Ginsenosides Restore Lipid and Redox Homeostasis in Mice with Intrahepatic Cholestasis through SIRT1/AMPK Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Preparation of GS and Fingerprint Detection
2.3. Animals and Experimental Protocol
2.4. Cell Culture
2.5. Detection of Biochemical Index
2.6. Histopathological Studies
2.7. Oil Red O Staining
2.8. Bodipy Staining
2.9. Immunofluorescent Staining
2.10. Detection of ROS in HepG2
2.11. Western Blotting Analysis
2.12. Quantification Real-Time PCR
2.13. RNA Silencing
2.14. Statistical Analysis
3. Results
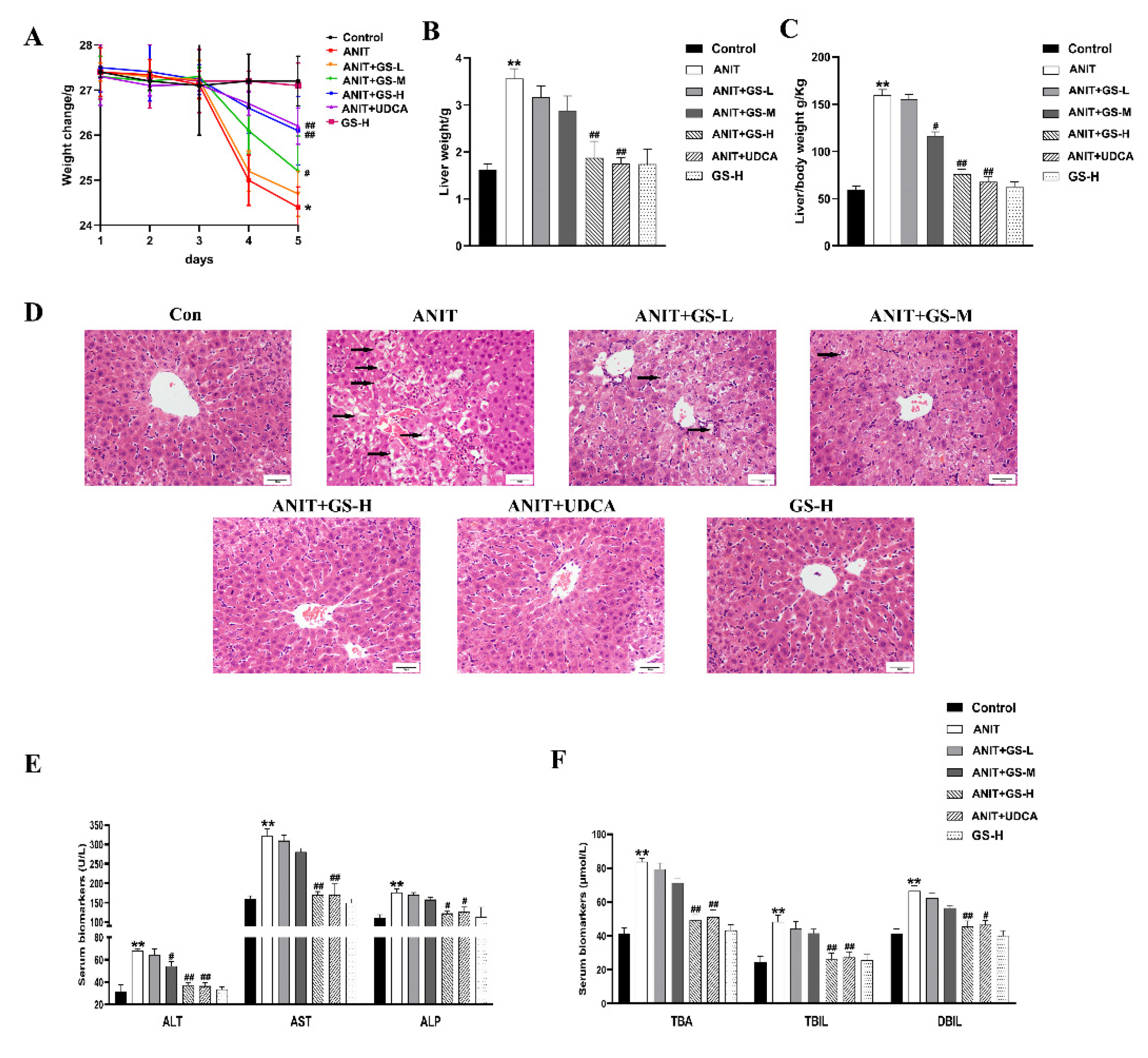
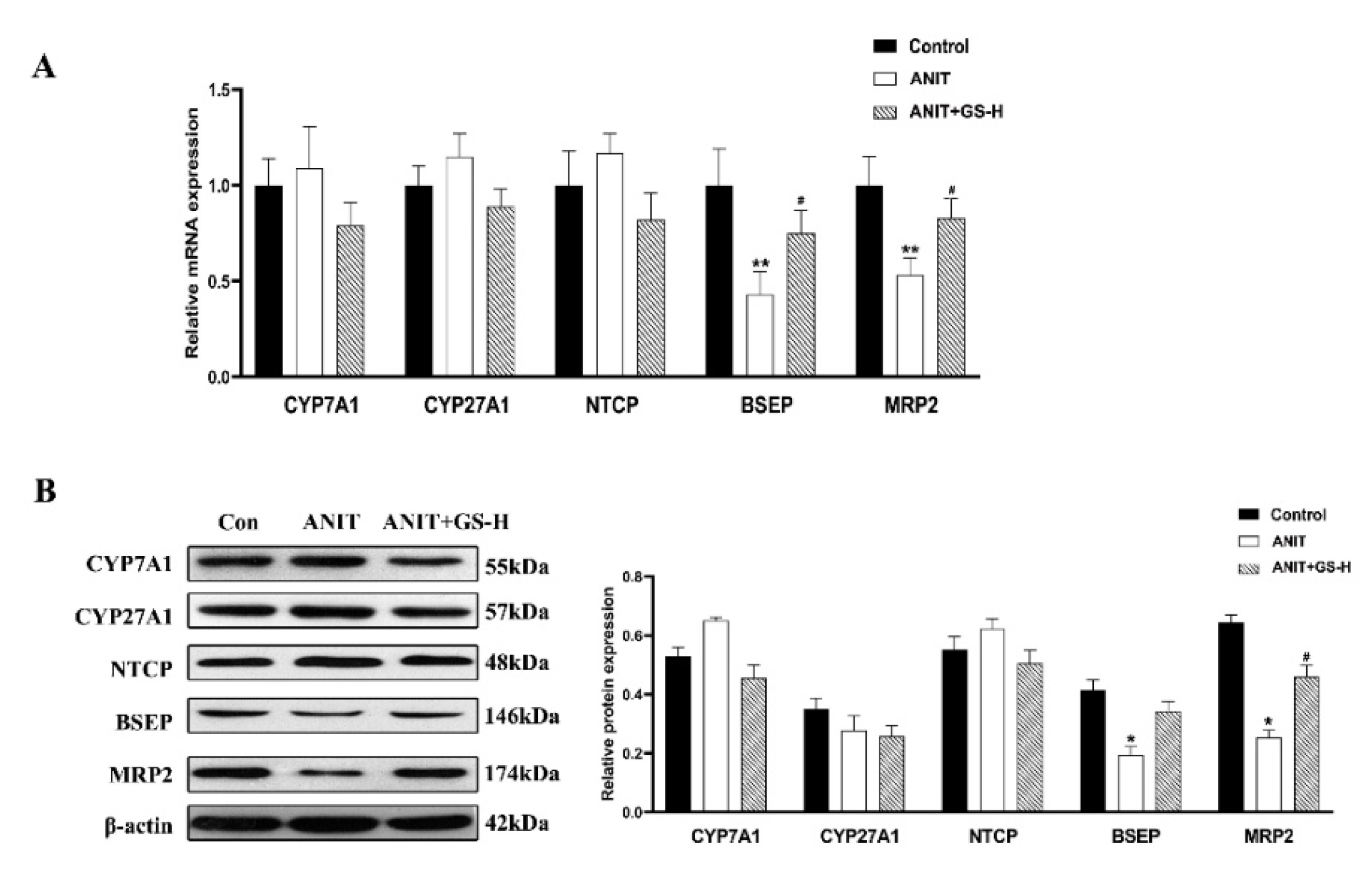
3.1. GS Alleviated ANIT-Induced Cholestatic Liver Injury in Mice
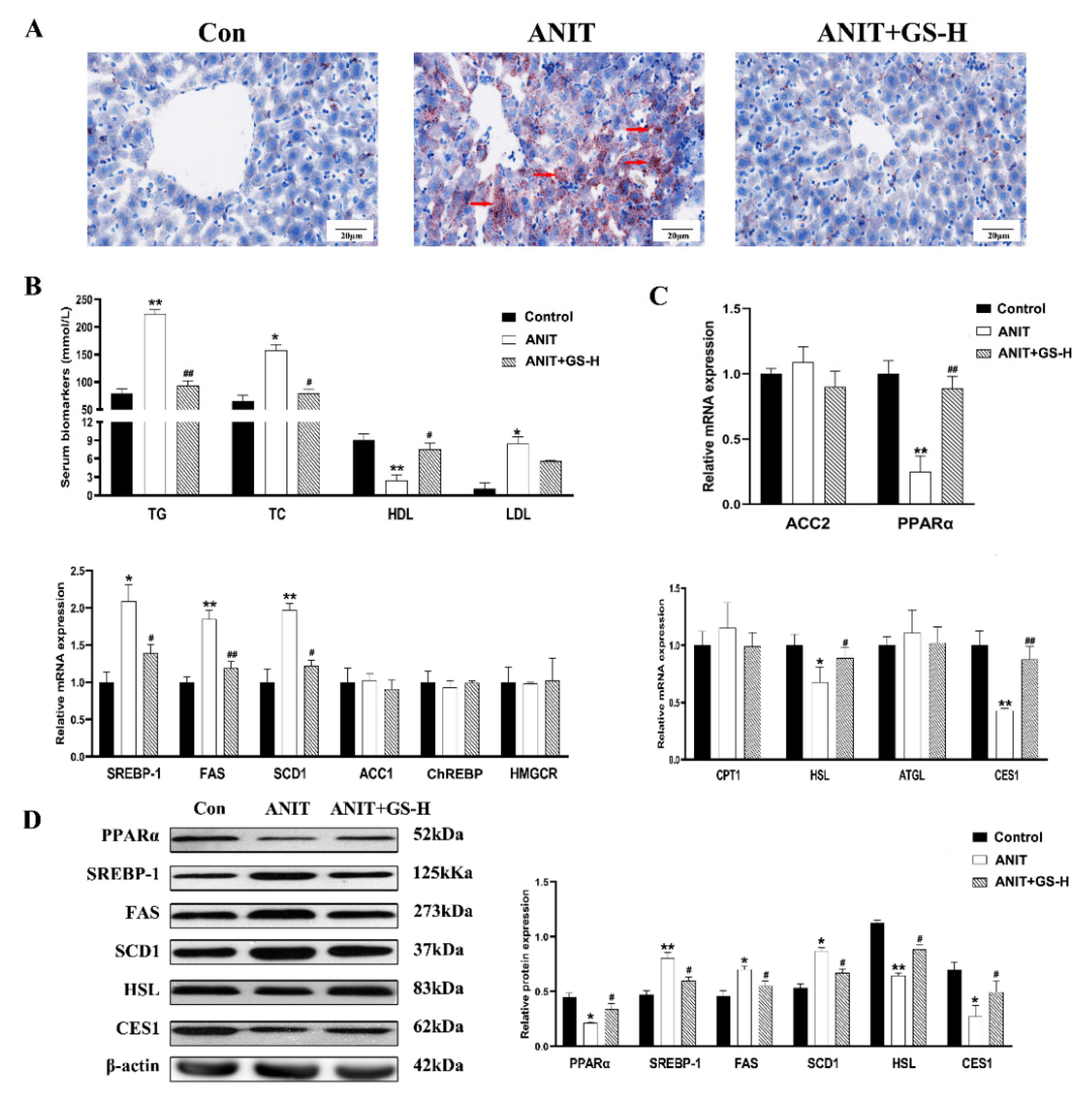
3.2. GS Restored ANIT-Induced Lipid Homeostasis
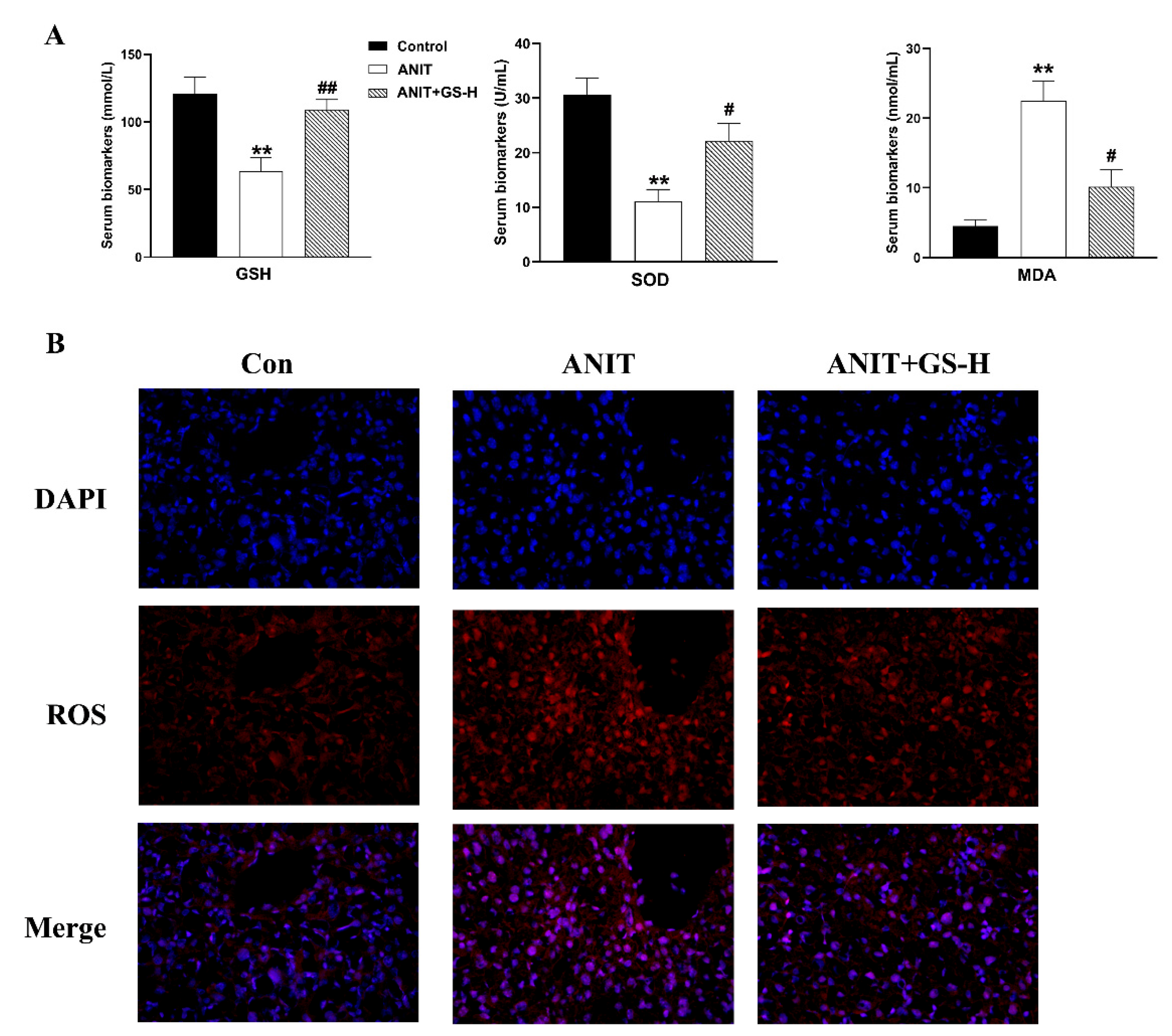
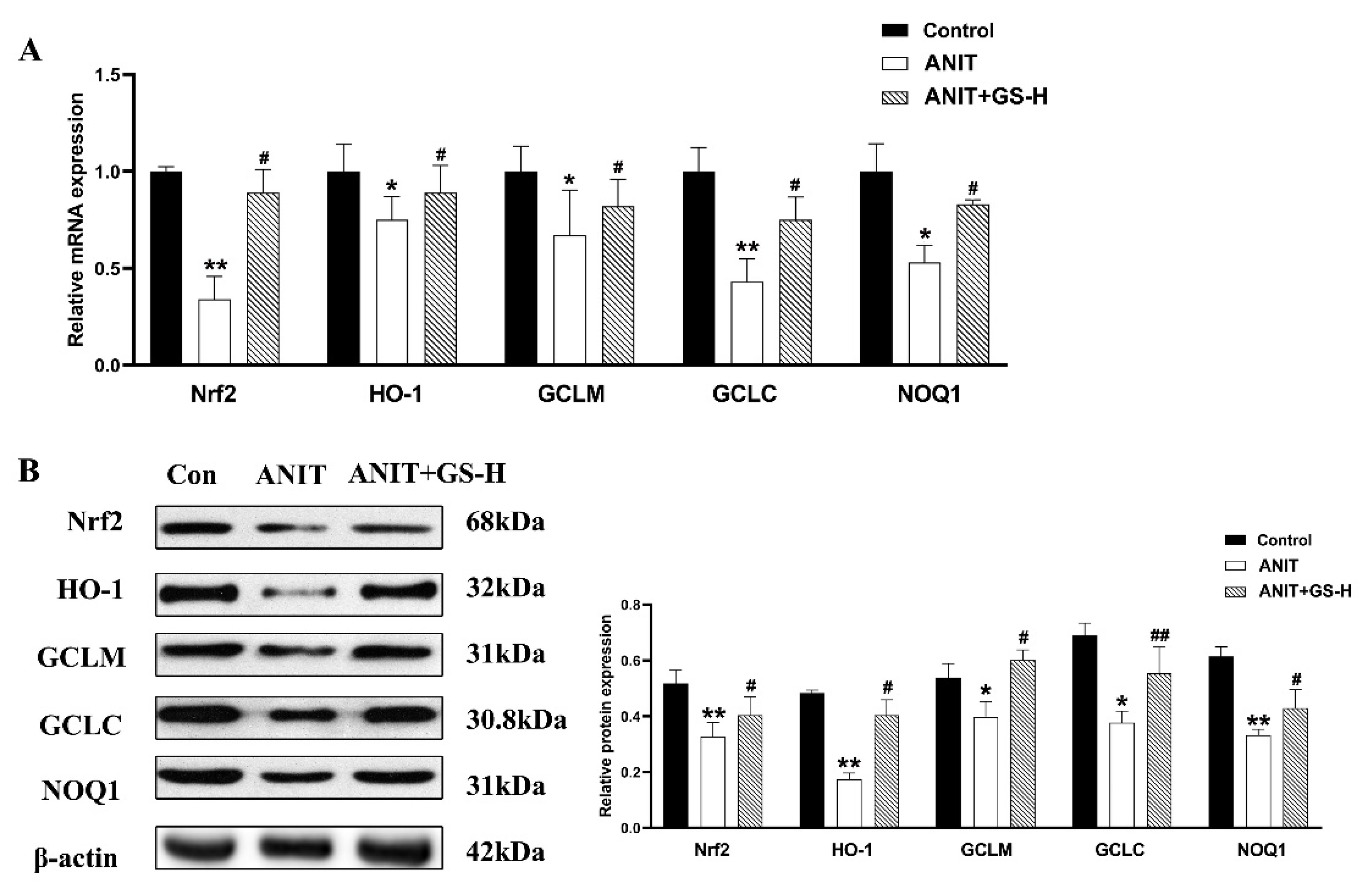
3.3. GS Inhibited ANIT-Induced Oxidative Stress in Mice
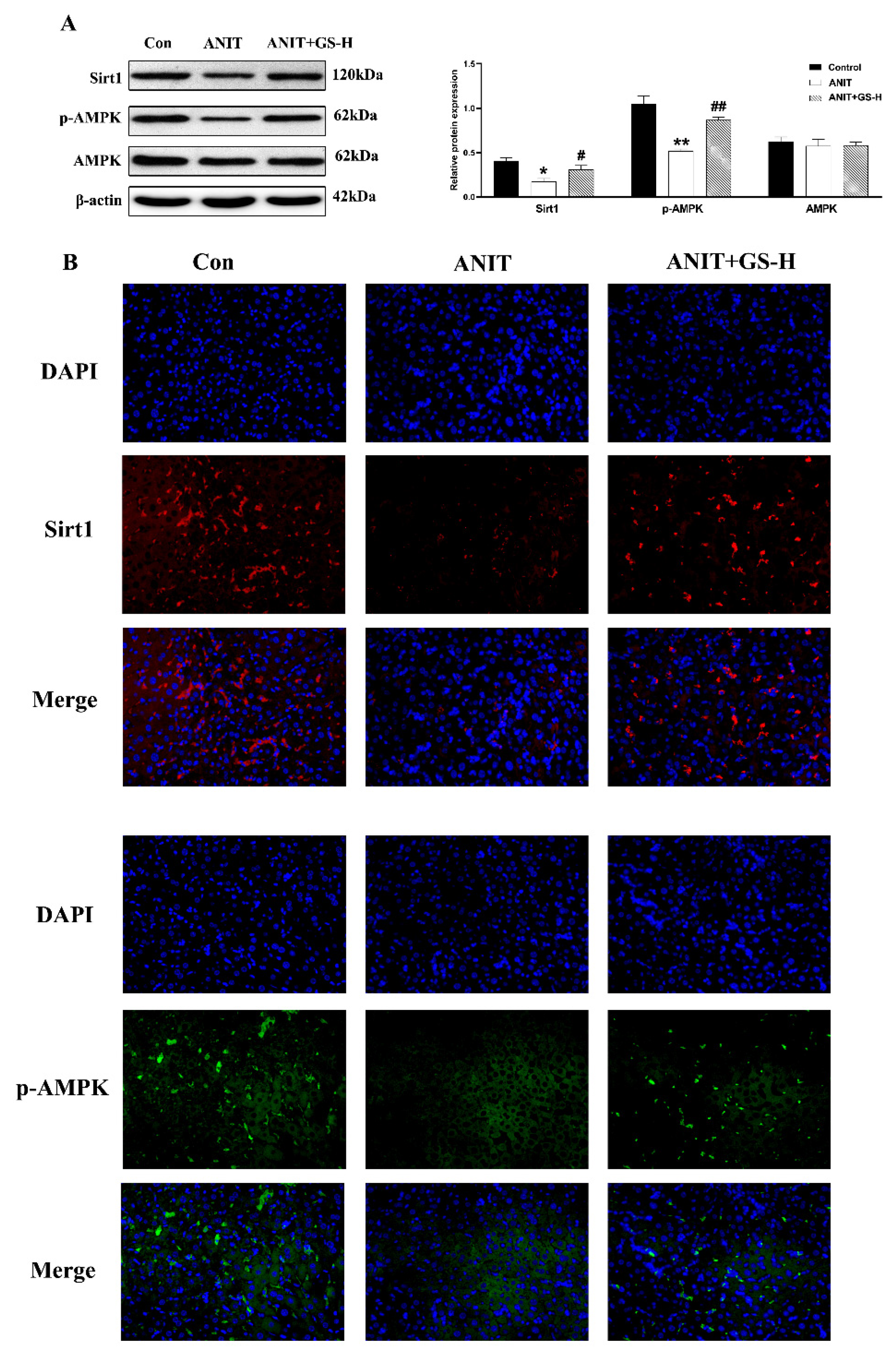
3.4. GS Ameliorated ANIT-Induced Low SIRT1/AMPK Expression in Mice
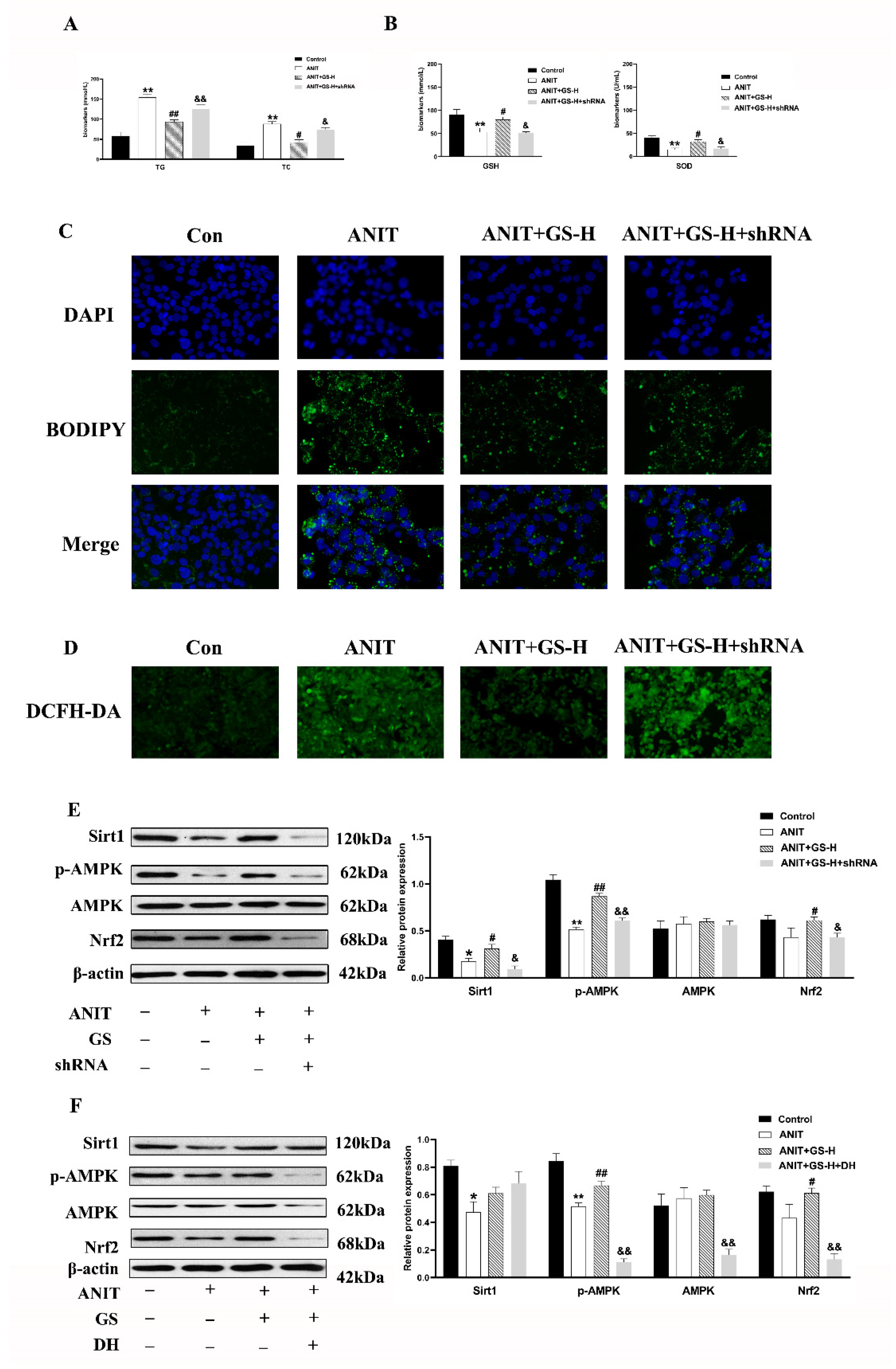
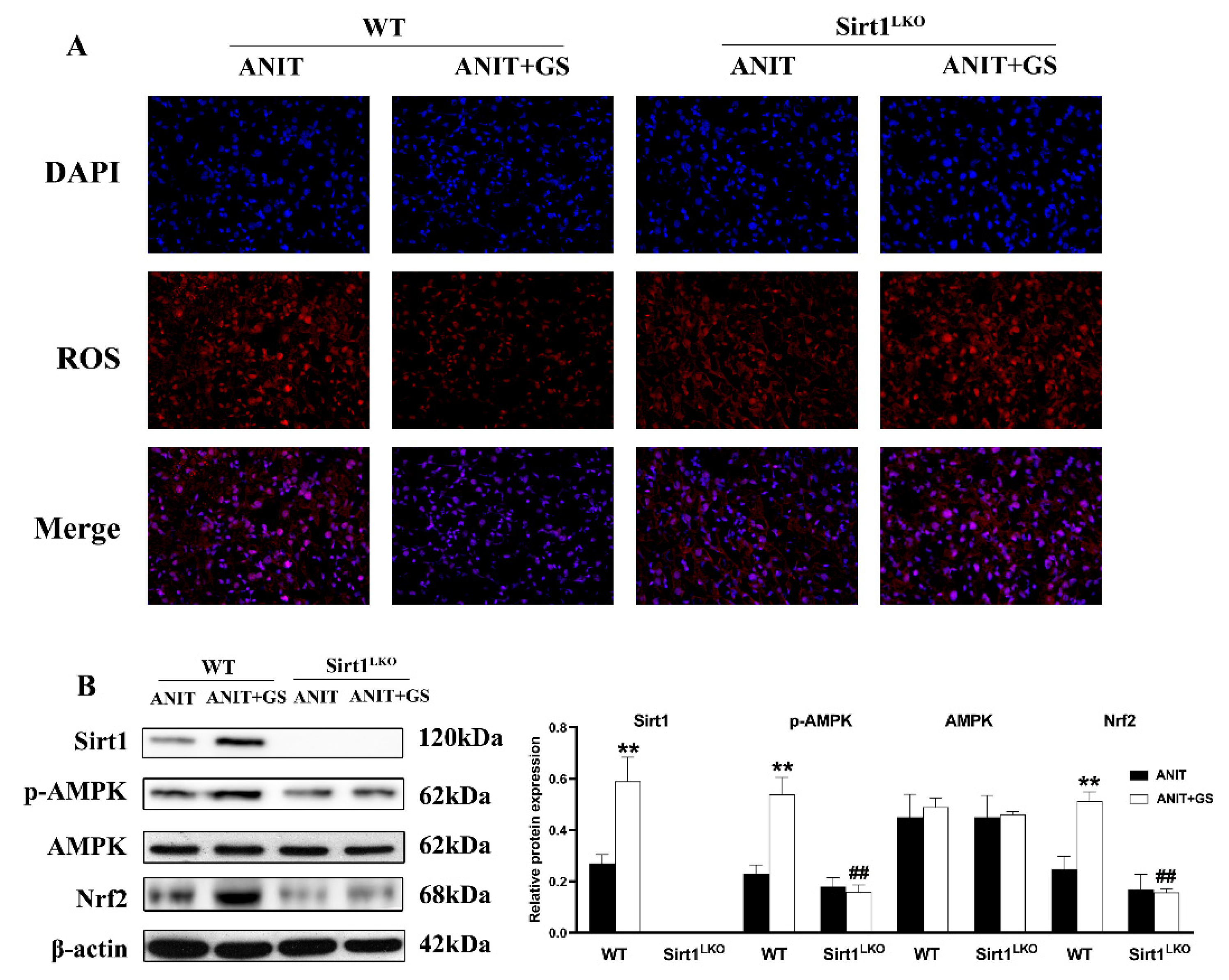
3.5. GS Protected against ANIT-Induced IC by Activating AMPK/Nrf2 via SIRT1 Signaling Pathway In Vitro
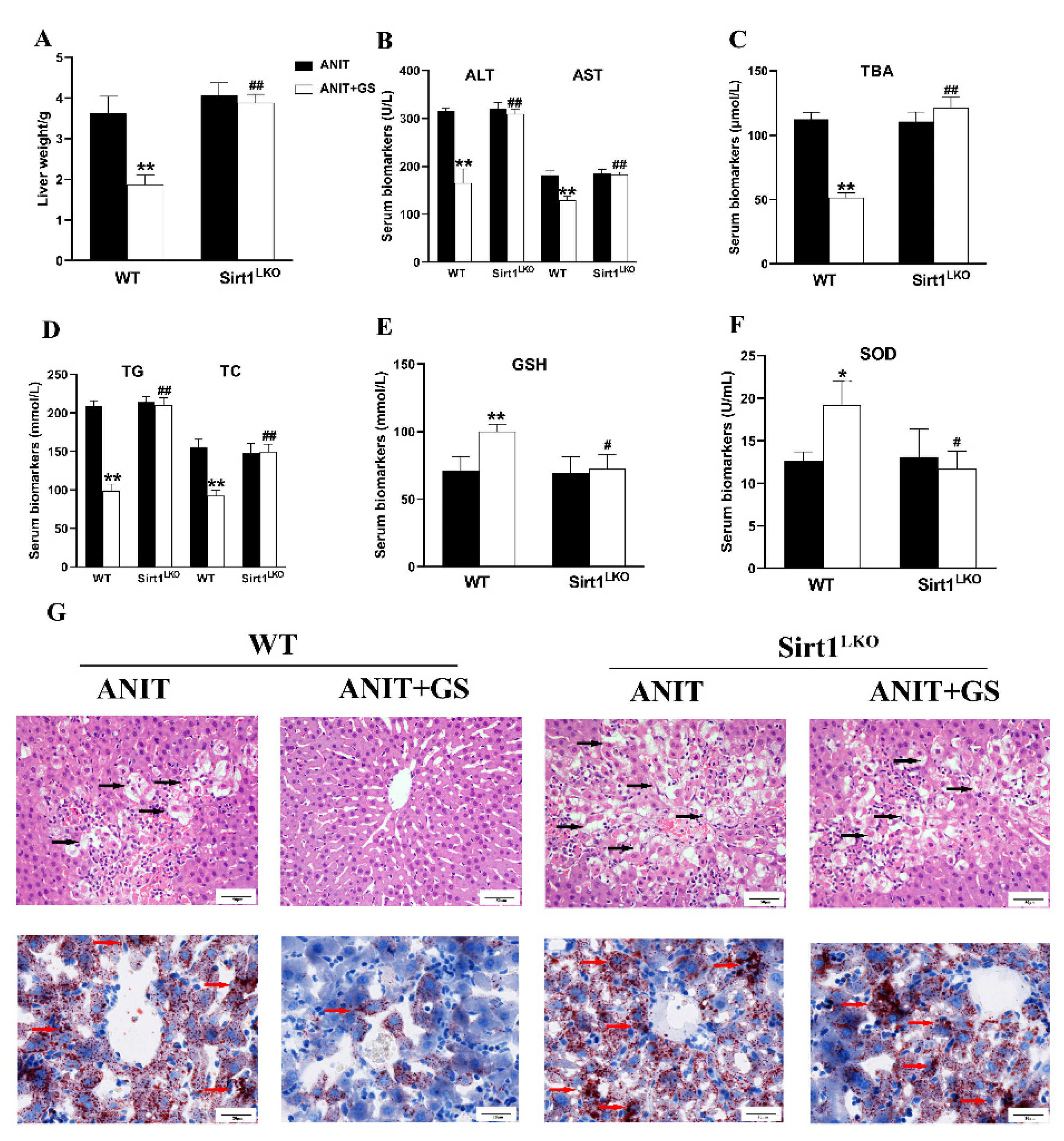
3.6. GS Ameliorated ANIT-Induced IC by Activating the AMPK/Nrf2 via SIRT1 in Mice
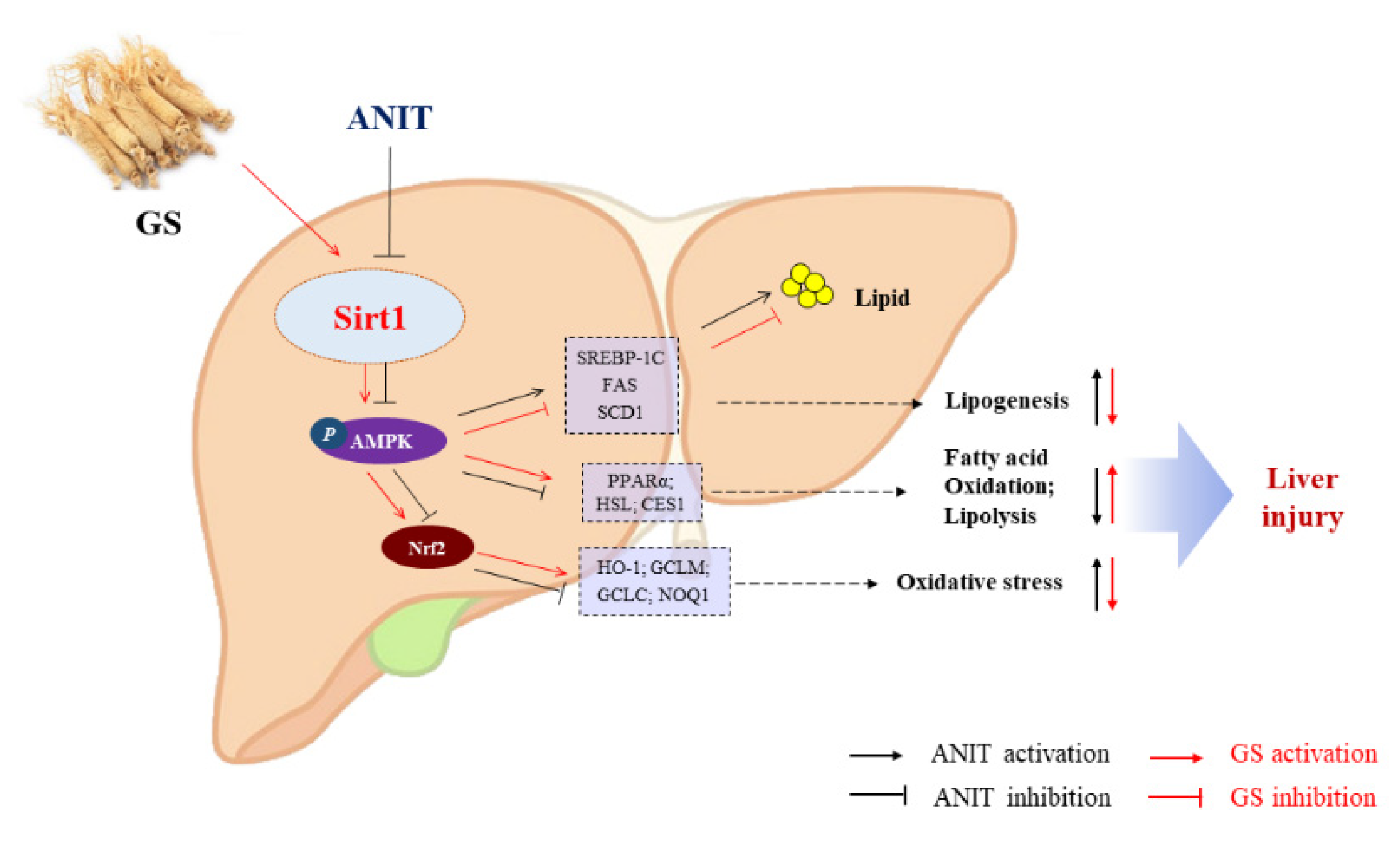
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dyson, J.K.; Hirschfield, G.M.; Adams, D.H.; Beuers, U.; Mann, D.A.; Lindor, K.D.; Jones, D.E.J. Novel therapeutic targets in primary biliary cirrhosis. Nat. Rev. Gastro. Hepat. 2015, 12, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Liu, X.X.; Yuan, Z.H.; Li, X.J.Y.; Yang, H.; Yuan, Z.Q.; Sun, L.X.; Zhang, L.Y.; Jiang, Z.Z. SRT1720 Alleviates ANIT-Induced Cholestasis in a Mouse Model. Front. Pharmacol. 2017, 8, 256. [Google Scholar] [CrossRef] [PubMed]
- Manos, M.M.; Leyden, W.A.; Murphy, R.C.; Terrault, N.A.; Bell, B.P. Limitations of conventionally derived chronic liver disease mortality rates: Results of a comprehensive assessment. Hepatology 2008, 47, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Menżyk, T.; Bator, M.; Derra, A.; Kierach, R.; Kukla, M. The role of metabolic disorders in the pathogenesis of intrahepatic cholestasis of pregnancy. Clin. Exp. Hepatol. 2018, 4, 217–223. [Google Scholar] [CrossRef]
- Dann, A.T.; Kenyon, A.P.; Wierzbicki, A.S.; Seed, P.T.; Shennan, A.H.; Tribe, R.M. Plasma Lipid Profiles of Women with intrahepatic cholestasis of pregnancy. Obstet. Gynecol. 2006, 107, 106–114. [Google Scholar] [CrossRef]
- Kamisako, T.; Ogawa, H. Effect of obstructive jaundice on the regulation of hepatic cholesterol metabolism in the rat. Disappearance of abcg5 and abcg8 mRNA after bile duct ligation. Hepatol. Res. 2003, 25, 99–104. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, L.Q.; Cui, Y.; Qi, Z.Q.; Huang, X.P.; Cai, L.Y.; Zhang, T.; Yin, Y.X.; Lu, Z.Y.; Xiang, J.Y. Roles of PPARγ/NF-κB Signaling Pathway in the Pathogenesis of Intrahepatic Cholestasis of Pregnancy. PLoS ONE 2014, 9, e87343. [Google Scholar] [CrossRef]
- Aynur, K.Y.; Büşra, C.; Özlem, Ü.U.; Uncuoğlu, A.; Gündüz, M. A novel etiologic factor of highly elevated cholestanol levels: Progressive familial intrahepatic cholestasis. J. Pediatric Endocrinol. Metab. 2020, 33, 665. [Google Scholar] [CrossRef]
- Jin, M.Y.; Feng, H.H.; Wang, Y.; Yan, S.R.; Shen, B.Y.; Li, Z.; Qin, H.Y.; Wang, Q.; Li, J.X.; Liu, G.W. Gentiopicroside Ameliorates Oxidative Stress and Lipid Accumulation through Nuclear Factor Erythroid 2-Related Factor 2 Activation. Oxid. Med. Cell Longev. 2020, 2020, 2940746. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.F.; She, Z.G.; Cai, J.J.; Li, H.L. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, K.; Hogstrand, C.; Xu, Y.H.; Chen, G.H.; Wei, C.C.; Luo, Z. Lipophagy mediated carbohydrate-induced changes of lipid metabolism via oxidative stress, endoplasmic reticulum (ER) stress and ChREBP/PPARγ pathways. Cell Mol. Life Sci. 2020, 77, 1987–2003. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Gershwin, M.E. Finding the cure for primary biliary cholangitis-Still waiting. Liver Int. 2017, 37, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Samant, H.; Manatsathit, W.; Dies, D.; Shokouh-Amiri, H.; Zibari, G.; Boktor, M.; Alexander, J.S. Cholestatic liver diseases: An era of emerging therapies. World J. Clin. Cases 2019, 7, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.X.; Huang, Y.L.; Zheng, H.; Li, S.Q.; Li, Z.H.; Yuan, L.; Cheng, X.; He, C.S.; Sun, J.F. Ginsenosides for the treatment of metabolic syndrome and cardiovascular diseases: Pharmacology and mechanisms. Biomed. Pharmacother. 2020, 132, 110915. [Google Scholar] [CrossRef]
- Lee, H.S.; Lim, S.M.; Jung, J.I.; Kim, S.M.; Lee, J.K.; Kim, Y.H.; Cha, K.M.; Oh, T.K.; Moon, J.M.; Kim, T.Y.; et al. Gynostemma Pentaphyllum Extract Ameliorates High-Fat Diet-Induced Obesity in C57BL/6N Mice by Upregulating SIRT1. Nutrients 2019, 11, 2475. [Google Scholar] [CrossRef]
- Yao, Y. Ginsenosides reduce body weight and ameliorate hepatic steatosis in high fat diet-induced obese mice via endoplasmic reticulum stress and p-STAT3 TAT3 signaling. Mol. Med. Rep. 2020, 21, 1059–1070. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.Q.; Zhou, Y.D.; Hou, J.G.; Liu, Y.; Wang, Y.P.; Gong, X.J.; Lin, X.H.; Jiang, S.; Wang, Z. Rare Ginsenoside 20(R)-Rg3 Inhibits D-Galactose-Induced Liver and Kidney Injury by Regulating Oxidative Stress-Induced Apoptosis. Am. J. Chin. Med. 2020, 48, 1141–1157. [Google Scholar] [CrossRef]
- Qu, L.L.; Zhu, Y.Y.; Liu, Y.N.; Yang, H.X.; Zhu, C.H.; Ma, P.; Deng, J.J.; Fan, D.D. Protective effects of ginsenoside Rk3 against chronic alcohol-induced liver injury in mice through inhibition of inflammation, oxidative stress, and apoptosis. Food Chem. Toxicol. 2019, 126, 277–284. [Google Scholar] [CrossRef]
- Xu, Y.J.; Yu, Z.Q.; Zhang, C.L.; Li, X.P.; Feng, C.Y.; Lei, K.; He, W.X.; Liu, D. Protective Effects of Ginsenosides on 17 [Formula: See text]-Ethynyelstradiol-Induced Intrahepatic Cholestasis via Anti-Oxidative and Anti-Inflammatory Mechanisms in Rats. Am. J. Chin. Med. 2017, 45, 1613–1629. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Zhang, Z.G.; Li, C.B.; Zhu, T.T.; Gao, J. S100A11 Promotes Liver Steatosis via FOXO1-Mediated Autophagy and Lipogenesis. Cell. Mol. Gastroenterol. Hepatol. 2020, 3, 697–724. [Google Scholar] [CrossRef]
- Guang, W.; Yang, C.K.; Wong, W.Y.; Zhang, N.; Wei, Y.F.; Zhang, J.L.; Yan, Y.; Tang, J.J.; Chuai, L.; Lee, K.K.H.; et al. Liver Fibrosis Can Be Induced by High Salt Intake through Excess Reactive Oxygen Species (ROS) Production. J. Agric. Food Chem. 2016, 64, 1610–1617. [Google Scholar] [CrossRef]
- Luo, Y.; Xiang, Z.; Min, L.; Yarong, H. RETRACTED: Echinacoside improves diabetic liver injury by regulating the AMPK/SIRT1 signaling pathway in db/db mice. Life Sci. 2021, 271, 119237. [Google Scholar] [CrossRef]
- Meng, Q.H.; Qi, X.; Fu, Y.; Chen, Q.; Cheng, P.; Yu, X.C.; Sun, X.; Wu, J.Z.; Li, W.W.; Zhang, Q.C.; et al. Flavonoids extracted from mulberry (Morus alba L.) leaf improve skeletal muscle mitochondrial function by activating AMPK in type 2 diabetes. J. Ethnopharmacol. 2020, 248, 112326. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiang, D.; Xiang, D.; He, W.; Liu, Y.; Lan, L.; Li, G.; Jiang, C.; Ren, X.; Liu, D.; et al. Baicalin Protects Against 17aEthinylestradiol-Induced Cholestasis via the Sirtuin 1/Hepatic Nuclear Receptor-1a/Farnesoid X Receptor Pathway. Front. Pharmacol. 2020, 10, 1685. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yang, R.; Wang, J.; Hu, D.D.; Li, F. PPARα activation protects against cholestatic liver injury. Sci. Rep. 2017, 7, 9913–9967. [Google Scholar] [CrossRef]
- Carling, D. The AMP-activated protein kinase cascade—A unifying system for energy control. Trends. Biochem. Sci. 2004, 29, 18–24. [Google Scholar] [CrossRef]
- Rui, B.B.; Chen, H.; Jang, L.; Li, Z.; Yang, J.M.; Xu, W.P.; Wei, W. Melatonin Upregulates the Activity of AMPK and Attenuates Lipid Accumulation in Alcohol-induced Rats. Alcohol Alcohol. 2015, 51, 11–19. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Kemp, B.E. AMPK in Health and Disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef]
- Boyer, J.L. New perspectives for the treatment of cholestasis: Lessons from basic science applied clinically. J. Hepatol. 2007, 46, 365–371. [Google Scholar] [CrossRef]
- Ning, C.Q.; Gao, X.G.; Wang, C.Y.; Huo, X.K.; Liu, Z.H.; Sun, H.J.; Yang, X.B.; Sun, P.Y.; Ma, X.D.; Meng, Q.; et al. Hepatoprotective effect of ginsenoside Rg1 from Panax ginseng on carbon tetrachloride-induced acute liver injury by activating Nrf2 signaling pathway in mice. Environ. Toxicol. 2018, 33, 1050–1060. [Google Scholar] [CrossRef]
- Qu, L.L.; Fu, R.Z.; Ma, X.X.; Fan, D.D. Hepatoprotective effects of ginsenoside Rk3 in acetaminophen-induced liver injury in mice by activation of autophagy. Food Funct. 2021, 12, 9128–9140. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; He, X.Q.; Zhao, J.Q.; Huang, W.X. Hepatoprotection by Ginsenoside Rg1 in alcoholic liver disease. Int. Immunopharmacol. 2021, 92, 107327. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, S.J.; Ren, H.N.; Du, R.Y.; Li, J.J.; Zhao, J.Q.; Gao, Y.; Zhu, Y.L.; Huang, W.X. Ginsenoside Rg1 alleviates ANIT-induced intrahepatic cholestasis in rats via activating farnesoid X receptor and regulating transporters and metabolic enzymes. Chem. Biol. Interact. 2020, 324, 109062. [Google Scholar] [CrossRef] [PubMed]
- Anwer, M.S. Role of protein kinase C isoforms in bile formation and cholestasis. Hepatology 2014, 60, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Aleksunes, L.M.; Cui, Y.J.; Klaassen, C.D. ANIT-Induced Intrahepatic Cholestasis Alters Hepatobiliary Transporter Expression via Nrf2-Dependent and Independent Signaling. Toxicol. Sci. 2009, 108, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Zhang, C.W.; Wang, L.; Tang, K.L.; Naoki, T.; Frank, J.G.; Xu, Y.; Fang, Z.Z. Lipidomics reveal Aryl hydrocarbon receptor (Ahr)-regulated lipid metabolic pathway in alpha-naphthyl isothiocyanate(ANIT)-induced intrahepatic cholestasis. Xenobiotica 2019, 49, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Han, M.X.; Zhang, X.L.; Tong, H.N.; Sun, X.B.; Sun, G.B. Ginsenoside Rb1 Protects Against Diabetic Cardiomyopathy by Regulating the Adipocytokine Pathway. J. Inflamm. Res. 2022, 15, 71–83. [Google Scholar] [CrossRef]
- Chang, H.C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef]
- Hardie, D.G. AMPK: A key regulator of energy balance in the single cell and the whole organism. Int. J. Obes. 2008, 32 (Suppl. 4), S7–S12. [Google Scholar] [CrossRef]
- Viollet, B.; Guigas, B.; Leclerc, J.; Hebrard, S.; Lantier, L.; Mounier, R.; Andreelli, F.; Foretz, M. AMP-activated protein kinase in the regulation of hepatic energy metabolism: From physiology to therapeutic perspectives. Acta Physiol. 2009, 196, 81–98. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.F.; Tan, J.; Li, M.M.; Song, S.L.; Miao, Y.Y.; Zhang, Q. Sirt1: Role Under the Condition of Ischemia/Hypoxia. Cell Mol. Neurobiol. 2017, 37, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Zhou, C.X.; Shi, Y.B.; Lu, H.; Xu, R.F.; He, X.Z. Nuclear transcription factor Nrf2 suppresses prostate cancer cells growth and migration through upregulating ferroportin. Oncotarget 2016, 7, 78804–78812. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.X.; Su, H.; Qi, B.; Wang, Y.; Yan, K.L.; Wang, X.L.; Li, X.Y.; Zhao, D.Q. A SIRT1 Activator, Ginsenoside Rc, Promotes Energy Metabolism in Cardiomyocytes and Neurons. J. Am. Chem. Soc. 2021, 143, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.J.; Hou, J.G.; Ma, Z.N.; Wang, Z.; Ren, S.; Wang, Y.P.; Liu, W.C.; Chen, C.; Li, W. Ginsenoside Rb3 provides protective effects against cisplatin-induced nephrotoxicity via regulation of AMPK-/mTOR-mediated autophagy and inhibition of apoptosis In Vitro and In Vivo. Cell Prolif. 2019, 52, e12627. [Google Scholar] [CrossRef] [PubMed]
- Korivi, M.; Hou, C.W.; Huang, C.Y.; Lee, S.D.; Hsu, M.F.; Yu, S.H.; Chen, C.Y.; Liu, Y.Y.; Kuo, C.H. Ginsenoside-Rg1 Protects the Liver against Exhaustive Exercise-Induced Oxidative Stress in Rats. Evid. Based Complement Alternat. Med. 2012, 2012, 932165. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Xu, Y.; Gao, Q.; Guo, S.; Zu, Y.; Wang, X.; Wang, C.; Zhang, C.; Liu, D. Ginsenosides Restore Lipid and Redox Homeostasis in Mice with Intrahepatic Cholestasis through SIRT1/AMPK Pathways. Nutrients 2022, 14, 3938. https://doi.org/10.3390/nu14193938
Li G, Xu Y, Gao Q, Guo S, Zu Y, Wang X, Wang C, Zhang C, Liu D. Ginsenosides Restore Lipid and Redox Homeostasis in Mice with Intrahepatic Cholestasis through SIRT1/AMPK Pathways. Nutrients. 2022; 14(19):3938. https://doi.org/10.3390/nu14193938
Chicago/Turabian StyleLi, Guodong, Yanjiao Xu, Qianyan Gao, Sheng Guo, Yue Zu, Ximin Wang, Congyi Wang, Chengliang Zhang, and Dong Liu. 2022. "Ginsenosides Restore Lipid and Redox Homeostasis in Mice with Intrahepatic Cholestasis through SIRT1/AMPK Pathways" Nutrients 14, no. 19: 3938. https://doi.org/10.3390/nu14193938



