Impact of Blueberry Consumption on the Human Fecal Bileacidome: A Pilot Study of Bile Acid Modulation by Freeze-Dried Blueberry
Abstract
:1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Participants and Original Design
2.3. Intervention
2.4. Measurement of Fecal Bile Acids
2.5. Bile Acid Analysis
2.6. Statistical Analysis
3. Results
3.1. Fecal Bile Acid Profiles Sustain Large Inter-Individual Variability
3.2. No Sexual Dimorphism Was Detected in the Fecal Bileacidome
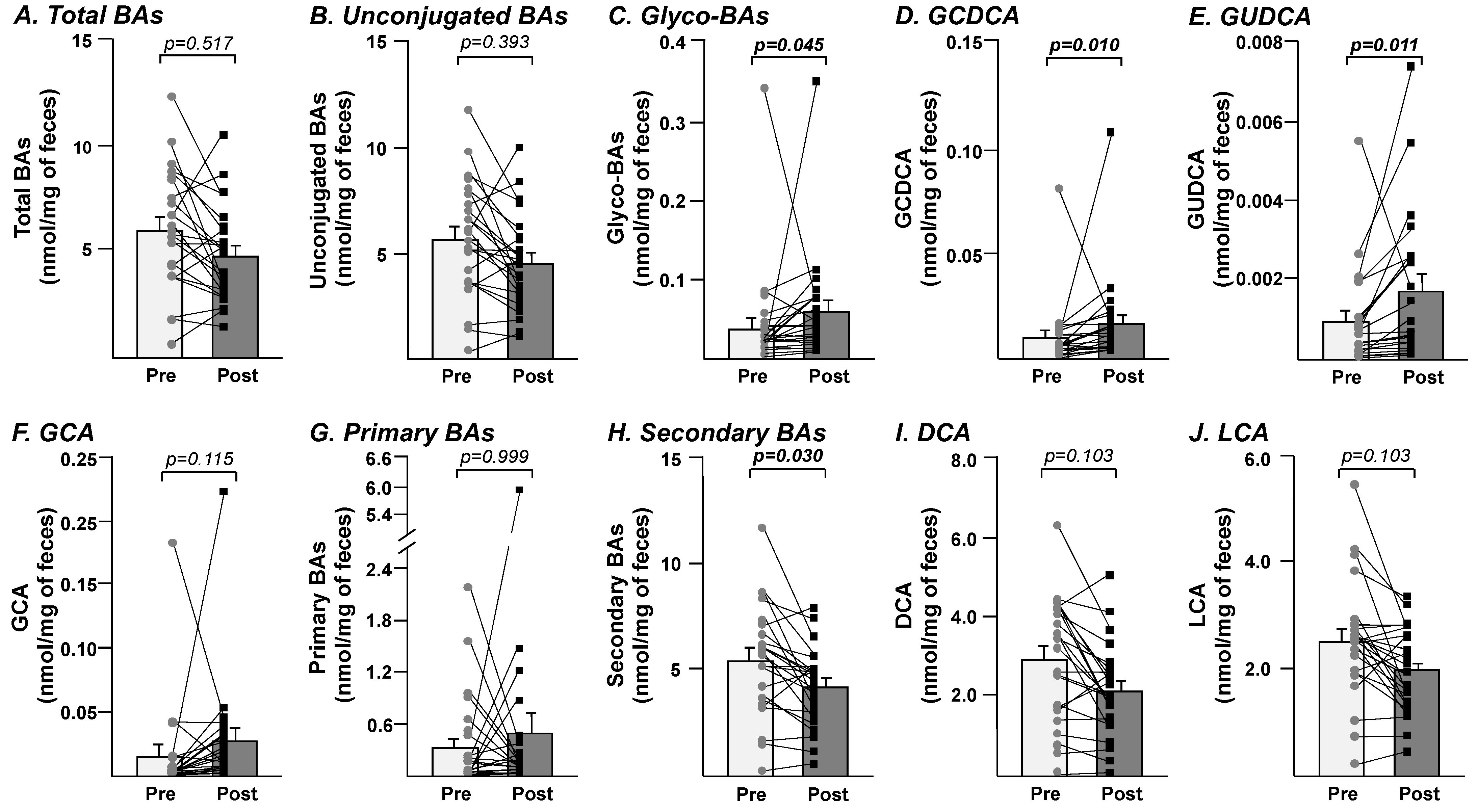
3.3. Fecal Bileacidome Exhibit Significant Alteration after 8-Week Consumption of Freeze-Dried Blueberry Powder
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiang, J.Y.L.; Ferrell, J.M. Bile acids as metabolic regulators and nutrient sensors. Annu. Rev. Nutr. 2019, 39, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, P.; Cariou, B.; Lien, F.; Kuipers, F.; Staels, B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 2009, 89, 147–191. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Karpen, S.J. Intestinal transport and metabolism of bile acids. J. Lipid Res. 2015, 56, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Bile acid metabolism in liver pathobiology. Gene Expr. 2018, 18, 71–87. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef]
- Biagioli, M.; Marchiano, S.; Carino, A.; Di Giorgio, C.; Santucci, L.; Distrutti, E.; Fiorucci, S. Bile acids activated receptors in inflammatory bowel disease. Cells 2021, 10, 1281. [Google Scholar] [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef]
- Sun, L.; Xie, C.; Wang, G.; Wu, Y.; Wu, Q.; Wang, X.; Liu, J.; Deng, Y.; Xia, J.; Chen, B.; et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med. 2018, 24, 1919–1929. [Google Scholar] [CrossRef]
- Ticho, A.L.; Malhotra, P.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. Intestinal absorption of bile acids in health and disease. Compr. Physiol. 2019, 10, 21–56. [Google Scholar] [CrossRef]
- Gruner, N.; Mattner, J. Bile acids and microbiota: Multifaceted and versatile regulators of the liver-gut axis. Int. J. Mol. Sci. 2021, 22, 1397. [Google Scholar] [CrossRef]
- Rodriguez-Morato, J.; Matthan, N.R. Nutrition and gastrointestinal microbiota, microbial-derived secondary bile acids, and cardiovascular disease. Curr. Atheroscler Rep. 2020, 22, 47. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomas-Barberan, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Pushpass, R.G.; Alzoufairi, S.; Jackson, K.G.; Lovegrove, J.A. Circulating bile acids as a link between the gut microbiota and cardiovascular health: Impact of prebiotics, probiotics and polyphenol-rich foods. Nutr. Res. Rev. 2021, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Zhang, L.; Zhang, X.; Edirisinghe, I.; Burton-Freeman, B.M.; Sandhu, A.K. Comprehensive characterization of bile acids in human biological samples and effect of 4-week strawberry intake on bile acid composition in human plasma. Metabolites 2021, 11, 99. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Morissette, A.; Kropp, C.; Songpadith, J.P.; Junges Moreira, R.; Costa, J.; Marine-Casado, R.; Pilon, G.; Varin, T.V.; Dudonne, S.; Boutekrabt, L.; et al. Blueberry proanthocyanidins and anthocyanins improve metabolic health through a gut microbiota-dependent mechanism in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E965–E980. [Google Scholar] [CrossRef]
- Anhe, F.F.; Varin, T.V.; Le Barz, M.; Desjardins, Y.; Levy, E.; Roy, D.; Marette, A. Gut microbiota dysbiosis in obesity-linked metabolic diseases and prebiotic potential of polyphenol-rich extracts. Curr. Obes Rep. 2015, 4, 389–400. [Google Scholar] [CrossRef]
- Rousseau, M.; Horne, J.; Guenard, F.; de Toro-Martin, J.; Garneau, V.; Guay, V.; Kearney, M.; Pilon, G.; Roy, D.; Couture, P.; et al. An 8-week freeze-dried blueberry supplement impacts immune-related pathways: A randomized, double-blind placebo-controlled trial. Genes Nutr. 2021, 16, 7. [Google Scholar] [CrossRef]
- Scarsella, C.; Almeras, N.; Mauriege, P.; Blanchet, C.; Sauve, L.; Dewailly, E.; Bergeron, J.; Despres, J.P. Prevalence of metabolic alterations predictive of cardiovascular disease risk in the Quebec population. Can. J. Cardiol. 2003, 19, 51–57. [Google Scholar]
- Daniel, N.; Nachbar, R.T.; Tran, T.T.T.; Ouellette, A.; Varin, T.V.; Cotillard, A.; Quinquis, L.; Gagne, A.; St-Pierre, P.; Trottier, J.; et al. Gut microbiota and fermentation-derived branched chain hydroxy acids mediate health benefits of yogurt consumption in obese mice. Nat. Commun. 2022, 13, 1343. [Google Scholar] [CrossRef]
- Gagnon, G.; Carreau, A.M.; Cloutier-Langevin, C.; Plante, A.S.; John Weisnagel, S.; Marceau, S.; Biertho, L.; Simon Hould, F.; Camirand-Lemyre, F.; Tchernof, A.; et al. Trimester-specific gestational weight gain in women with and without previous bariatric surgeries. Eur J Obstet Gynecol. Reprod Biol. 2022, 270, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Trottier, J.; Bialek, A.; Caron, P.; Straka, R.J.; Heathcote, J.; Milkiewicz, P.; Barbier, O. Metabolomic profiling of 17 bile acids in serum from patients with primary biliary cirrhosis and primary sclerosing cholangitis: A pilot study. Dig. Liver Dis. 2012, 44, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.H. Fecal bile acids in health and disease. In Liver, Nutrition, and Bile Acids; Galli, G., Bosisio, E., Eds.; Springer: Boston, MA, USA, 1985; Volume 90. [Google Scholar]
- Chen, L.; van den Munckhof, I.C.L.; Schraa, K.; Ter Horst, R.; Koehorst, M.; van Faassen, M.; van der Ley, C.; Doestzada, M.; Zhernakova, D.V.; Kurilshikov, A.; et al. Genetic and microbial associations to plasma and fecal bile acids in obesity relate to plasma lipids and liver fat content. Cell Rep. 2020, 33, 108212. [Google Scholar] [CrossRef]
- Trottier, J.; Caron, P.; Straka, R.J.; Barbier, O. Profile of serum bile acids in noncholestatic volunteers: Gender-related differences in response to fenofibrate. Clin. Pharmacol. Ther. 2011, 90, 279–286. [Google Scholar] [CrossRef]
- Phelps, T.; Snyder, E.; Rodriguez, E.; Child, H.; Harvey, P. The influence of biological sex and sex hormones on bile acid synthesis and cholesterol homeostasis. Biol. Sex. Differ. 2019, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol 2014, 30, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.M.; Angeloni, S.; Abouelenein, D.; Acquaticci, L.; Xiao, J.; Sagratini, G.; Maggi, F.; Vittori, S.; Caprioli, G. A new HPLC-MS/MS method for the simultaneous determination of 36 polyphenols in blueberry, strawberry and their commercial products and determination of antioxidant activity. Food Chem. 2022, 367, 130743. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Verediano, T.A.; Stampini Duarte Martino, H.; Dias Paes, M.C.; Tako, E. Effects of anthocyanin on intestinal health: A systematic review. Nutrients 2021, 13, 1331. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Cardona, F.; Andres-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuno, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahlon, T.S.; Smith, G.E. In vitro binding of bile acids by blueberries (Vaccinium spp.), plums (Prunus spp.), prunes (Prunus spp.), strawberries (Fragaria X ananassa), cherries (Malpighia punicifolia), cranberries (Vaccinium macrocarpon) and apples (Malus sylvestris). Food Chem. 2007, 100, 1182–1187. [Google Scholar] [CrossRef]
- Naumann, S.; Haller, D.; Eisner, P.; Schweiggert-Weisz, U. Mechanisms of interactions between bile acids and plant compounds-a review. Int. J. Mol. Sci. 2020, 21, 6495. [Google Scholar] [CrossRef] [PubMed]
- Surampudi, P.; Enkhmaa, B.; Anuurad, E.; Berglund, L. Lipid lowering with soluble dietary fiber. Curr. Atheroscler Rep. 2016, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Hanafi, N.I.; Mohamed, A.S.; Sheikh Abdul Kadir, S.H.; Othman, M.H.D. Overview of bile acids signaling and perspective on the signal of ursodeoxycholic acid, the most hydrophilic bile acid, in the heart. Biomolecules 2018, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Hohenester, S.; Kanitz, V.; Kremer, A.E.; Paulusma, C.C.; Wimmer, R.; Kuehn, H.; Denk, G.; Horst, D.; Elferink, R.O.; Beuers, U. Glycochenodeoxycholate promotes liver fibrosis in mice with hepatocellular cholestasis. Cells 2020, 9, 281. [Google Scholar] [CrossRef]
- Thomas John, P. The emerging role of Bile Acids in the pathogenesis of inflammatory bowel disease. Front. Immunol. 2022, 13, 246. [Google Scholar] [CrossRef]
- Usui, Y.; Ayibieke, A.; Kamiichi, Y.; Okugawa, S.; Moriya, K.; Tohda, S.; Saito, R. Impact of deoxycholate on Clostridioides difficile growth, toxin production, and sporulation. Heliyon 2020, 6, e03717. [Google Scholar] [CrossRef]
| Parameter | Mean | [Range] | |
|---|---|---|---|
| Weight (kg) | 89.5 | [62.5–126.5] | |
| Height (m) | 1.71 | [1.53–1.88] | |
| BMI (kg/m2) | 30.8 | [23.4–47.1] | |
| Waist circumference (cm) | 101.5 | [81.0–131.3] | |
| Hip circumference (cm) | 110.2 | [92.2–141.8] | |
| SBP (mmHg) | 115.2 | [99.0–131.7] | |
| DBP (mmHg) | 72.5 | [57.0–82.3] | |
| Apo B (g/L) | 0.91 | [0.60–1.41] | |
| Total-C (mmol/L) | 4.56 | [2.99–6.41] | |
| TG (mmol/L) | 1.54 | [0.53–3.98] | |
| HDL-C (mmol/L) | 1.17 | [0.74–1.99] | |
| LDL-C (mmol/L) | 2.68 | [1.58–4.05] | |
| Total-C/HDL-C | 4.06 | [2.42–6.97] | |
| HbA1c | 0.051 | [0.045–0.056] | |
| Age (year) | 35.0 | [23.0–53.0] | |
| Sex | [n] | ||
| Men | 11 | ||
| Women | 13 | ||
| Annual household income (CAD) | [n] | ||
| 0–39,999 | 7 | ||
| 40,000–79,000 | 8 | ||
| 80,000–99,000 | 4 | ||
| ≥100,000 | 4 | ||
| Nondisclosed | 1 | ||
| Highest education level completed | [n] | ||
| High school | 2 | ||
| College | 6 | ||
| University | 16 |
| Before (Baseline) | After (Week 8) | Mean Diff. | Adjusted p Value | |||
|---|---|---|---|---|---|---|
| Bile Acids | Mean | SEM | Mean | SEM | ||
| CA | 0.1887 | ±0.0734 | 0.2732 | ±0.1672 | 0.0844 | >0.999 |
| CDCA | 0.0967 | ±0.0278 | 0.1296 | ±0.0673 | 0.0328 | >0.999 |
| DCA | 2.9097 | ±0.3159 | 2.1280 | ±0.2336 | −0.7817 | 0.103 |
| LCA | 2.5275 | ±0.2297 | 1.9844 | ±0.1574 | −0.5431 | 0.103 |
| HDCA | 0.0087 | ±0.0011 | 0.0064 | ±0.0008 | −0.0023 | 0.375 |
| HCA | 0.0023 | ±0.0007 | 0.0019 | ±0.0006 | −0.0005 | >0.999 |
| UDCA | 0.0323 | ±0.0093 | 0.0518 | ±0.0192 | 0.0195 | >0.999 |
| GCA | 0.0159 | ±0.0076 | 0.0279 | ±0.0090 | 0.0120 | 0.115 |
| GCDCA | 0.0102 | ±0.0032 | 0.0170 | ±0.0043 | 0.0068 | 0.010 |
| GDCA | 0.0132 | ±0.0031 | 0.0145 | ±0.0018 | 0.0013 | >0.999 |
| GLCA | 0.0003 | ±0.0001 | 0.0003 | ±0.0000 | −0.0001 | >0.999 |
| GUDCA | 0.0010 | ±0.0002 | 0.0017 | ±0.0004 | 0.0008 | 0.011 |
| TCA | 0.0153 | ±0.0081 | 0.0212 | ±0.0098 | 0.0059 | >0.999 |
| TCDCA | 0.0073 | ±0.0030 | 0.0188 | ±0.0095 | 0.0115 | >0.999 |
| TDCA | 0.0306 | ±0.0117 | 0.0203 | ±0.0065 | −0.0103 | >0.999 |
| TLCA | 0.0015 | ±0.0007 | 0.0011 | ±0.0004 | −0.0004 | >0.999 |
| TUDCA | 0.0007 | ±0.0004 | 0.0014 | ±0.0008 | 0.0007 | >0.999 |
| TOTAL BA | 6.0068 | ±0.5721 | 4.8358 | ±0.4598 | −1.1710 | 0.517 |
| Unconjugated | 5.7660 | ±0.5556 | 4.5752 | ±0.4564 | −1.1908 | 0.393 |
| Taurine-conjugated | 0.0554 | ±0.0228 | 0.0628 | ±0.0231 | 0.0074 | >0.999 |
| Glycine-conjugated | 0.0406 | ±0.0139 | 0.0614 | ±0.0141 | 0.0208 | 0.045 |
| Primary | 0.3343 | ±0.1075 | 0.4877 | ±0.2482 | 0.1534 | >0.999 |
| Secondary | 5.4828 | ±0.5251 | 4.1486 | ±0.3707 | −1.3343 | 0.030 |
| 6α-hydroxylated | 0.0110 | ±0.0015 | 0.0083 | ±0.0008 | −0.0027 | 0.940 |
| Total CA | 0.2200 | ±0.0820 | 0.3223 | ±0.1735 | 0.1023 | >0.999 |
| Total CDCA | 0.1144 | ±0.0299 | 0.1659 | ±0.0760 | 0.0515 | >0.999 |
| Total DCA | 2.9536 | ±0.3213 | 2.1632 | ±0.2368 | −0.7905 | 0.115 |
| Total LCA | 2.5294 | ±0.2299 | 1.9859 | ±0.1574 | −0.5435 | 0.103 |
| Total HDCA | 0.0087 | ±0.0011 | 0.0065 | ±0.0008 | −0.0023 | 0.414 |
| Total HCA | 0.0023 | ±0.0007 | 0.0019 | ±0.0006 | −0.0004 | >0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagnon, W.; Garneau, V.; Trottier, J.; Verreault, M.; Couillard, C.; Roy, D.; Marette, A.; Drouin-Chartier, J.-P.; Vohl, M.-C.; Barbier, O. Impact of Blueberry Consumption on the Human Fecal Bileacidome: A Pilot Study of Bile Acid Modulation by Freeze-Dried Blueberry. Nutrients 2022, 14, 3857. https://doi.org/10.3390/nu14183857
Gagnon W, Garneau V, Trottier J, Verreault M, Couillard C, Roy D, Marette A, Drouin-Chartier J-P, Vohl M-C, Barbier O. Impact of Blueberry Consumption on the Human Fecal Bileacidome: A Pilot Study of Bile Acid Modulation by Freeze-Dried Blueberry. Nutrients. 2022; 14(18):3857. https://doi.org/10.3390/nu14183857
Chicago/Turabian StyleGagnon, William, Véronique Garneau, Jocelyn Trottier, Mélanie Verreault, Charles Couillard, Denis Roy, André Marette, Jean-Philippe Drouin-Chartier, Marie-Claude Vohl, and Olivier Barbier. 2022. "Impact of Blueberry Consumption on the Human Fecal Bileacidome: A Pilot Study of Bile Acid Modulation by Freeze-Dried Blueberry" Nutrients 14, no. 18: 3857. https://doi.org/10.3390/nu14183857





