The Effect of Vitamin D and Its Analogs in Ovarian Cancer
Abstract
:1. Ovarian Cancer
2. Vitamin D and Vitamin D Analogs
3. Effect of Vitamin D on Ovarian Cancer Epidemiology
4. Mechanism of Anticancer Activity in Ovarian Cancer Models In Vitro
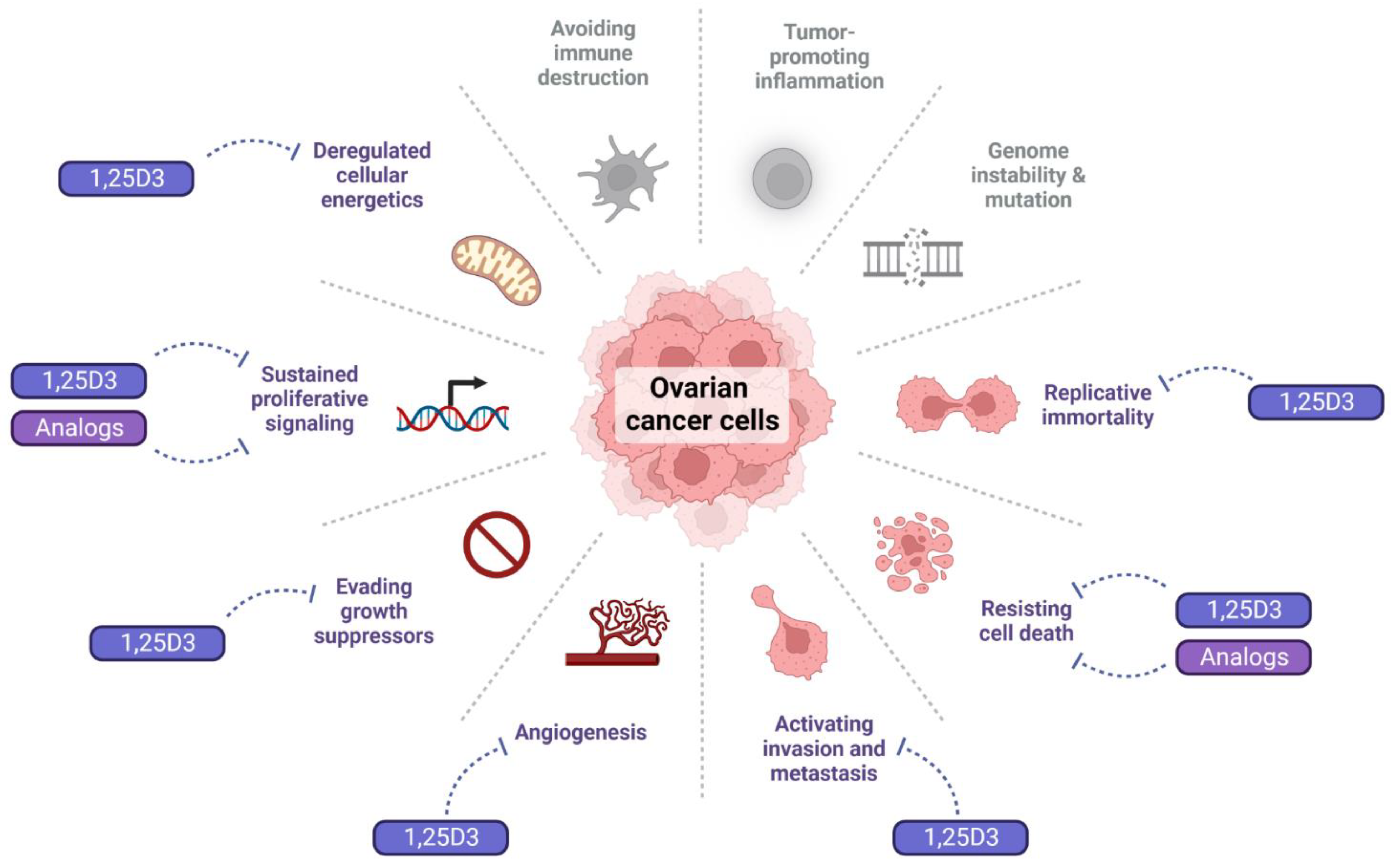
4.1. Effect of 1,25D3 and Its Analogs on the Hallmarks of Cancer
4.2. Effect of 1,25D3 and Its Analogs on Proliferative Signals
4.3. Effect of 1,25D3 and Its Analogs on Cell Death
4.4. Effect of 1,25D3 and Its Analogs on Metastatic Potential
4.5. Effect of 1,25D3 and Its Analogs on Replicative Immortality and Angiogenesis
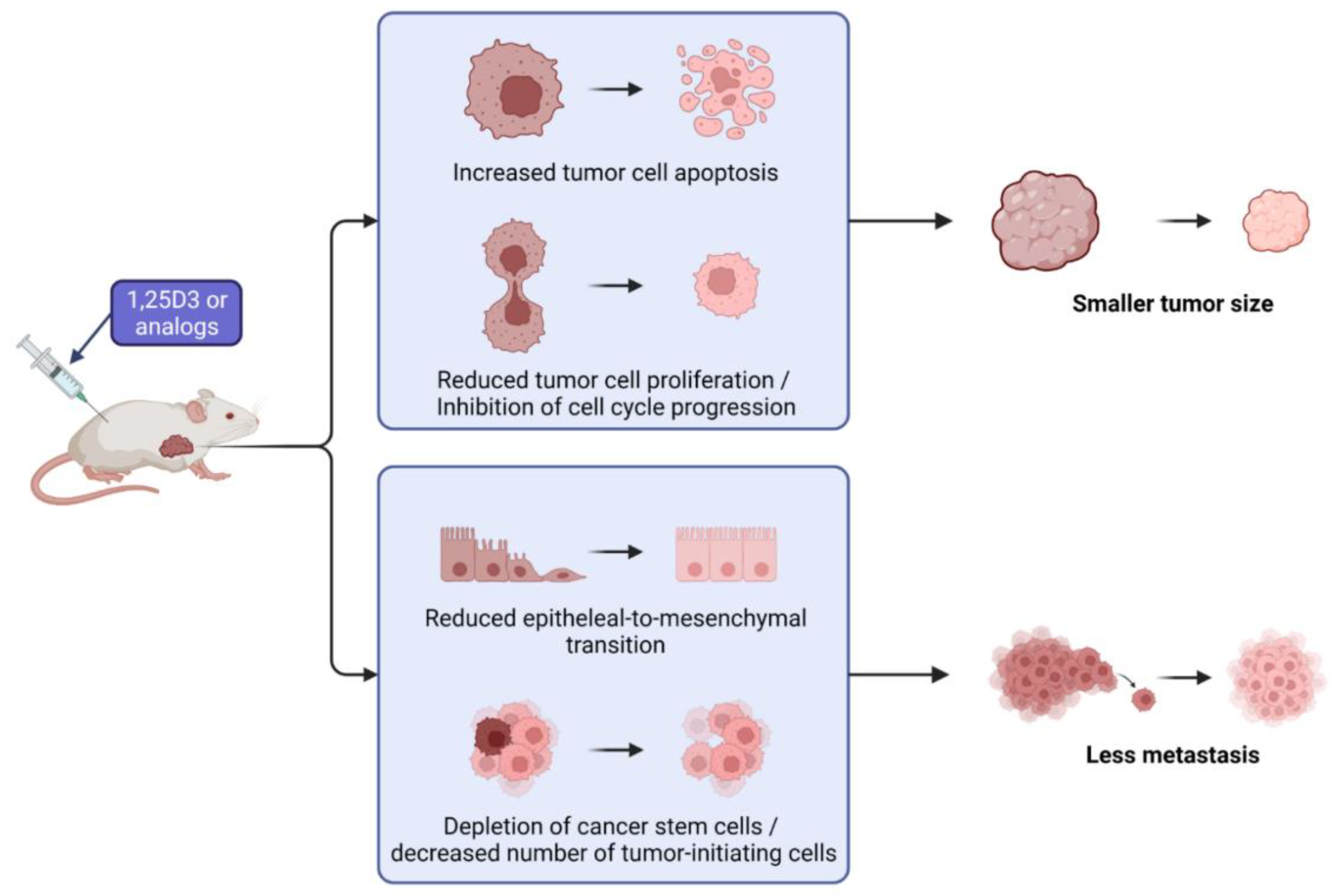
5. Mechanism of Anticancer Activity in Ovarian Cancer Models In Vivo
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Ducie, J.; Dao, F.; Considine, M.; Olvera, N.; Shaw, P.A.; Kurman, R.J.; Shih, I.M.; Soslow, R.A.; Cope, L.; Levine, D.A. Molecular analysis of high-grade serous ovarian carcinoma with and without associated serous tubal intra-epithelial carcinoma. Nat. Commun. 2017, 8, 990. [Google Scholar] [CrossRef] [PubMed]
- Kossai, M.; Leary, A.; Scoazec, J.Y.; Genestie, C. Ovarian Cancer: A Heterogeneous Disease. Pathobiology 2018, 85, 41–49. [Google Scholar] [CrossRef]
- Adhikari, L.H.L. Ovarian Neoplasms WHO Classification Review. Available online: https://www.pathologyoutlines.com/topic/ovarytumorwhoclassif.html (accessed on 17 May 2022).
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Crum, C.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2012, 119 (Suppl. 2), S118–S129. [Google Scholar] [CrossRef]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef]
- Carlberg, C.; Munoz, A. An update on vitamin D signaling and cancer. Semin. Cancer Biol. 2022, 79, 217–230. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Kostenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.; Naska, A.; Neuhauser-Berthold, M.; et al. Dietary reference values for vitamin D. Efsa J. 2016, 14, e04547. [Google Scholar] [CrossRef]
- Moore, R.G.; Lange, T.S.; Robinson, K.; Kim, K.K.; Uzun, A.; Horan, T.C.; Kawar, N.; Yano, N.; Chu, S.R.; Mao, Q.; et al. Efficacy of a non-hypercalcemic vitamin-D2 derived anti-cancer agent (MT19c) and inhibition of fatty acid synthesis in an ovarian cancer xenograft model. PLoS ONE 2012, 7, e34443. [Google Scholar] [CrossRef]
- Lange, T.S.; Stuckey, A.R.; Robison, K.; Kim, K.K.; Singh, R.K.; Raker, C.A.; Brard, L. Effect of a vitamin D(3) derivative (B3CD) with postulated anti-cancer activity in an ovarian cancer animal model. Invest. New Drugs 2010, 28, 543–553. [Google Scholar] [CrossRef]
- Davicco, M.J.; Coxam, V.; Gaumet, N.; Lebecque, P.; Barlet, J.P. EB 1089, a calcitriol analogue, decreases fetal calcium content when injected into pregnant rats. Exp. Physiol. 1995, 80, 449–456. [Google Scholar] [CrossRef]
- Baurska, H.; Klopot, A.; Kielbinski, M.; Chrobak, A.; Wijas, E.; Kutner, A.; Marcinkowska, E. Structure-function analysis of vitamin D(2) analogs as potential inducers of leukemia differentiation and inhibitors of prostate cancer proliferation. J. Steroid Biochem. Mol. Biol. 2011, 126, 46–54. [Google Scholar] [CrossRef]
- Pietraszek, A.; Malinska, M.; Chodynski, M.; Krupa, M.; Krajewski, K.; Cmoch, P.; Wozniak, K.; Kutner, A. Synthesis and crystallographic study of 1,25-dihydroxyergocalciferol analogs. Steroids 2013, 78, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.; Antoszczak, M.; Markowska, J.; Huczynski, A. Role of Vitamin K in Selected Malignant Neoplasms in Women. Nutrients 2022, 14, 3401. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.; Antoszczak, M.; Markowska, J.; Huczynski, A. Role of Vitamin E in Selected Malignant Neoplasms in Women. Nutr. Cancer 2022, 74, 1163–1170. [Google Scholar] [CrossRef]
- Markowska, A.; Antoszczak, M.; Markowska, J.; Huczynski, A. Role of Vitamin C in Selected Malignant Neoplasms in Women. Nutrients 2022, 14, 882. [Google Scholar] [CrossRef]
- Lefkowitz, E.S.; Garland, C.F. Sunlight, vitamin D, and ovarian cancer mortality rates in US women. Int. J. Epidemiol. 1994, 23, 1133–1136. [Google Scholar] [CrossRef]
- Tran, B.; Jordan, S.J.; Lucas, R.; Webb, P.M.; Neale, R.; Australian Ovarian Cancer Study Group. Association between ambient ultraviolet radiation and risk of epithelial ovarian cancer. Cancer Prev. Res. (Phila) 2012, 5, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.M.; de Fazio, A.; Protani, M.M.; Ibiebele, T.I.; Nagle, C.M.; Brand, A.H.; Blomfield, P.I.; Grant, P.; Perrin, L.C.; Neale, R.E.; et al. Circulating 25-hydroxyvitamin D and survival in women with ovarian cancer. Am. J. Clin. Nutr. 2015, 102, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.S.; Cuellar-Partida, G.; Lu, Y.; Fasching, P.A.; Hein, A.; Burghaus, S.; Beckmann, M.W.; Lambrechts, D.; Van Nieuwenhuysen, E.; Vergote, I.; et al. Association of vitamin D levels and risk of ovarian cancer: A Mendelian randomization study. Int. J. Epidemiol. 2016, 45, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.S.; Dixon-Suen, S.C.; Han, X.; An, J.; Liyanage, U.; Me Research, T.; Dusingize, J.C.; Schumacher, J.; Gockel, I.; Böhmer, A.; et al. A comprehensive re-assessment of the association between vitamin D and cancer susceptibility using Mendelian randomization. Nat. Commun. 2021, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulou, V.I.; Tsilidis, K.K.; Haycock, P.C.; Dimou, N.L.; Al-Dabhani, K.; Martin, R.M.; Lewis, S.J.; Gunter, M.J.; Mondul, A.; Shui, I.M.; et al. Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. BMJ 2017, 359, j4761. [Google Scholar] [CrossRef]
- Ong, J.S.; Gharahkhani, P.; An, J.; Law, M.H.; Whiteman, D.C.; Neale, R.E.; MacGregor, S. Vitamin D and overall cancer risk and cancer mortality: A Mendelian randomization study. Hum. Mol. Genet. 2018, 27, 4315–4322. [Google Scholar] [CrossRef]
- Sajo, E.A.; Okunade, K.S.; Olorunfemi, G.; Rabiu, K.A.; Anorlu, R.I. Serum vitamin D deficiency and risk of epithelial ovarian cancer in Lagos, Nigeria. Ecancermedicalscience 2020, 14, 1078. [Google Scholar] [CrossRef]
- Xu, J.; Chen, K.; Zhao, F.; Huang, D.; Zhang, H.; Fu, Z.; Xu, J.; Wu, Y.; Lin, H.; Zhou, Y.; et al. Association between vitamin D/calcium intake and 25-hydroxyvitamin D and risk of ovarian cancer: A dose-response relationship meta-analysis. Eur. J. Clin. Nutr. 2021, 75, 417–429. [Google Scholar] [CrossRef]
- Piatek, K.; Kutner, A.; Cacsire Castillo-Tong, D.; Manhardt, T.; Kupper, N.; Nowak, U.; Chodynski, M.; Marcinkowska, E.; Kallay, E.; Schepelmann, M. Vitamin D Analogs Regulate the Vitamin D System and Cell Viability in Ovarian Cancer Cells. Int. J. Mol. Sci. 2021, 23, 172. [Google Scholar] [CrossRef]
- Friedrich, M.; Rafi, L.; Mitschele, T.; Tilgen, W.; Schmidt, W.; Reichrath, J. Analysis of the vitamin D system in cervical carcinomas, breast cancer and ovarian cancer. Recent. Results Cancer Res. 2003, 164, 239–246. [Google Scholar] [CrossRef]
- Brozyna, A.A.; Kim, T.K.; Zablocka, M.; Jozwicki, W.; Yue, J.; Tuckey, R.C.; Jetten, A.M.; Slominski, A.T. Association among Vitamin D, Retinoic Acid-Related Orphan Receptors, and Vitamin D Hydroxyderivatives in Ovarian Cancer. Nutrients 2020, 12, 3541. [Google Scholar] [CrossRef] [PubMed]
- Kuittinen, T.; Rovio, P.; Luukkaala, T.; Laurila, M.; Grenman, S.; Kallioniemi, A.; Maenpaa, J. Paclitaxel, Carboplatin and 1,25-D3 Inhibit Proliferation of Ovarian Cancer Cells In Vitro. Anticancer Res. 2020, 40, 3129–3138. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Vitamin D—Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef]
- Ferrer-Mayorga, G.; Larriba, M.J.; Crespo, P.; Munoz, A. Mechanisms of action of vitamin D in colon cancer. J. Steroid Biochem Mol. Biol. 2019, 185, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, A.; Choudhury, B.K.; Sharma, T.; Bansal, N.; Bansal, R.; Gupta, S. Vitamin D and gastrointestinal cancer. J. Lab. Physicians 2018, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Vanhevel, J.; Verlinden, L.; Doms, S.; Wildiers, H.; Verstuyf, A. The role of vitamin D in breast cancer risk and progression. Endocr. Relat. Cancer 2022, 29, R33–R55. [Google Scholar] [CrossRef]
- Swami, S.; Krishnan, A.V.; Feldman, D. Vitamin D metabolism and action in the prostate: Implications for health and disease. Mol. Cell Endocrinol. 2011, 347, 61–69. [Google Scholar] [CrossRef]
- Li, P.; Li, C.; Zhao, X.; Zhang, X.; Nicosia, S.V.; Bai, W. p27(Kip1) stabilization and G(1) arrest by 1,25-dihydroxyvitamin D(3) in ovarian cancer cells mediated through down-regulation of cyclin E/cyclin-dependent kinase 2 and Skp1-Cullin-F-box protein/Skp2 ubiquitin ligase. J. Biol. Chem. 2004, 279, 25260–25267. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, X.; Tang, J.; Kasiappan, R.; Jinwal, U.; Li, P.; Hann, S.; Nicosia, S.V.; Wu, J.; Zhang, X.; et al. The coupling of epidermal growth factor receptor down regulation by 1alpha,25-dihydroxyvitamin D3 to the hormone-induced cell cycle arrest at the G1-S checkpoint in ovarian cancer cells. Mol. Cell Endocrinol. 2011, 338, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Park, W.H.; Suh, D.H.; Kim, K.; No, J.H.; Kim, Y.B. Calcitriol Combined With Platinum-based Chemotherapy Suppresses Growth and Expression of Vascular Endothelial Growth Factor of SKOV-3 Ovarian Cancer Cells. Anticancer Res. 2021, 41, 2945–2952. [Google Scholar] [CrossRef] [PubMed]
- Olbromski, P.J.; Pawlik, P.; Bogacz, A.; Sajdak, S. Identification of New Molecular Biomarkers in Ovarian Cancer Using the Gene Expression Profile. J. Clin. Med. 2022, 11, 3888. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Guo, X.; Du, R.; Guo, K.; Qi, P.; Bian, H. Identification of key genes and pathways related to cancer-associated fibroblasts in chemoresistance of ovarian cancer cells based on GEO and TCGA databases. J. Ovarian Res. 2022, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, F.; Li, P.; Li, C.; Ma, Q.; Nicosia, S.V.; Bai, W. Growth suppression of ovarian cancer xenografts in nude mice by vitamin D analogue EB1089. Clin. Cancer Res. 2005, 11, 323–328. [Google Scholar] [CrossRef]
- Tamura, R.E.; de Vasconcellos, J.F.; Sarkar, D.; Libermann, T.A.; Fisher, P.B.; Zerbini, L.F. GADD45 proteins: Central players in tumorigenesis. Curr. Mol. Med. 2012, 12, 634–651. [Google Scholar] [CrossRef]
- McGlorthan, L.; Paucarmayta, A.; Casablanca, Y.; Maxwell, G.L.; Syed, V. Progesterone induces apoptosis by activation of caspase-8 and calcitriol via activation of caspase-9 pathways in ovarian and endometrial cancer cells in vitro. Apoptosis 2021, 26, 184–194. [Google Scholar] [CrossRef]
- Rodriguez, G.C.; Turbov, J.; Rosales, R.; Yoo, J.; Hunn, J.; Zappia, K.J.; Lund, K.; Barry, C.P.; Rodriguez, I.V.; Pike, J.W.; et al. Progestins inhibit calcitriol-induced CYP24A1 and synergistically inhibit ovarian cancer cell viability: An opportunity for chemoprevention. Gynecol. Oncol. 2016, 143, 159–167. [Google Scholar] [CrossRef]
- Ji, M.T.; Nie, J.; Nie, X.F.; Hu, W.T.; Pei, H.L.; Wan, J.M.; Wang, A.Q.; Zhou, G.M.; Zhang, Z.L.; Chang, L.; et al. 1alpha,25(OH)2D3 Radiosensitizes Cancer Cells by Activating the NADPH/ROS Pathway. Front. Pharmacol. 2020, 11, 945. [Google Scholar] [CrossRef]
- Hou, Y.F.; Gao, S.H.; Wang, P.; Zhang, H.M.; Liu, L.Z.; Ye, M.X.; Zhou, G.M.; Zhang, Z.L.; Li, B.Y. 1alpha,25(OH)(2)D(3) Suppresses the Migration of Ovarian Cancer SKOV-3 Cells through the Inhibition of Epithelial-Mesenchymal Transition. Int. J. Mol. Sci. 2016, 17, 1285. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Kitami, K.; Yoshihara, M.; Tamauchi, S.; Sugiyama, M.; Koya, Y.; Yamakita, Y.; Fujimoto, H.; Iyoshi, S.; Uno, K.; Mogi, K.; et al. Peritoneal Restoration by Repurposing Vitamin D Inhibits Ovarian Cancer Dissemination via Blockade of the TGF-beta1/Thrombospondin-1 Axis. Matrix Biol. 2022, 109, 70–90. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaset-Ismail, A.; Pedziwiatr, D.; Suszynska, E.; Sluczanowska-Glabowska, S.; Schneider, G.; Kakar, S.S.; Ratajczak, M.Z. Vitamin D3 stimulates embryonic stem cells but inhibits migration and growth of ovarian cancer and teratocarcinoma cell lines. J. Ovarian Res. 2016, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wang, P.; Jiang, F.; Yu, J.; Ding, H.; Zhang, Z.; Pei, H.; Li, B. A Newly Identified lncBCAS1-4_1 Associated With Vitamin D Signaling and EMT in Ovarian Cancer Cells. Front. Oncol. 2021, 11, 691500. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Katsaros, D.; Biglia, N.; Wang, Z.; Pagano, I.; Tius, M.; Tiirikainen, M.; Rosser, C.; Yang, H.; Yu, H. Vitamin D receptor upregulates lncRNA TOPORS-AS1 which inhibits the Wnt/beta-catenin pathway and associates with favorable prognosis of ovarian cancer. Sci. Rep. 2021, 11, 7484. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Liu, L.; Hou, Y.; Li, B. 1alpha,25Dihydroxyvitamin D3 restrains stem celllike properties of ovarian cancer cells by enhancing vitamin D receptor and suppressing CD44. Oncol. Rep. 2019, 41, 3393–3403. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Bao, J.; Li, P.; Nicosia, S.V.; Bai, W. Induction of ovarian cancer cell apoptosis by 1,25-dihydroxyvitamin D3 through the down-regulation of telomerase. J. Biol. Chem. 2004, 279, 53213–53221. [Google Scholar] [CrossRef] [PubMed]
- Kasiappan, R.; Shen, Z.; Tse, A.K.; Jinwal, U.; Tang, J.; Lungchukiet, P.; Sun, Y.; Kruk, P.; Nicosia, S.V.; Zhang, X.; et al. 1,25-Dihydroxyvitamin D3 suppresses telomerase expression and human cancer growth through microRNA-498. J. Biol. Chem. 2012, 287, 41297–41309. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, F.; Yang, W.; Shi, W.; Wan, J.; Li, J.; Pan, J.; Wang, P.; Qiu, J.; Zhang, Z.; et al. Effect of 1alpha,25(OH)2D3-Treated M1 and M2 Macrophages on Cell Proliferation and Migration Ability in Ovarian Cancer. Nutr. Cancer 2022, 74, 2632–2643. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Wu, J.L.; Quan, W.Q.; Ma, L.; Yang, F.; Wu, K.Y.; Wan, H.Y. Tumor-produced versican V1 enhances hCAP18/LL-37 expression in macrophages through activation of TLR2 and vitamin D3 signaling to promote ovarian cancer progression in vitro. PLoS ONE 2013, 8, e56616. [Google Scholar] [CrossRef]
- Liu, L.; Hu, Z.; Zhang, H.; Hou, Y.; Zhang, Z.; Zhou, G.; Li, B. Vitamin D postpones the progression of epithelial ovarian cancer induced by 7, 12-dimethylbenz [a] anthracene both in vitro and in vivo. Onco Targets Ther. 2016, 9, 2365–2375. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, T.; Bhatt, H. Cancer Antigen 125; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Srivastava, A.K.; Rizvi, A.; Cui, T.; Han, C.; Banerjee, A.; Naseem, I.; Zheng, Y.; Wani, A.A.; Wang, Q.E. Depleting ovarian cancer stem cells with calcitriol. Oncotarget 2018, 9, 14481–14491. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.; Naseem, I. Causing DNA damage and stopping DNA repair—Vitamin D supplementation with Poly(ADP-ribose) polymerase 1 (PARP1) inhibitors may cause selective cell death of cancer cells: A novel therapeutic paradigm utilizing elevated copper levels within the tumour. Med. Hypotheses 2020, 144, 110278. [Google Scholar] [CrossRef] [PubMed]
- Bellio, C.; DiGloria, C.; Foster, R.; James, K.; Konstantinopoulos, P.A.; Growdon, W.B.; Rueda, B.R. PARP Inhibition Induces Enrichment of DNA Repair-Proficient CD133 and CD117 Positive Ovarian Cancer Stem Cells. Mol. Cancer Res. 2019, 17, 431–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piatek, K.; Schepelmann, M.; Kallay, E. The Effect of Vitamin D and Its Analogs in Ovarian Cancer. Nutrients 2022, 14, 3867. https://doi.org/10.3390/nu14183867
Piatek K, Schepelmann M, Kallay E. The Effect of Vitamin D and Its Analogs in Ovarian Cancer. Nutrients. 2022; 14(18):3867. https://doi.org/10.3390/nu14183867
Chicago/Turabian StylePiatek, Karina, Martin Schepelmann, and Enikö Kallay. 2022. "The Effect of Vitamin D and Its Analogs in Ovarian Cancer" Nutrients 14, no. 18: 3867. https://doi.org/10.3390/nu14183867
APA StylePiatek, K., Schepelmann, M., & Kallay, E. (2022). The Effect of Vitamin D and Its Analogs in Ovarian Cancer. Nutrients, 14(18), 3867. https://doi.org/10.3390/nu14183867







