanti, P.; Bernardini, A.; D’Arrigo, G.; Egeo, G.; Frediani, F.; Garbo, R.; Pierangeli, G.; Prudenzano, M.P.; et al. Applications of ketogenic diets in patients with headache: Clinical recommendations. Nutrients 2021, 13, 2307. [Google Scholar] [CrossRef]
- Longo, R.; Peri, C.; Cricrì, D.; Coppi, L.; Caruso, D.; Mitro, N.; De Fabiani, E.; Crestani, M. Ketogenic diet: A new light shining on old but gold biochemistry. Nutrients 2019, 11, 2497. [Google Scholar] [CrossRef]
- Dąbek, A.; Wojtala, M.; Pirola, L.; Balcerczyk, A. Modulation of Cellular Biochemistry, Epigenetics and Metabolomics by Ketone Bodies. Implications of the Ketogenic Diet in the Physiology of the Organism and Pathological States. Nutrients 2020, 12, 788. [Google Scholar] [CrossRef]
- Grabacka, M.; Pierzchalska, M.; Dean, M.; Reiss, K. Regulation of ketone body metabolism and the role of PPARα. Int. J. Mol. Sci. 2016, 17, 2093. [Google Scholar] [CrossRef]
- Kossoff, B.; Turnern, W.; Doerrer, Z.; Cervenka, S.; Henry, M. The Ketogenic and Modified Atkins Diet Treatments for Epilepsy and Other Disorders, 6th ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Caprio, M.; Infante, M.; Moriconi, E.; Armani, A.; Fabbri, A.; Mantovani, G.; Mariani, S.; Lubrano, C.; Poggiogalle, E.; Migliaccio, S.; et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: Systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2019, 42, 1365–1386. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B. Teaching the basics of cancer metabolism: Developing antitumor strategies by exploiting the differences between normal and cancer cell metabolism. Redox Biol. 2017, 12, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Warburg, N.E.; Wind, F.O. The metabolism of tumours in the body. J. Gen. Physiol. 1926, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 5th ed.; W. H. Freeman and Company: New York, NY, USA, 2008; pp. 540–541. [Google Scholar]
- Nelson, D.L.; Cox, M.M. I Principi di Biochimica di Lehninger, 5th ed.; Zanichelli editore S.p.A.: Bologna, Italy, 2010. [Google Scholar]
- Bott, A.J.; Maimouni, S.; Zong, W.-X. The pleiotropic effects of glutamine metabolism in cancer. Cancers 2019, 11, 770. [Google Scholar] [CrossRef]
- Mattaini, K.R.; Sullivan, M.R.; Vander Heiden, M.G. The importance of serine metabolism in cancer. J. Cell Biol. 2016, 214, 249–257. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, C.; Yin, H.; Yu, J.; Chen, S.; Fang, J. Leucine deprivation inhibits proliferation and induces apoptosis of human breast cancer cells via fatty acid synthase. Oncotarget 2016, 7, 63679–63689. [Google Scholar] [CrossRef]
- McDonald, T.; Cervenka, M. The expanding role of Ketogenic diets in adult neurological disorders. Brain Sci. 2018, 8, 148. [Google Scholar] [CrossRef]
- Barrea, L.; Caprio, M.; Tuccinardi, D.; Moriconi, E.; Di Renzo, L.; Muscogiuri, G.; Colao, A.; Savastano, S. Could ketogenic diet ‘starve’ cancer? Emerging evidence. 2020, 1–22. [Google Scholar] [CrossRef]
- Poff, A.; Ari, C.; Arnold, P.; Seyfried, T.; D’Agostino, D. Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer. Int. J. Cancer 2014, 135, 1711–1720. [Google Scholar] [CrossRef]
- Wong, N.; Ojo, D.; Yan, J.; Tang, D. PKM2 contributes to cancer metabolism. Cancer Lett. 2015, 356, 184–191. [Google Scholar] [CrossRef]
- Luo, W.; Hu, H.; Chang, R.; Zhong, J.; Knabel, M.; O’Meally, R.; Cole, R.N.; Pandey, A.; Semenza, G.L. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 2011, 145, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.C.; Hu, Y.; Cheng, G.; Liang, L.; Gao, B.; Ren, Y.; Liu, J.; Cao, X.; Zheng, M.; Li, S.; et al. A ketogenic diet attenuates proliferation and stemness of glioma stem-like cells by altering metabolism resulting in increased ROS production. Int. J. Oncol. 2019, 56, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Woolf, E.C.; Curley, K.L.; Liu, Q.; Turner, G.H.; Charlton, J.A.; Preul, M.C.; Scheck, A.C. The ketogenic diet alters the hypoxic response and affects expression of proteins associated with angiogenesis, invasive potential and vascular permeability in a mouse glioma model. PLoS ONE 2015, 10, e0130357. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S.; Sandur, S.K.; Pandey, M.K.; Sethi, G. Inflammation and cancer: How hot is the link? Biochem. Pharmacol. 2006, 72, 1605–1621. [Google Scholar] [CrossRef]
- Cullingford, T.E. The ketogenic diet; fatty acids, fatty acid-activated receptors and neurological disorders. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 253–264. [Google Scholar] [CrossRef]
- Mukherjee, P.; Augur, Z.M.; Li, M.; Hill, C.; Greenwood, B.; Domin, M.A.; Kondakci, G.; Narain, N.R.; Kiebish, M.A.; Bronson, R.T.; et al. Therapeutic benefit of combining calorie-restricted ketogenic diet and glutamine targeting in late-stage experimental glioblastoma. Commun. Biol. 2019, 2, 200. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kanneganti, T.D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y. Aging-related gene signature regulated by Nlrp3 predicts glioma progression. Am. J. Cancer Res. 2015, 5, 442–449. [Google Scholar]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.-D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Lussier, D.M.; Woolf, E.C.; Johnson, J.L.; Brooks, K.S.; Blattman, J.N.; Scheck, A.C. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer 2016, 16, 310. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, T.N.; Shelton, L.; Arismendi-Morillo, G.; Kalamian, M.; Elsakka, A.; Maroon, J.; Mukherjee, P. Provocative Question: Should Ketogenic Metabolic Therapy Become the Standard of Care for Glioblastoma? Neurochem. Res. 2019, 44, 2392–2404. [Google Scholar] [CrossRef]
- Elsakka, A.M.A.; Bary, M.A.; Abdelzaher, E.; Elnaggar, M.; Kalamian, M.; Mukherjee, P.; Seyfried, T.N. Management of Glioblastoma Multiforme in a Patient Treated With Ketogenic Metabolic Therapy and Modified Standard of Care: A 24-Month Follow-Up. Front. Nutr. 2018, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, T.; Nakagawara, A. Role of p53 in cell death and human cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef]
- Yue, X.; Zhao, Y.; Xu, Y.; Zheng, M.; Feng, Z.; Hu, W. Mutant p53 in Cancer: Accumulation, Gain-of-Function, and Therapy. J. Mol. Biol. 2017, 429, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, F.; Sun, Q.; Lin, N.; Han, H.; You, K.; Tian, F.; Mao, Z.; Li, T.; Tong, T.; et al. P53 Β-Hydroxybutyrylation Attenuates P53 Activity. Cell Death Dis. 2019, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Mahmod, A.I.; Kamal, A.; Rashid, H.M.; Alashqar, A.M.D.; Khater, S.; Jamal, D.; Waly, M. Ketogenic diet in cancer prevention and therapy: Molecular targets and therapeutic opportuities. Curr. Issues Mol. Biol. 2021, 43, 558–589. [Google Scholar] [CrossRef]
- Li, W.; Saud, S.M.; Young, M.R.; Chen, G.; Hua, B. Targeting AMPK for cancer prevention and treatment. Oncotarget 2015, 6, 7365–7378. [Google Scholar] [CrossRef]
- Scheck, A.C.; Abdelwahab, M.; Stafford, P.; Kim, D.; Iwai, S.; Preul, M.C.; Rho, J.M. Mechanistic studies of the ketogenic diet as an adjuvant therapy for malignant gliomas. Cancer Res. 2010, 70, 638. [Google Scholar] [CrossRef]
- Strowd, R.E.; Cervenka, M.C.; Henry, B.J.; Kossoff, E.H.; Hartman, A.L.; Blakeley, J.O. Glycemic modulation in neuro-oncology: Experience and future directions using a modified Atkins diet for high-grade brain tumors. Neuro-Oncol. Pract. 2015, 2, 127–136. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Pauli, C.; Du, X.; Wang, D.G.; Li, X.; Wu, D.; Amadiume, S.C.; Goncalves, M.D.; Hodakoski, C.; Lundquist, M.R.; et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 2018, 560, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Vendramin, R.; Litchfield, K.; Swanton, C. Cancer evolution: Darwin and beyond. EMBO J. 2021, 40, e108389. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, C.; Huang, Y.; He, M.; Xu, W.W.; Li, B. Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. MedComm 2021, 2, 315–340. [Google Scholar] [CrossRef] [PubMed]
- Alfarouk, K.O.; Ahmed, S.B.M.; Elliott, R.L.; Benoit, A.; Alqahtani, S.S.; Ibrahim, M.E.; Bashir, A.H.H.; AlHoufie, S.T.S.; Elhassan, G.O.; Wales, C.C.; et al. The pentose phosphate pathway dynamics in cancer and its dependency on intracellular pH. Metabolites 2020, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Fine, E.J.; Miller, A.; Quadros, E.V.; Sequeira, J.M.; Feinman, R.D. Acetoacetate reduces growth and ATP concentration in cancer cell lines which over-express uncoupling protein 2. Cancer Cell Int. 2009, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Klement, R.J.; Champ, C.E. Calories, carbohydrates, and cancer therapy with radiation: Exploiting the five R’s through dietary manipulation. Cancer Metastasis Rev. 2014, 33, 217–229. [Google Scholar] [CrossRef]
- Boison, D. New insights into the mechanisms of the ketogenic diet. Curr. Opin. Neurol. 2017, 30, 187–192. [Google Scholar] [CrossRef]
- Stafford, P.; Abdelwahab, M.G.; Kim, D.Y.; Preul, M.C.; Rho, J.M.; Scheck, A.C. The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr. Metab. 2010, 7, 74. [Google Scholar] [CrossRef]
- Shea, A.; Harish, V.; Afzal, Z.; Chijioke, J.; Kedir, H.; Dusmatova, S.; Roy, A.; Ramalinga, M.; Harris, B.; Blancato, J.; et al. MicroRNAs in glioblastoma multiforme pathogenesis and therapeutics. Cancer Med. 2016, 5, 1917–1946. [Google Scholar] [CrossRef]
- Møller, H.G.; Rasmussen, A.P.; Andersen, H.H.; Johnsen, K.B.; Henriksen, M.; Duroux, M. A systematic review of MicroRNA in glioblastoma multiforme: Micro-modulators in the mesenchymal mode of migration and invasion. Mol. Neurobiol. 2012, 47, 131–144. [Google Scholar] [CrossRef]
- Preston, M.J.; Stylianou, J.; Zeng, M.Q.; Glover, M.S.; Scheck, A.C.; Woolf, E.C.; O’Neill, K.; Syed, N. The ketogenic diet induces epigenetic changes that play key roles in tumour development. Neuro-Oncology 2017, 19, i28. [Google Scholar] [CrossRef][Green Version]
- Zeng, Q.; Stylianou, T.; Preston, J.; Glover, S.; O’Neill, K.; Woolf, E.C.; Scheck, A.C.; Syed, N. The ketogenic diet alters the epigenetic landscape of gbm to potentiate the effects of chemotherapy and radiotherapy. Neuro-Oncology 2019, 21, iv8. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Sanderson, T.M.; El-Abbadi, M.M.; McGowan, R.; Mukherjee, P. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br. J. Cancer 2003, 89, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhu, X.; Wang, H.; Wang, F.; Guan, W. Roles of caloric restriction, ketogenic diet and intermittent fasting during initiation, progression and metastasis of cancer in animal models: A systematic review and meta-analysis. PLoS ONE 2014, 9, e115147. [Google Scholar] [CrossRef] [PubMed]
- Maurer, G.D.; Brucker, D.P.; Bähr, O.; Harter, P.N.; Hattingen, E.; Walenta, S.; Mueller-Klieser, W.; Steinbach, J.P.; Rieger, J. Differential utilization of ketone bodies by neurons and glioma cell lines: A rationale for ketogenic diet as experimental glioma therapy. BMC Cancer 2011, 11, 315. [Google Scholar] [CrossRef]
- Rieger, J.; Bähr, O.; Maurer, G.D.; Hattingen, E.; Franz, K.; Brucker, D.; Walenta, S.; Kämmerer, U.; Coy, J.F.; Weller, M.; et al. ERGO: A pilot study of ketogenic diet in recurrent glioblastoma. Int. J. Oncol. 2014, 44, 1843–1852. [Google Scholar] [CrossRef]
- Zhou, W.; Mukherjee, P.; Kiebish, M.A.; Markis, W.T.; Mantis, J.G.; Seyfried, T.N. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr. Metab. 2007, 4, 5. [Google Scholar] [CrossRef]
- Abdelwahab, M.G.; Fenton, K.E.; Preul, M.C.; Rho, J.M.; Lynch, A.; Stafford, P.; Scheck, A.C. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS ONE 2012, 7, e36197. [Google Scholar] [CrossRef]
- Dang, M.T.; Wehrli, S.; Dang, C.V.; Curran, T. The ketogenic diet does not affect growth of Hedgehog pathway medulloblastoma in mice. PLoS ONE 2015, 10, e0133633. [Google Scholar] [CrossRef]
- Morscher, R.J.; Aminzadeh-Gohari, S.; Feichtinger, R.; Mayr, J.A.; Lang, R.; Neureiter, D.; Sperl, W.; Kofler, B. Inhibition of neuroblastoma tumor growth by ketogenic diet and/or calorie restriction in a CD1-nu mouse model. PLoS ONE 2015, 10, e0129802. [Google Scholar] [CrossRef]
- Ciusani, E.; Vasco, C.; Rizzo, A.; Girgenti, V.; Padelli, F.; Pellegatta, S.; Fariselli, L.; Bruzzone, M.G.; Salmaggi, A. MR-Spectroscopy and Survival in Mice with High Grade Glioma Undergoing Unrestricted Ketogenic Diet. Nutr. Cancer 2020, 73, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- De Feyter, H.M.; Behar, K.L.; Rao, J.U.; Madden-Hennessey, K.; Ip, K.L.; Hyder, F.; Drewes, L.R.; Geschwind, J.F.; De Graaf, R.A.; Rothman, D.L. A ketogenic diet increases transport and oxidation of ketone bodies in RG2 and 9L gliomas without affecting tumor growth. Neuro-Oncology 2016, 18, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Klement, R.J.; Champ, C.E.; Otto, C.; Kämmerer, U. Anti-tumor effects of ketogenic diets in mice: A meta-analysis. PLoS ONE 2016, 11, e0155050. [Google Scholar] [CrossRef] [PubMed]
- Javier, R.; Wang, W.; Drumm, M.; McCortney, K.; Sarkaria, J.N.; Horbinski, C. The efficacy of an unrestricted cycling ketogenic diet in preclinical models of IDH wild-type and IDH mutant glioma. PLoS ONE 2022, 17, e0257725. [Google Scholar] [CrossRef]
- Champ, C.E.; Palmer, J.D.; Volek, J.S.; Werner-Wasik, M.; Andrews, D.W.; Evans, J.J.; Glass, J.; Kim, L.; Shi, W. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J. Neurooncol. 2014, 117, 125–131. [Google Scholar] [CrossRef]
- Panhans, C.M.; Gresham, G.; Amaral, J.L.; JHu, J. Exploring the Feasibility and Effects of a Ketogenic Diet in Patients With CNS Malignancies: A Retrospective Case Series. Front. Neurosci. 2020, 14, 390. [Google Scholar] [CrossRef]
- Martin-McGill, K.J.; Marson, A.G.; Tudur Smith, C.; Young, B.; Mills, S.J.; Cherry, M.G.; Jenkinson, M.D. Ketogenic diets as an adjuvant therapy for glioblastoma (KEATING): A randomized, mixed methods, feasibility study. J. Neurooncol. 2020, 147, 213–227. [Google Scholar] [CrossRef]
- Van Der Louw, E.J.T.M.; Olieman, J.F.; van den Bemt, P.M.L.A.; Bromberg, J.E.C.; de Hoop, E.O.; Neuteboom, R.F.; Catsman-Berrevoets, C.E.; Vincent, A.J.P.E. Ketogenic diet treatment as adjuvant to standard treatment of glioblastoma multiforme: A feasibility and safety study. Ther. Adv. Med. Oncol. 2019, 11, 1758835919853958. [Google Scholar] [CrossRef]
- Van Der Louw, E.J.; Reddingius, R.E.; Olieman, J.F.; Neuteboom, R.F.; Catsman-Berrevoets, C.E. Ketogenic diet treatment in recurrent diffuse intrinsic pontine glioma in children: A safety and feasibility study. Pediatr. Blood Cancer 2018, 66, e2756. [Google Scholar] [CrossRef]
- Perez, A.; van der Louw, E.; Nathan, J.; El-Ayadi, M.; Golay, H.; Korff, C.; Ansari, M.; Catsman-Berrevoets, C.; von Bueren, A.O. Ketogenic diet treatment in diffuse intrinsic pontine glioma in children: Retrospective analysis of feasibility, safety, and survival data. Cancer Rep. 2021, 4, e1383. [Google Scholar] [CrossRef]
- EWoolf, E.C.; Scheck, A.C. The ketogenic diet for the treatment of malignant glioma. J. Lipid Res. 2015, 56, 5–10. [Google Scholar]
- Artzi, M.; Liberman, G.; Vaisman, N.; Bokstein, F.; Vitinshtein, F.; Aizenstein, O.; Ben Bashat, D. Changes in cerebral metabolism during ketogenic diet in patients with primary brain tumors: 1H-MRS study. J. Neurooncol. 2017, 132, 267–275. [Google Scholar] [CrossRef]
- Martin-McGill, K.J.; Marson, A.; Smith, C.T.; Jenkinson, M.D. The Modified Ketogenic Diet in Adults with Glioblastoma: An Evaluation of Feasibility and Deliverability within the National Health Service. Nutr. Cancer 2018, 70, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.; Tyrlikova, I.; Zuccoli, K.; Tyrlik, A.; Maroon, J.C. Treatment of glioblastoma multiforme with ‘classic’ 4:1 ketogenic diet total meal replacement. Cancer Metab. 2020, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Porper, K.; Shpatz, Y.; Plotkin, L.; Pechthold, R.G.; Talianski, A.; Champ, C.E.; Furman, O.; Shimoni-Sebag, A.; Symon, Z.; Amit, U.; et al. A Phase I clinical trial of dose-escalated metabolic therapy combined with concomitant radiation therapy in high-grade glioma. J. Neurooncol. 2021, 153, 487–496. [Google Scholar] [CrossRef]
- Liang, S.; Fan, X.; Zhao, M.; Shan, X.; Li, W.; Ding, P.; You, G.; Hong, Z.; Yang, X.; Luan, G.; et al. Clinical practice guidelines for the diagnosis and treatment of adult diffuse glioma-related epilepsy. Cancer Med. 2019, 8, 4527–4535. [Google Scholar] [CrossRef]
- Martin-McGill, K.J.; Bresnahan, R.; Levy, R.G.; Cooper, P.N. Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst. Rev. 2020, 6, 1–55. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zhang, K.; Yang, W.; Li, B. The Anticonvulsant Effects of Ketogenic Diet on Epileptic Seizures and Potential Mechanisms. Curr. Neuropharmacol. 2017, 16, 66–70. [Google Scholar] [CrossRef]
- Ułamek-Kozioł, M.; Czuczwar, S.J.; Januszewski, S.; Pluta, R. Ketogenic Diet and Epilepsy. Nutirents 2019, 11, 2510. [Google Scholar] [CrossRef]
- Nebeling, L.C.; Miraldi, F.; Shurin, S.B.; Lerner, E. Effects of a Ketogenic Diet on Tumor Metabolism and Nutritional Status in Pediatrie Oncology Patients: Two Case Reports. J. Am. Coll. Nutr. 1995, 14, 202–208. [Google Scholar] [CrossRef]
- Martin-McGill, K.J.; Bresnahan, R.; Levy, R.G.; Cooper, P.N. Ketogenic diets for drug-resistant epilepsy (Review). Cochrane Database Syst. Rev. 2018, 11, 1–40. [Google Scholar]
- Klement, R.J.; Weigel, M.M.; Sweeney, R.A. A ketogenic diet consumed during radiotherapy improves several aspects of quality of life and metabolic health in women with breast cancer. Clin. Nutr. 2021, 40, 4267–4274. [Google Scholar] [CrossRef] [PubMed]
- Bonuccelli, G.; Tsirigos, A.; Whitaker-Menezes, D.; Pavlides, S.; Pestell, R.G.; Chiavarina, B.; Frank, P.G.; Flomenberg, N.; Howell, A.; Martinez-Outschoorn, U.E.; et al. Ketones and lactate ‘fuel’ tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle 2010, 9, 3506–3514. [Google Scholar] [CrossRef]
- Watanabe, M.; Tuccinardi, D.; Ernesti, I.; Basciani, S.; Mariani, S.; Genco, A.; Manfrini, S.; Lubrano, C.; Gnessi, L. Scientific evidence underlying contraindications to the ketogenic diet: An update. Obes. Rev. 2020, 21, e13053. [Google Scholar] [CrossRef]
- Liskiewicz, A.D.; Kasprowska, D.; Wojakowska, A.; Polanski, K.; Lewin-Kowalik, J.; Kotulska, K.; Jedrzejowska-Szypulka, H. Long-term High Fat Ketogenic Diet Promotes Renal Tumor Growth in a Rat Model of Tuberous Sclerosis. Sci. Rep. 2016, 6, 21807. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Lin, R.; Jin, L.; Zhao, L.; Kang, H.-B.; Pan, Y.; Liu, S.; Qian, G.; Qian, Z.; Konstantakou, E.; et al. Prevention of Dietary-Fat-Fueled Ketogenesis Attenuates BRAF V600E Tumor Growth. Cell Metab. 2017, 25, 358–373. [Google Scholar] [CrossRef]

| Cancer Type | Year | Cell Line | KD Ratio | Study Group | Results | Reference |
|---|---|---|---|---|---|---|
| Glioma | 2003 | CT-2A | 5.5:1 | SD, KD, SD + CR, KD + CR | Reduced tumor growth in groups with CR. | [57] |
| 2007 | CT-2A, U87-MG | 4:1 | KD + CR vs. SD + CR | KD reduced growth and improved survival. | [61] | |
| 2010 | GL261 | 6:1 | SD vs. KD | KD increased survival. | [52] | |
| 2011 | T98G, U87MG, NIH3T3, A172, LNT-229, U251MG | 3:1 | SD vs. KD | No effects on survival or tumor progression. | [59] | |
| 2012 | GL261-Luc2 | 4:1 | KD, SD + RT, KD + RT | KD alone and KD + RT increase survival with an additive effect compared with RT alone. | [62] | |
| 2014 | U87MG | 3:1 | SD, SD + bevacizumab, KD, KD + bevacizumab | KD alone not effective on tumor progression or survival. KD + bevacizumab: synergistic effect. | [60] | |
| 2015 | GL261-Luc2 | 4:1 | SD vs. KD | In KD the expression of VEGFR2, MMP-2, MMP-9, vimetina and peritumoral edema were reduced. | [28] | |
| 2015 | SH-SY5Y, SK-N-BE(2) | 1.5:1 | SD, CR, KD, KD + CR | Ketogenic diet and/or calorie restriction significantly reduced tumor growth and prolonged survival. | [64] | |
| 2016 | L9, RG2 | 4.5:1 | SD vs. KD | Gliomas can oxidize ketone bodies and overexpress Monocarboxylate transporter 1 (MCT1). | [66] | |
| 2019 | VM-M3, CT-2A | 3.6:1 | SD, SD + DON, KD, KD + DON | KD + DON increased the survival, reduced the tumor growth and edema. | [31] | |
| 2020 | GL261 | 7:1 | SD vs. KD | Improved survival and changes in the availability of energy and structural resources of neoplastic cells. | [65] | |
| 2022 | GBM6, GBM43, TB09 e HT1080 | 4.2:1 | KD vs. SD | There are no differences in the use of KD in wild-type and mutated IDH. | [68] | |
| Medulloblastoma | 2015 | Spontaneous medulloblastoma (Ptch1+/−Trp53−/−) | 4:1 and 6:1 | SD vs. KD | No effects on the tumor progression or survival. | [63] |
| Cancer Type | Year | Number of Patients | Study Group | Results | Reference |
|---|---|---|---|---|---|
| Recurrent glioblastoma | 2014 | 20 | KD, conventional therapy, KD + conventional therapy | KD alone not efficacy. KD + bevacizumab prolongs PFS compared with bevacizumab alone. | [60] |
| Glioblastoma | 2014 | 6 | Standard therapy vs. terapia standard + KD | KD well tolerated and safe even in combination with standard therapies. Improved glucose profile also in combination with steroids. | [69] |
| Glioma | 2015 | 8 | Standard therapy + MAD (modified atkins diet) | KD well tolerated with improved seizure control. | [44] |
| Glioblastoma and gliomatosis cerebri | 2017 | 9 | SD, KD, KD+bevacizumab | KD determines accumulation of ketone bodies in the CNS of patients with brain tumors. | [76] |
| High-grade glioma | 2018 | 6 | Standard therapy + MKD | Well-tolerated diet with limited side effects (fatigue, constipation). | [77] |
| Glioblastoma | 2019 | 11 | Standard therapy + KD | No severe adverse effects, no effects on survival, neurological functioning, or quality of life. | [72] |
| Difuse intrinsic pontine glioma | 2019 | 3 | Standard therapy + KD | KD is safe but the effect on survival requires a larger cohort. | [73] |
| Glioblastoma | 2020 | 8 | Standard therapy + KD | KD was well tolerated, sample sparsity did not allow testing for survival benefits. | [78] |
| Glioblastoma | 2020 | 12 | Standard therapy+MKD (modified ketogenic diet) o MCTKD (medium-chain triglycerideketogenic diet) | Some patients developed indroelectrolyte disorders. There was an improvement in GHS, which was better in MKD. | [71] |
| Glioma | 2020 | 12 | Standard therapy + KD | Improved symptoms and seizures. Improved disease control with reduction in vasogenic edema. | [70] |
| High-grade glioma | 2021 | 13 | RT + modified atkins diet + MCT + metformin supplementation | Promising intervention. | [79] |
| Diffuse intrinsic pontine glioma | 2021 | 5 | Standard therapy + KD | KD is safe but the effect on survival requires a larger cohort. | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Bello, S.; Valdemarin, F.; Martinuzzi, D.; Filippi, F.; Gigli, G.L.; Valente, M. Ketogenic Diet in the Treatment of Gliomas and Glioblastomas. Nutrients 2022, 14, 3851. https://doi.org/10.3390/nu14183851
Dal Bello S, Valdemarin F, Martinuzzi D, Filippi F, Gigli GL, Valente M. Ketogenic Diet in the Treatment of Gliomas and Glioblastomas. Nutrients. 2022; 14(18):3851. https://doi.org/10.3390/nu14183851
Chicago/Turabian StyleDal Bello, Simone, Francesca Valdemarin, Deborah Martinuzzi, Francesca Filippi, Gian Luigi Gigli, and Mariarosaria Valente. 2022. "Ketogenic Diet in the Treatment of Gliomas and Glioblastomas" Nutrients 14, no. 18: 3851. https://doi.org/10.3390/nu14183851
APA StyleDal Bello, S., Valdemarin, F., Martinuzzi, D., Filippi, F., Gigli, G. L., & Valente, M. (2022). Ketogenic Diet in the Treatment of Gliomas and Glioblastomas. Nutrients, 14(18), 3851. https://doi.org/10.3390/nu14183851









