Abstract
Background: Growth failure in infants born preterm is a significant issue, increasing the risk of poorer neurodevelopmental outcomes and metabolic syndrome later in life. During the first 1000 days of life biological systems mature rapidly involving developmental programming, cellular senescence, and metabolic maturation, regulating normal growth and development. However, little is known about metabolic maturation in infants born preterm and the relationship with growth. Objective: To examine the available evidence on urinary markers of metabolic maturation and their relationship with growth in infants born preterm. Eligibility criteria: Studies including in this scoping review using qualitative or quantitative methods to describe urinary markers of metabolic maturation and the relationship with growth in infants born preterm. Results: After a screening process 15 titles were included in this review, from 1998–2021 drawing from China (n = 1), Italy (n = 3), Germany (n = 3), Greece (n = 1), Japan (n = 2), Norway (n = 1), Portugal (n = 1), Spain (n = 2) and USA (n = 1). The included studies examined urinary metabolites in 1131 infants. A content analysis identified 4 overarching themes relating to; (i) metabolic maturation relative to gestational age, (ii) metabolic signature and changes in urinary metabolites over time, (iii) nutrition and (iv) growth. Conclusion: The results of this scoping review suggest there are considerable gaps in our knowledge relating to factors associated with metabolic instability, what constitutes normal maturation of preterm infants, and how the development of reference phenome age z scores for metabolites of interest could improve nutritional and growth outcomes.
1. Introduction
Globally, an estimated 15 million infants are born preterm (before 37 weeks gestational age) each year, with a prevalence of 5 to 18% depending on country of birth [1]. Current recommendations suggest the growth of preterm infants should aim to approximate the in utero growth of infants of the equivalent gestation [2,3,4], although defining optimal growth relative to short and long term outcomes continues to be debated [5]. During the first 1000 days growth not only involves increasing weight and body length, but also rapid maturation of the immune system, endocrine system and metabolic pathways [6,7,8]. Post-natal growth failure in preterm infants is a persistent problem and may result in poorer neurocognitive outcomes [5], as well as increasing the risk of morbidity and mortality [5,9,10]. Conversely rapid weight gain, particularly between 2.5 and 6 years of age, is associated with the development of metabolic syndrome and cardiovascular disease later in life [11,12]. Reasons for constrained growth are numerous but may include (i) failure to deliver sufficient nutrition, (ii) intestinal immaturity resulting in alterations of nutrient utilisation by the intestine or losses via renal system, (iii) metabolic immaturity leading to transient intolerance of lipids and glucose, (iv) dysregulated maturation of metabolic pathways and urinary losses of important metabolites, (v) dysbiosis of the microbiome with poor diversity and low abundance of intestinal microbiota, (vi) medical management including use of pharmacopeia (i.e., diuretics) increasing urinary losses of electrolytes and (vii) disruption in achieving nutrition targets [5,6,13,14,15].
Although there are well established nutritional recommendations from various expert groups pertaining to macro- and micronutrient requirements of preterm infants [4,16], the recommendations do not account for an individual preterm infant’s ability to assimilate nutrients or the ability to overcome potential aberrance in metabolic pathways [5,9,17]. As an example, some preterm infants, (especially those around the threshold of viability) experience metabolic immaturity and instability leading to sustained periods of hyperglycaemia and hypertriglyceridemia, meaning that some nutritional goals are not met during a time of rapid growth and organ development [9,17]. Current strategies to manage metabolic complications include the use of insulin (which is not without risk), or potentially an even cruder strategy of reducing the amount substrate (e.g., glucose and lipid) delivered with associated negative sequalae on macro- and micronutrient intake [9,10,17,18].
With the advent of high throughput analytic techniques to quantify components of biological samples, it is increasingly possible to consider the development a more nuanced approach to medical and nutritional management for a whole range of conditions [19]. To this end 1H nuclear magnetic resonance (1H-NMR) spectroscopy and mass spectroscopy (MS) can be used to analyse the metabolome in biological fluids such as urine [20,21] and identify signatures associated with different health and disease states [13]. Although blood has been comprehensively studied with regard to metabolomic analysis, preterm infants have small circulating blood volumes. As urine is chemically complex, metabolomic analysis has been shown to provide information on varying physiological states, metabolism signatures and functions [22]. In addition, urine is readily available, collection is non-invasive and easy making it an accessible biological fluid to study. Giallourou et al. used urinary metabolic profiling to study metabolic maturation of infants (n = 1131) from resource constrained settings over 3 continents in the first 1000 day. From this work they identified eight metabolic signatures which were independent of feeding practices. These were developed into time dependent variation in healthy compared to growth constrained infants phenome age for z scores (PAZ) [13]. In this setting the development of PAZ for the metabolites of interest provided the opportunity to plot individual metabolic maturity in real time and provide the opportunity to offer interventions targeted to an infant’s precise metabolic predisposition [13]. Developing PAZ scores for preterm infants may provide a better understanding of metabolic factors which may be contributing to extra uterine growth retardation [23].
Developing a better understand of dynamic changes to post-natal metabolic stability and maturity in preterm infants, may help to (i) develop normative z-scores for age for metabolites associated with metabolic stability and maturation in preterm infants, (ii) refine our understanding of nutritional needs based on metabolic maturity rather than chronological maturation, (iii) provide an opportunity to identify potential future targets for nutritional supplementation to promote metabolic maturation and improve growth outcomes [13,24,25,26,27].
A scoping review was chosen over a systematic review as the use of urinary metabolomics to quantify metabolic stability and maturity in preterm infants is a relatively unexplored area of nutritional and metabolic research. As a result, it was not possible to complete a systematic review with/without meta-analysis. The rationale for this methodological approach is explored by Munn et al. [28] further. This scoping review was carried out to gain a better understanding of where the current evidence base is in terms of achieving these goals.
2. Materials and Methods
We chose to complete a scoping review, as a method to systematically review the available literature completing a content and narrative review.
2.1. Preparing to Scope the Literature and Protocol Development
A scoping review was conducted to understand the range of evidence currently available and to map key concepts within it. Specifically, it aimed to address the question “Are there specific metabolic signatures which could be used to develop reference phenome age z score for metabolites of interest associated with metabolic maturation and growth?” For the purposes of this review, we defined preterm infants as born <37 weeks gestational age.
Scoping review methodology was chosen because it offers a framework to examine a broad range of evidence in an emerging field [28] and allows the analysis of current knowledge gaps and future research priorities. The Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) [29] was used to report the evidence examined in this review.
2.2. Protocol Development
The protocol was developed using the PRISMA-ScR checklist [29] and previously published work [30]. The protocol described; (1) the research question, (2) the information sources to be searched, (3) a description of the full electronic search strategy, (4) study inclusion and exclusion criteria (5) data extraction and charting, (6) collation of data, analysis, and critical appraisal to answer the research questions posed.
2.3. Data Sources Searched
The research questions were used to complete a literature search across multiple databases and thus identify relevant studies. The databases searched were PubMed, the Cochrane Library, NHS Evidence and the NICE Healthcare Databases Advanced Search website (HDAS) (https://hdas.nice.org.uk/) (accessed 30 January 2022). HDAS was used to allow searches within multiple databases, including AMED, BNI, Cinahl, Embase, Health Business Elite, HMIC, Medline and PsycInfo.
2.4. The Search Strategy
A search strategy was devised with the assistance of a PubMed information specialist. The search strategy used key words from articles relating to infants born preterm (Appendix Table A1 and Table A2). Searches were adapted for the additional electronic databases. Forward and backward citation searching was completed on full text articles selected with no predefined start date until February 2022.
2.5. Study Selection
Studies were eligible for inclusion if they were written in the English language, describing urinary markers of metabolic maturation or growth in preterm infants. Opinion pieces, editorials and congress abstracts were excluded as per the scoping methodology advocated by Aksey and O’Malley [31]. Article titles and abstracts were screened, duplicates deleted, and then full text articles reviewed for eligibility (SP, LVM, JJA, CW). Where multiple articles described the same cohort of children these were only counted once. Bibliographies of included studies were hand searched for additional studies which may fulfil the inclusion criteria. Exclusion criteria included studies, infants with other primary pathologies and metabolites described in other fluids.
2.6. Data Extraction and Charting
Data extraction was completed using a two-stage process. For quality control article titles and abstracts were screened, duplicates deleted, and then full text articles reviewed for eligibility (SP, LVM, JJA, CW). A data extraction template (Microsoft 2010, Redmond, WA, USA) was used to capture the study design, results, and conclusions. This was followed by content analysis.
2.7. Collating, Summarising and Reporting Results
Data synthesis was completed using a content analysis approach. Content analysis was chosen as it is an established technique for reporting subjects common to multiple data sets [31,32]. Descriptive aspects about the population studied, methodology, outcomes and any key findings were coded. Content analysis was completed by coding initial themes, which were grouped into sub-categories and then into overarching themes. The overarching themes and sub-categories from this process were used to develop a summary table. A narrative data synthesis was also completed [33] summarising results of the identified studies.
3. Results
3.1. Study Characteristics
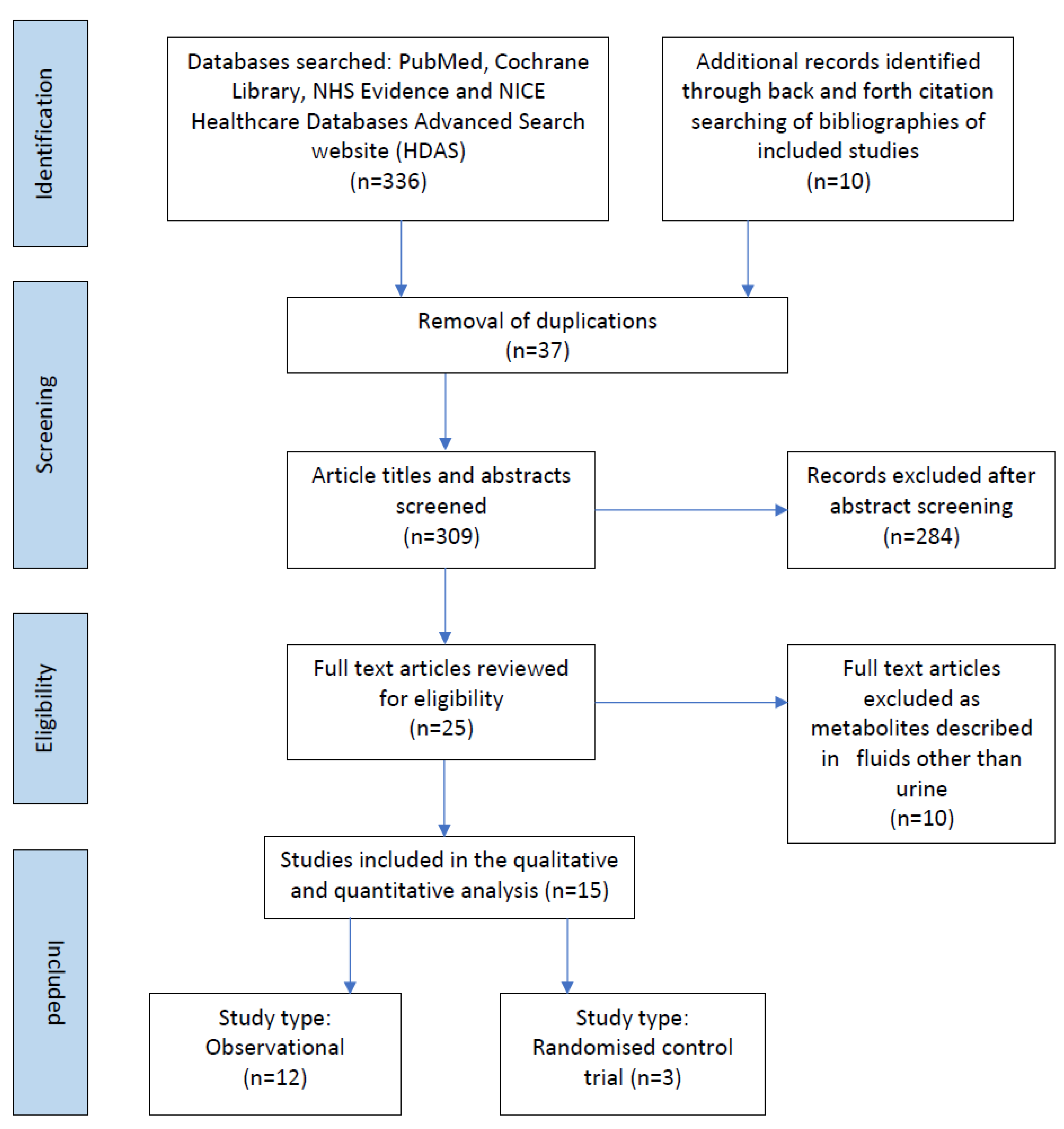
336 records were identified, of which 37 were duplicates. Following the removal of duplicate records, 309 records abstracts and titles were screened for inclusion (Figure 1). The full texts of 25 articles were reviewed for eligibility, of which 15 related to preterm infants, from 1998–2021 drawing from China (n = 1), Italy (n = 3), Germany (n = 3), Greece (n = 1), Japan (n = 2), Norway (n = 1), Portugal (n = 1), Spain (n = 2) and USA (n = 1). The included studies examined urinary metabolites in 1131 infants.
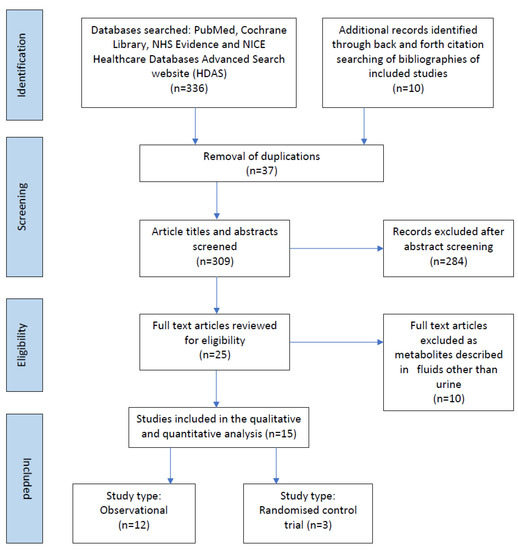
Figure 1.
Prisma flow chart of studies included in the scoping review.
3.2. Narrative Data Synthesis
A narrative data synthesis identified preterm birth was associated with deficiencies in amino acid, carbohydrate, and fatty acid metabolism pathways and metabolites associated with energy and protein pathways are downregulated (Table 1) [34,35,36,37,38,39,40,41,42,43,44,45,46].

Table 1.
Studies describing urinary metabolites in preterm infants [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48].
3.3. Content Analysis and Overarching Themes
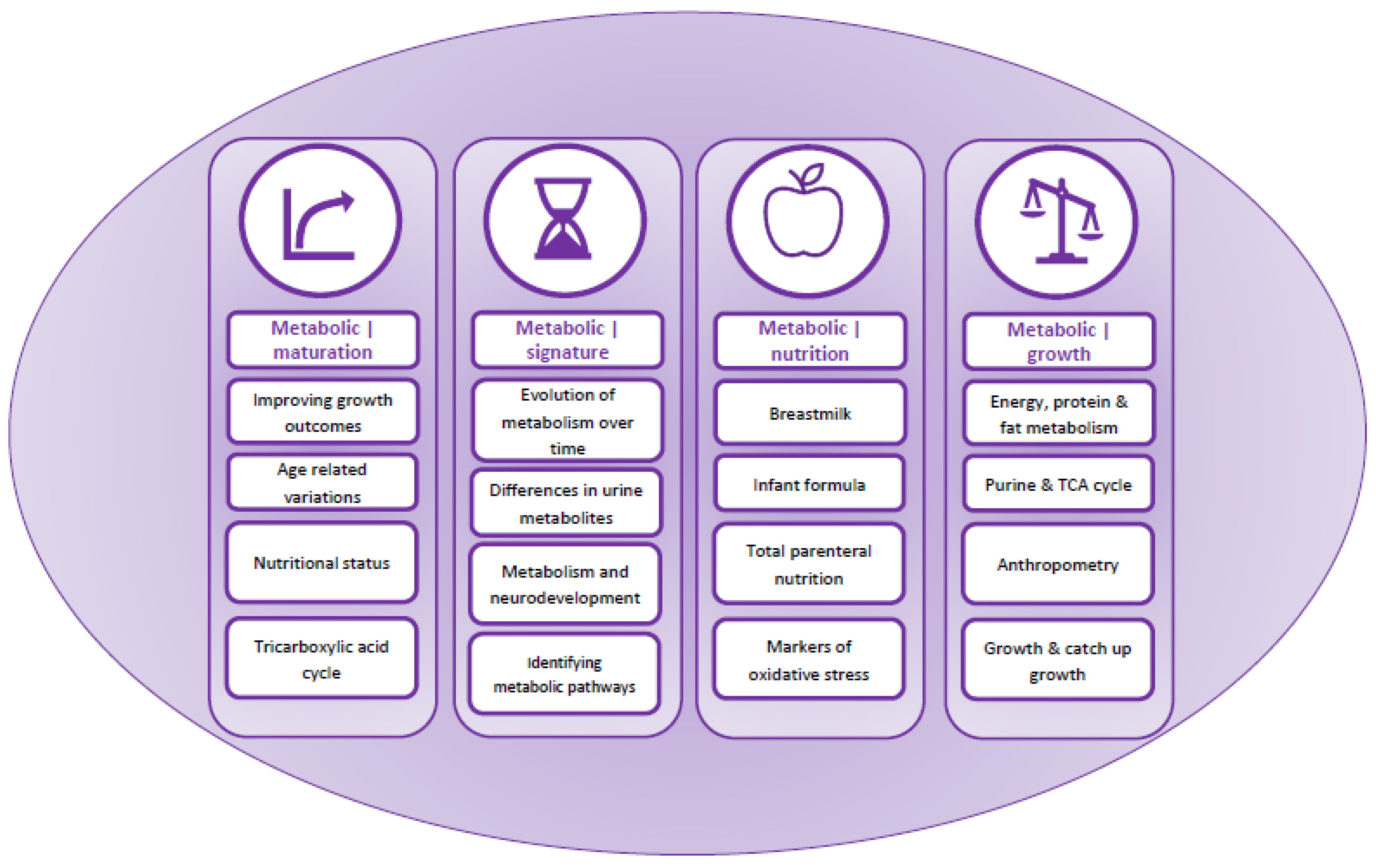
Content analysis identified four overarching themes relating to, (i) metabolic maturation relative to gestational age, (ii) metabolic signature and changes in urinary metabolites over time, (iii) nutrition and (iv) growth (Table 2 and Figure 2). These were used to develop a summary of factors affecting metabolic maturation in infants born preterm (Figure 3), and describe metabolites associated with each of the themes (Table 2).

Table 2.
Content analysis of metabolite maturation in infants born preterm.
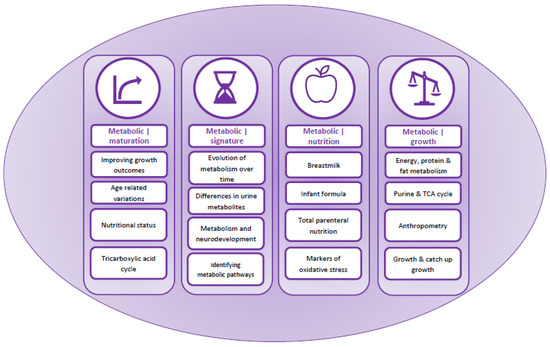
Figure 2.
Graphical representation of the narrative synthesis and content analysis.
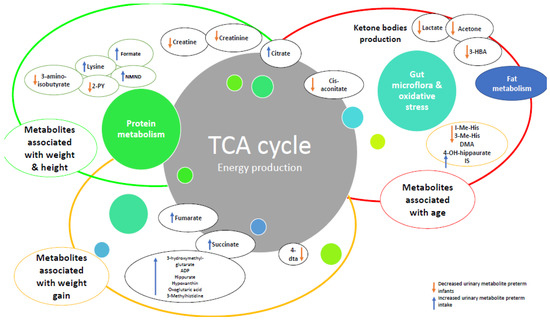
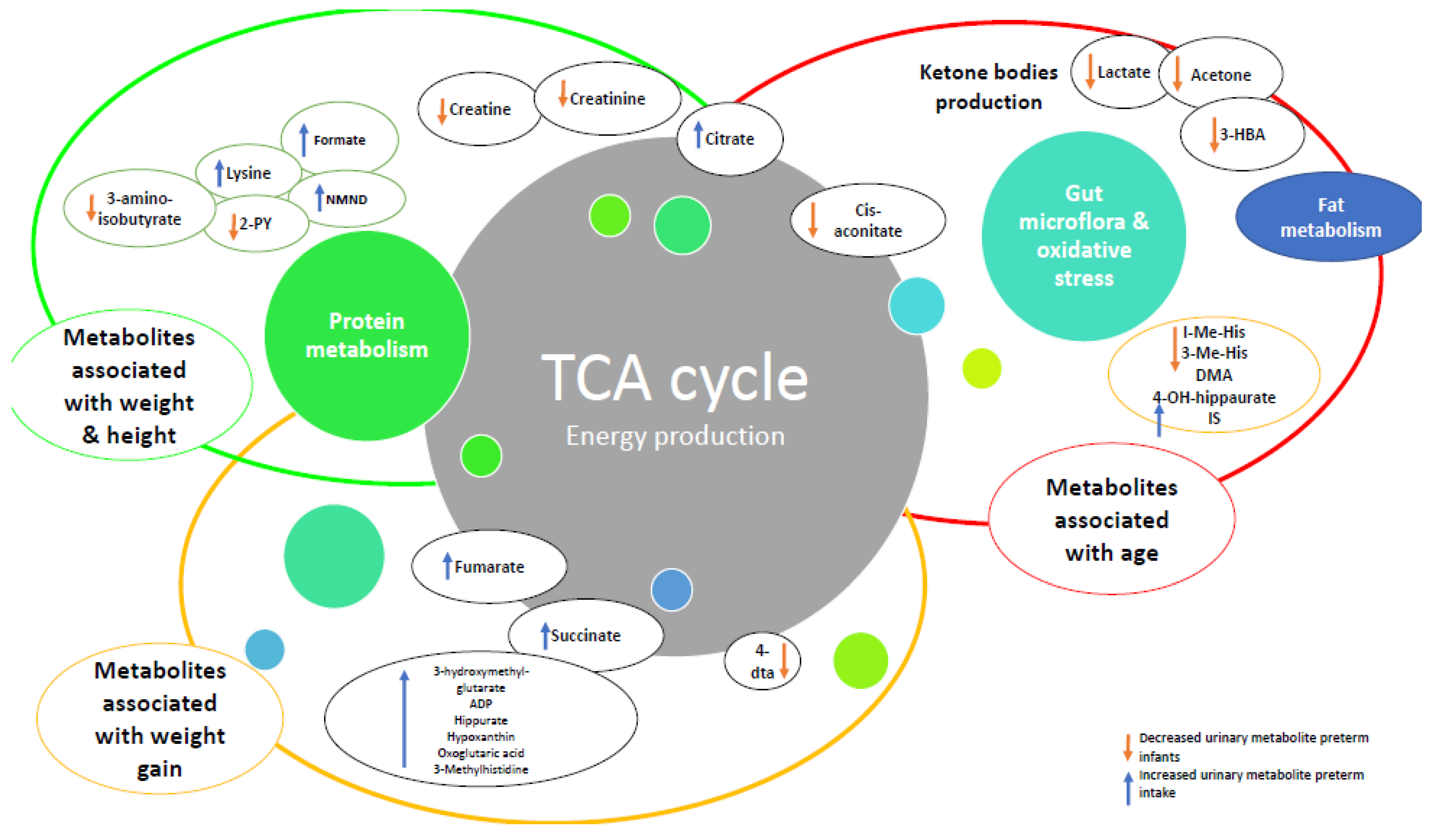
Figure 3.
Relationships with changes in metabolites over time and growth in preterm infants compared to healthy term infants. Abbreviations: ADP: adenosine triphosphate, 4-DTA: 4-deoxythreonic acid, 4-DTA: 4-deoxythreonic acid, 3-HBA: 3-hydroxybutyrate; IS: indoxyl sulfate; 3-Me-His: 3-methylhistidine; 1-Me-His: 1-methylhistidine.
3.4. Category 1: Metabolic|Maturation
Four studies characterised changes to the urinary metabolome in preterm infants associated with postnatal maturation in the first few days [35], and first few weeks of life [34,37,45,46]. They identified postnatal changes to the metabolism of glucogenic amino acids, the tricarboxylic acid (TCA) cycle and choline metabolism. These changes correlate with both post-menstrual age (PMA) and gestational age at birth, demonstrating a unique preterm pattern of metabolic maturation.
3.5. Category 2: Metabolic|Signatures
Nine studies [35,36,37,38,39,40,44,45,48] included suggested a distinct metabolic signature of prematurity. Preterm birth was associated with deficiencies in amino acid, carbohydrate, and fatty acid metabolism pathways. Extremely preterm infants had the most significant metabolic aberration with variation in metabolites of tyrosine metabolism including tyrosine, tryptophan, and phenylalanine biosynthesis along with the TCA cycle including arginine and proline metabolism, consistent with a role in foetal maturation. None of the included studies addressed issues relating to early life metabolic instability, hyperglycaemia, and hypertriglyceridemia although Morniroli et al. [48] reported higher losses of glucose in urine of preterm infants compared to those at term.
3.6. Category 3: Metabolic|Nutrition
Seven studies [37,40,42,43,44,46,47] examined the effects of differing nutritional sources on the urinary metabolites of preterm infants. Markers of oxidative stress are higher in preterm than term infants. Nutrition can alter these markers of oxidative stress, with parenteral nutrition (PN), as well as formula feeding leading to higher levels being excreted when compared to breast feeding. Urinary metabolites of choline metabolism are increased in response to breast feeding.
3.7. Category 4: Metabolic|Growth
Six studies [34,37,40,44,46,48] commented on the metabolic profiles of preterm infants with regard to growth parameters. Only two studies directly compared the urinary metabolomes of preterm infants with differing growth profiles. Hulsemann et al. [34] who found infants with stagnating or decreasing weight to have higher 3-methylhistidine/creatinine ratios. Moltu et al. [37] found no difference in urinary metabolic profiles between preterm infants fed an interventional enhanced nutritional plan and the controls, despite the intervention arm demonstrating significantly better growth.
4. Discussion
This scoping review has outlined the current understanding of metabolic maturation and the distinct metabolic profiles associated with prematurity. Metabolic maturation can be defined the relationship to ‘biochemical maturity relative to chronological age’ [13]. Preterm birth was associated with deficiencies in amino acid, carbohydrate, and fatty acid metabolism pathways. This seems to be followed by an increase in glucogenic amino acids TCA cycle metabolites and urinary choline metabolites following birth, which correlate with both premenstrual age (PMA) and gestational age at birth, demonstrating a unique preterm pattern of metabolic maturation. Markers of oxidative stress are higher in preterm than term infants, though these seem modifiable by nutrition, with parenteral nutrition (PN) and formula feeding leading to higher levels being excreted compared to breast feeding.
However, there are several gaps in the current knowledge base, including (i) what is the normal pattern of metabolic maturation for preterm infants, (ii) how metabolic signatures may vary in those infants with metabolic instability (as illustrated by an intolerance of glucose or lipid for example) compared to those who are tolerant of parenteral and enteral nutrition, (iii) what the efficacy of nutritional interventions could be to facilitate metabolic maturation and improve growth outcomes and (iv) whether there is an opportunity to develop reference standards for metabolic maturity, i.e., metabolism may be related to gestational age/corrected gestational age, rather than chronological age.
Briefly, metabolic functions can be split into two categories, bioenergetic functions and metabolic signalling functions. Bioenergetic functions, are highly regulated, supporting canonical metabolic activity such as providing energy or cellular building blocks. Metabolic signalling functions play an instructive or modulatory role in the regulation of metabolic pathways, with metabolites being the rate limiting substrate for epigenetic modification and post-translational modifications [6]. By combining advances in both metabolomic analytics and data analysis with anthropometry it may be possible to define nutritional phenotypes based upon metabolic maturity [6]. With the advent of high throughput analytic techniques to quantify components of biological samples, it increasingly possible to consider the development a more nuanced approach to medical and nutritional management for a whole range of conditions [19].
An elegant study completed by Giallourou et al. [13] demonstrated a potential way metabolites of interest within a paediatric population may be used to assess the efficacy of nutrition interventions. The group characterised changes in urinary metabolic profile of infants (n = 1131) from resource constrained settings over 3 continents over time during the first 1000 days of life. Findings suggest that biochemical immaturity during the first two-years-of-life, is associated with poorer growth outcomes, which were evident from as early as three months of age and persisted until the end of the second year of life. Linear and ponderal growth were associated with eight age-dependent metabolic signatures, from which phenome age z score (PAZ) reference curves were developed. The use of PAZ for these metabolites of interest provided the opportunity to determine an infant’s position along this metabolic maturation continuum. In the future, there may be the potential to quantify the effectiveness of a nutrition intervention in real time, as well as targeting the individual infants metabolic age rather than chronological age. This is an attractive model for optimising nutrition support in preterm infants, especially those with metabolic instability, as it provides a non-invasive way to measure nutritional responsiveness in preterm infants together with the opportunity to offer interventions targeted to more precise metabolic predisposition [13].
As growth failure is linked to increased risk of metabolic disease later in life, developing nutrition interventions favouring growth in all children affected by malnutrition is imperative [51], including infants born preterm [13]. Metabolic pathways are also influenced by epigenetic marks early in life [52], and this overlaps with changes in metabolic signatures during the evolution of carbohydrate metabolism which coincide with increasing intestinal uptake of disaccharides in the growing infant. Myoinositol plays an essential role in glucose metabolism and transport, as well as being a precursor for several secondary messaging pathways related to intracellular insulin signalling. Myo-inositol is also a component of structural and signalling lipids such as phyosphatidylinositol [53]. Prematurity also affects metabolic pathways involving hydroxyproline, creatine and myo-inositol [54,55,56,57,58], which may contribute to future cardiometabolic disease [44,54]. This temporal relationship has been eloquently described in a small cohort of pre-pubertal children (4–9 years of age) who were SGA at birth. Myo-inositol (urine) levels were decreased by 4-fold in SGA catch-up growth compared with non-catch-up growth. Transcriptomic analysis identified myo-inositol was associated with gene clusters coding for insulin and insulin like growth factor 1 (IGF-1) children [53].
Preterm infants are known to have altered body composition, which has implications for future cardiometabolic disease risk [59,60] and developing PAZ for metabolites associated with neurodevelopment and body composition (particularly lean mass) may serve as a useful reference against which to identify metabolic age compared to chronological age. Betaine and choline, are important precursors for acetylcholine (a neurotransmitter) and phospholipid (an important structural and signalling component of cell membrane), and low levels in animals are associated with neurodevelopmental delay [61]. Choline is a also a precursor for betaine synthesis which is used to form homocysteine and methionine, essential for protein synthesis and linear growth [62] and higher urinary levels of choline in the first few weeks of life are seen in breastfed infants [63]. A low urinary 3-methylhistidine/creatinine ratio has been shown to be positively correlated with body weight and tissue accretion [63]. Preterm infants with plateauing or decreasing weight have been shown to have 3-methylhistidine/creatinine ratios above normal range [34], and developing PAZ for these metabolites would complement existing work [13,44]. Other significant differences in urinary metabolic signatures in preterm infants include increased 3-hydroxyisovalerate (3-HVA), with decreased dimethylamine (DMA) and 1-methylhistidine which are related to gut microbiome and muscle protein turnover [44]. Aberrance with regard to these metabolites leads to poor nutrient utilisation and development of skeletal and lean muscle mass. Finally, with regard to energy balance, preterm infants have significantly lower urinary concentrations of succinic acid and lactose compared to term infants [44,62] and this in part may be due to age related differences in TCA cycle activity. Higher losses of these end products of metabolism appear to be related to growth faltering resulting in poor weight gain [63], suggesting there may be windows of opportunity for intervention if higher urinary levels than phenome age z scores were found. Further research is required to understand the temporal relationships between urinary metabolites of interest and growth in preterm infants.
5. Limitations
This is a scoping review to present the current range of evidence specific to urinary metabolites in preterm infants compared to healthy newborns. A significant issue that this review highlights is the relative lack of longitudinal data describing metabolic maturation within these infant cohorts, which is why the literature included in this scoping review explores what is known about urinary metabolomics in preterm infants. Given this, it was not possible to meta-analyse the results or reliably identify metabolites associated with metabolic stability and growth to allow the development of phenome age z scores.
6. Future Research Priorities
Future research is required to describe and define the normal range for urinary metabolites in healthy infants and those with complex disease and of different gestational age to allow the development of PAZ charts for metabolites of interest. This in turn may allow age, and disease specific nutritional interventions. As suggested by Gallouri et al. [13] a priority should be to develop age-specific reference curves for urinary metabolites in preterm infants compared to healthy infants. However, the development of aggregated PAZ for metabolites of interest requires large numbers and longitudinal data. Collaborative efforts to develop these would provide a unique opportunity to further our insight into better supporting ideal growth within these vulnerable infant cohorts.
Developing a better understanding of this relationship [6,13] will help (i) refine our understanding of phenotypic and metabolic responses to nutritional interventions, (ii) provide an opportunity to identify nutritional supplementation, (iii) define age related reference ranges for specific metabolites and (iv) identify specific windows in which targeted supplementation might improve growth outcomes considering metabolic maturity rather than chronological maturity [13,24,25,26].
7. Conclusions
The results of this scoping suggest that preterm birth is associated with particular metabolic signatures, and that these signatures change in relation to both increasing PMA and in response to certain patterns of nutrition. However, considerable gaps in our knowledge remain, relating to metabolic maturation of infants, especially those born preterm. Although medical and nutritional management of these infants has significantly improved, a proportion continues to be growth constrained despite adequate nutritional support, for reasons that are unclear. Characterising metabolites of interest and developing PAZ for metabolites associated with the metabolic maturation and growth may elucidate windows of opportunity for nutrition supplementation allowing early intervention before growth failure is identified using anthropometry alone.
Author Contributions
Authors made the following contribution to the manuscript: (1) L.V.M., C.W., J.J.A., A.Y., R.M.B., J.S., M.J.J. and J.V.P. formulated the original idea, (2) L.V.M., C.W., J.J.A. and S.P. completed the database search, data extraction and analysis (3) L.V.M. and S.P. drafted the manuscript (4) C.W., J.J.A., A.Y., R.M.B., J.S., M.J.J. and J.V.P. reviewed and revised the manuscript for important intellectual content, (5) and All authors have read and agreed to the published version of the manuscript.
Funding
This work has received funding from the British Dietetic Association General Education Trust Fund (Ref: 21/05). A.Y. and M.J.J. are supported by the National Institute for Health Research Southampton Biomedical Research Centre.
Institutional Review Board Statement
This scoping review did not require ethical approval.
Acknowledgments
British Dietetic Association General Education Trust Fund for supporting this research and the University of Southampton Library specialists in helping to define the search strategy.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist.
Table A1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist.
| Section | Item | PRISMA-ScR Checklist Item | Reported on Page |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a scoping review. | 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary that includes (as applicable): background, objectives, eligibility criteria, sources of evidence, charting methods, results, and conclusions that relate to the review questions and objectives. | 1 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. Explain why the review questions/objectives lend themselves to a scoping review approach. | 3 |
| Objectives | 4 | Provide an explicit statement of the questions and objectives being addressed with reference to their key elements (e.g., population or participants, concepts, and context) or other relevant key elements used to conceptualize the review questions and/or objectives. | 3 |
| Methods | |||
| Protocol and registration | 5 | Indicate whether a review protocol exists; state if and where it can be accessed (e.g., a Web address); and if available, provide registration information, including the registration number. | 4 |
| Eligibility criteria | 6 | Specify characteristics of the sources of evidence used as eligibility criteria (e.g., years considered, language, and publication status), and provide a rationale. | 4 |
| Information sources * | 7 | Describe all information sources in the search (e.g., databases with dates of coverage and contact with authors to identify additional sources), as well as the date the most recent search was executed. | 4 |
| Search | 8 | Present the full electronic search strategy for at least 1 database, including any limits used, such that it could be repeated. | 4 |
| Selection of sources of evidence † | 9 | State the process for selecting sources of evidence (i.e., screening and eligibility) included in the scoping review. | 4 |
| Data charting process ‡ | 10 | Describe the methods of charting data from the included sources of evidence (e.g., calibrated forms or forms that have been tested by the team before their use, and whether data charting was done independently or in duplicate) and any processes for obtaining and confirming data from investigators. | 4 |
| Data items | 11 | List and define all variables for which data were sought and any assumptions and simplifications made. | 4 |
| Critical appraisal of individual sources of evidence § | 12 | If done, provide a rationale for conducting a critical appraisal of included sources of evidence; describe the methods used and how this information was used in any data synthesis (if appropriate). | 4 |
| Synthesis of results | 13 | Describe the methods of handling and summarizing the data that were charted. | 4 |
| Results | |||
| Selection of sources of evidence | 14 | Give numbers of sources of evidence screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally using a flow diagram. | 6 |
| Characteristics of sources of evidence | 15 | For each source of evidence, present characteristics for which data were charted and provide the citations. | 7 |
| Critical appraisal within sources of evidence | 16 | If done, present data on critical appraisal of included sources of evidence (see item 12). | 7 |
| Results of individual sources of evidence | 17 | For each included source of evidence, present the relevant data that were charted that relate to the review questions and objectives. | 7 |
| Synthesis of results | 18 | Summarize and/or present the charting results as they relate to the review questions and objectives. | 7 |
| Discussion | |||
| Summary of evidence | 19 | Summarize the main results (including an overview of concepts, themes, and types of evidence available), link to the review questions and objectives, and consider the relevance to key groups. | 26 |
| Limitations | 20 | Discuss the limitations of the scoping review process. | 30 |
| Conclusions | 21 | Provide a general interpretation of the results with respect to the review questions and objectives, as well as potential implications and/or next steps. | 31 |
| Funding | |||
| Funding | 22 | Describe sources of funding for the included sources of evidence, as well as sources of funding for the scoping review. Describe the role of the funders of the scoping review. | 31 |
JBI = Joanna Briggs Institute; PRISMA-ScR = Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews. * Where sources of evidence are compiled from, such as bibliographic databases, social media platforms, and Web sites. † A more inclusive/heterogeneous term used to account for the different types of evidence or data sources (e.g., quantitative and/or qualitative research, expert opinion, and policy documents) that may be eligible in a scoping review as opposed to only studies. This is not to be confused with information sources. ‡ The frameworks by Arksey and O’Malley (6) and Levac and colleagues (7) and the JBI guidance (4, 5) refer to the process of data extraction in a scoping review as data charting. § The process of systematically examining research evidence to assess its validity, results, and relevance before using it to inform a decision. This term is used for items 12 and 19 instead of “risk of bias” (which is more applicable to systematic reviews of interventions) to include and acknowledge the various sources of evidence that may be used in a scoping review (e.g., quantitative and/or qualitative research, expert opinion, and policy document).
Appendix B

Table A2.
Search strategy for PuBMed and study inclusion criteria to do.
Table A2.
Search strategy for PuBMed and study inclusion criteria to do.
| Study Selection Criteria (PICOTS) | ||
|---|---|---|
| Inclusion Criteria | Exclusion Criteria | |
| Population |
|
|
| Intervention |
|
|
| Comparison |
|
|
| Outcome |
|
|
| Timing |
|
|
| Setting |
|
|
| Search Strategy | ||
| Search words |
| |
| Limits |
| |
| Year range |
| |
| Search example: PUBMED |
| |
| Expanded search terms | (“infant, premature”[MeSH Terms] OR (“infant”[All Fields] AND “premature”[All Fields]) OR “premature infant”[All Fields] OR (“preterm”[All Fields] AND “infants”[All Fields]) OR “preterm infants”[All Fields] OR (“infant, premature”[MeSH Terms] OR (“infant”[All Fields] AND “premature”[All Fields]) OR “premature infant”[All Fields] OR (“premature”[All Fields] AND “infants”[All Fields]) OR “premature infants”[All Fields])) AND (((“urinary tract”[MeSH Terms] OR (“urinary”[All Fields] AND “tract”[All Fields]) OR “urinary tract”[All Fields] OR “urinary”[All Fields]) AND (“metabolome”[MeSH Terms] OR “metabolome”[All Fields] OR “metabolomes”[All Fields] OR “metabolomics”[MeSH Terms] OR “metabolomics”[All Fields] OR “metabolomic”[All Fields])) OR ((“urinary tract”[MeSH Terms] OR (“urinary”[All Fields] AND “tract”[All Fields]) OR “urinary tract”[All Fields] OR “urinary”[All Fields]) AND (“metabolite”[All Fields] OR “metabolite s”[All Fields] OR “metabolites”[All Fields]))) AND (“growth and development”[MeSH Subheading] OR (“growth”[All Fields] AND “development”[All Fields]) OR “growth and development”[All Fields] OR “growth”[All Fields] OR “growth”[MeSH Terms] OR “growths”[All Fields] OR (“weight gain”[MeSH Terms] OR (“weight”[All Fields] AND “gain”[All Fields]) OR “weight gain”[All Fields])) AND (((“metabolic”[All Fields] OR “metabolical”[All Fields] OR “metabolically”[All Fields] OR “metabolics”[All Fields] OR “metabolism”[MeSH Terms] OR “metabolism”[All Fields] OR “metabolisms”[All Fields] OR “metabolism”[MeSH Subheading] OR “metabolic networks and pathways”[MeSH Terms] OR (“metabolic”[All Fields] AND “networks”[All Fields] AND “pathways”[All Fields]) OR “metabolic networks and pathways”[All Fields] OR “metabolities”[All Fields] OR “metabolization”[All Fields] OR “metabolize”[All Fields] OR “metabolized”[All Fields] OR “metabolizer”[All Fields] OR “metabolizers”[All Fields] OR “metabolizes”[All Fields] OR “metabolizing”[All Fields]) AND (“maturate”[All Fields] OR “maturated”[All Fields] OR “maturating”[All Fields] OR “maturation”[All Fields] OR “maturational”[All Fields] OR “maturations”[All Fields] OR “maturative”[All Fields] OR “mature”[All Fields] OR “matured”[All Fields] OR “maturer”[All Fields] OR “maturers”[All Fields] OR “matures”[All Fields] OR “maturing”[All Fields] OR “maturities”[All Fields] OR “maturity”[All Fields])) OR ((“metabolic”[All Fields] OR “metabolical”[All Fields] OR “metabolically”[All Fields] OR “metabolics”[All Fields] OR “metabolism”[MeSH Terms] OR “metabolism”[All Fields] OR “metabolisms”[All Fields] OR “metabolism”[MeSH Subheading] OR “metabolic networks and pathways”[MeSH Terms] OR (“metabolic”[All Fields] AND “networks”[All Fields] AND “pathways”[All Fields]) OR “metabolic networks and pathways”[All Fields] OR “metabolities”[All Fields] OR “metabolization”[All Fields] OR “metabolize”[All Fields] OR “metabolized”[All Fields] OR “metabolizer”[All Fields] OR “metabolizers”[All Fields] OR “metabolizes”[All Fields] OR “metabolizing”[All Fields]) AND (“maturate”[All Fields] OR “maturated”[All Fields] OR “maturating”[All Fields] OR “maturation”[All Fields] OR “maturational”[All Fields] OR “maturations”[All Fields] OR “maturative”[All Fields] OR “mature”[All Fields] OR “matured”[All Fields] OR “maturer”[All Fields] OR “maturers”[All Fields] OR “matures”[All Fields] OR “maturing”[All Fields] OR “maturities”[All Fields] OR “maturity”[All Fields])))Translations preterm infants: “infant, premature”[MeSH Terms] OR (“infant”[All Fields] AND “premature”[All Fields]) OR “premature infant”[All Fields] OR (“preterm”[All Fields] AND “infants”[All Fields]) OR “preterm infants”[All Fields]premature infants: “infant, premature”[MeSH Terms] OR (“infant”[All Fields] AND “premature”[All Fields]) OR “premature infant”[All Fields] OR (“premature”[All Fields] AND “infants”[All Fields]) OR “premature infants”[All Fields]Urinary: “urinary tract”[MeSH Terms] OR (“urinary”[All Fields] AND “tract”[All Fields]) OR “urinary tract”[All Fields] OR “urinary”[All Fields]metabolomics: “metabolome”[MeSH Terms] OR “metabolome”[All Fields] OR “metabolomes”[All Fields] OR “metabolomics”[MeSH Terms] OR “metabolomics”[All Fields] OR “metabolomic”[All Fields]urinary: “urinary tract”[MeSH Terms] OR (“urinary”[All Fields] AND “tract”[All Fields]) OR “urinary tract”[All Fields] OR “urinary”[All Fields]metabolites: “metabolite”[All Fields] OR “metabolite’s”[All Fields] OR “metabolites”[All Fields]Growth: “growth and development”[Subheading] OR (“growth”[All Fields] AND “development”[All Fields]) OR “growth and development”[All Fields] OR “growth”[All Fields] OR “growth”[MeSH Terms] OR “growths”[All Fields]weight gain: “weight gain”[MeSH Terms] OR (“weight”[All Fields] AND “gain”[All Fields]) OR “weight gain”[All Fields]Metabolic: “metabolic”[All Fields] OR “metabolical”[All Fields] OR “metabolically”[All Fields] OR “metabolics”[All Fields] OR “metabolism”[MeSH Terms] OR “metabolism”[All Fields] OR “metabolisms”[All Fields] OR “metabolism”[Subheading] OR “metabolic networks and pathways”[MeSH Terms] OR (“metabolic”[All Fields] AND “networks”[All Fields] AND “pathways”[All Fields]) OR “metabolic networks and pathways”[All Fields] OR “metabolities”[All Fields] OR “metabolization”[All Fields] OR “metabolize”[All Fields] OR “metabolized”[All Fields] OR “metabolizer”[All Fields] OR “metabolizers”[All Fields] OR “metabolizes”[All Fields] OR “metabolizing”[All Fields] maturation: “maturate”[All Fields] OR “maturated”[All Fields] OR “maturating”[All Fields] OR “maturation”[All Fields] OR “maturational”[All Fields] OR “maturations”[All Fields] OR “maturative”[All Fields] OR “mature”[All Fields] OR “matured”[All Fields] OR “maturer”[All Fields] OR “maturers”[All Fields] OR “matures”[All Fields] OR “maturing”[All Fields] OR “maturities”[All Fields] OR “maturity”[All Fields]metabolic: “metabolic”[All Fields] OR “metabolical”[All Fields] OR “metabolically”[All Fields] OR “metabolics”[All Fields] OR “metabolism”[MeSH Terms] OR “metabolism”[All Fields] OR “metabolisms”[All Fields] OR “metabolism”[Subheading] OR “metabolic networks and pathways”[MeSH Terms] OR (“metabolic”[All Fields] AND “networks”[All Fields] AND “pathways”[All Fields]) OR “metabolic networks and pathways”[All Fields] OR “metabolities”[All Fields] OR “metabolization”[All Fields] OR “metabolize”[All Fields] OR “metabolized”[All Fields] OR “metabolizer”[All Fields] OR “metabolizers”[All Fields] OR “metabolizes”[All Fields] OR “metabolizing”[All Fields] maturity: “maturate”[All Fields] OR “maturated”[All Fields] OR “maturating”[All Fields] OR “maturation”[All Fields] OR “maturational”[All Fields] OR “maturations”[All Fields] OR “maturative”[All Fields] OR “mature”[All Fields] OR “matured”[All Fields] OR “maturer”[All Fields] OR “maturers”[All Fields] OR “matures”[All Fields] OR “maturing”[All Fields] OR “maturities”[All Fields] OR “maturity”[All Fields] | |
References
- Preterm Birth. Available online: http://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 24 April 2022).
- Demerath, E.W.; Johnson, W.; Davern, B.A.; Anderson, C.G.; Shenberger, J.S.; Misra, S.; Ramel, S.E. New body composition reference charts for preterm infants. Am. J. Clin. Nutr. 2016, 105, 70–77. [Google Scholar] [CrossRef]
- Ashton, J.; Johnson, M.J.; Pond, J.; Crowley, P.; Dimitrov, B.D.; Pearson, F.; Beattie, R.M. Assessing the growth of preterm infants using detailed anthropometry. Acta Paediatr. 2017, 106, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral Nutrient Supply for Preterm Infants: Commentary From the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Beattie, R.M.; Johnson, M.J. Optimising growth in very preterm infants: Reviewing the evidence. Arch. Dis. Child. Fetal Neonatal Ed. Published online First: 28 February. 2022. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Swann, J.R. Metabolic phenotyping of malnutrition during the first 1000 days of life. Eur. J. Nutr. 2018, 58, 909–930. [Google Scholar] [CrossRef]
- Simeoni, U.; Yzydorczyk, C.; Siddeek, B.; Benahmed, M. Epigenetics and neonatal nutrition. Early Hum. Dev. 2014, 90, S23–S24. [Google Scholar] [CrossRef]
- Chiu, C.-Y.; Yeh, K.-W.; Lin, G.; Chiang, M.-H.; Yang, S.-C.; Chao, W.-J.; Yao, T.-C.; Tsai, M.-H.; Hua, M.-C.; Liao, S.-L.; et al. Metabolomics Reveals Dynamic Metabolic Changes Associated with Age in Early Childhood. PLoS ONE 2016, 11, e0149823. [Google Scholar] [CrossRef] [PubMed]
- Ramel, S.; Rao, R. Hyperglycemia in Extremely Preterm Infants. NeoReviews 2020, 21, e89–e97. [Google Scholar] [CrossRef] [PubMed]
- van der Lugt, N.M.; Smits-Wintjens, V.E.H.J.; van Zwieten, P.H.T.; Walther, F.J. Short and long term outcome of neonatal hyperglycemia in very preterm infants: A retrospective follow-up study. BMC Pediatr. 2010, 10, 52. [Google Scholar] [CrossRef]
- Ni, Y.; Beckmann, J.; Hurst, J.R.; Morris, J.K.; Marlow, N. Size at birth, growth trajectory in early life, and cardiovascular and metabolic risks in early adulthood: EPICure study. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 149–155. [Google Scholar] [CrossRef]
- Heidemann, L.A.; Procianoy, R.S.; Silveira, R.C. Prevalence of metabolic syndrome-like in the follow-up of very low birth weight preterm infants and associated factors. J. Pediatr. 2019, 95, 291–297. [Google Scholar] [CrossRef]
- Giallourou, N.; Fardus-Reid, F.; Panic, G.; Veselkov, K.; McCormick, B.J.J.; Olortegui, M.P.; Ahmed, T.; Mduma, E.; Yori, P.P.; Mahfuz, M.; et al. Metabolic maturation in the first 2 years of life in resource-constrained settings and its association with postnatal growth. Sci. Adv. 2020, 6, eaay5969. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, A.F.; Pan, A.; Lam, V.; Gouthro, K.C.; Simpson, P.M.; Salzman, N.H.; Nghiem-Rao, T.H. Longitudinal changes in the gut microbiome of infants on total parenteral nutrition. Pediatr. Res. 2019, 86, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.A.; Shah, J.K.; McGee, C.; Steele, B.T. Mineral excretion in premature infants receiving various diuretic therapies. J. Pediatr. 1988, 113, 540–545. [Google Scholar] [CrossRef]
- Grace, E.; Hilditch, C.; Gomersall, J.; Collins, C.T.; Rumbold, A.; Keir, A.K. Safety and efficacy of human milk-based fortifier in enterally fed preterm and/or low birthweight infants: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 106, 137–142. [Google Scholar] [CrossRef]
- Boscarino, G.; Conti, M.G.; Di Chiara, M.; Bianchi, M.; Onestà, E.; Faccioli, F.; Deli, G.; Repole, P.; Oliva, S.; Cresi, F.; et al. Early Enteral Feeding Improves Tolerance of Parenteral Nutrition in Preterm Newborns. Nutrients 2021, 13, 3886. [Google Scholar] [CrossRef]
- Moltu, S.J.; Bronsky, J.; Embleton, N.; Gerasimidis, K.; Indrio, F.; Köglmeier, J.; de Koning, B.; Lapillonne, A.; Norsa, L.; Verduci, E.; et al. Nutritional Management of the Critically Ill Neonate: A Position Paper of the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef]
- Blanton, L.V.; Charbonneau, M.R.; Salih, T.; Barratt, M.J.; Venkatesh, S.; Ilkaveya, O.; Subramanian, S.; Manary, M.J.; Trehan, I.; Jorgensen, J.M.; et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 2016, 351, aad3311. [Google Scholar] [CrossRef]
- Subramanian, S.; Huq, S.; Yatsunenko, T.; Haque, R.; Mahfuz, M.; Alam, M.A.; Benezra, A.; DeStefano, J.; Meier, M.F.; Muegge, B.; et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 2014, 510, 417–421. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, L.; Johnson, M.; Mandal, R.; Wishart, D.S. Comprehensive Targeted Metabolomic Assay for Urine Analysis. Anal. Chem. 2020, 92, 10627–10634. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-N. Current concepts of very low birth weight infants with extra-uterine growth restriction. Pediatr. Neonatol. 2022, 63, 3–4. [Google Scholar] [CrossRef]
- Freemark, M. Metabolomics in Nutrition Research: Biomarkers Predicting Mortality in Children with Severe Acute Malnutrition. Food Nutr. Bull. 2015, 36, S88–S92. [Google Scholar] [CrossRef]
- Owino, V.; Ahmed, T.; Freemark, M.; Kelly, P.; Loy, A.; Manary, M.; Loechl, C. Environmental Enteric Dysfunction and Growth Failure/Stunting in Global Child Health. Pediatrics 2016, 138, e20160641. [Google Scholar] [CrossRef]
- Bourdon, C.; Lelijveld, N.; Thompson, D.; Dalvi, P.S.; Gonzales, G.B.; Wang, D.; Alipour, M.; Wine, E.; Chimwezi, E.; Wells, J.C.; et al. Metabolomics in plasma of Malawian children 7 years after surviving severe acute malnutrition: “ChroSAM” a cohort study. eBioMedicine 2019, 45, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Moco, S.; Collino, S.; Rezzi, S.; Martin, F.-P. Metabolomics perspectives in pediatric research. Pediatr. Res. 2013, 73, 570–576. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Marino, L.; Valla, F.; Beattie, R.; Verbruggen, S. Micronutrient status during paediatric critical illness: A scoping review. Clin. Nutr. 2020, 39, 3571–3593. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Popay, J. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews A Product from the ESRC Methods Programme. Lancaster Univeristy. Available online: https://www.lancaster.ac.uk/media/lancaster-university/content-assets/documents/fhm/dhr/chir/NSsynthesisguidanceVersion1-April2006.pdf (accessed on 9 June 2022).
- Hülsemann, J.; Kordass, U.; Sander, G.; Schmidt, E.; Schöch, G. 3-Methylhistidine/Creatinine Ratio in Urine from Low-Birth-Weight Infants. Ann. Nutr. Metab. 1988, 32, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulou, I.; Chasapi, S.A.; Bariamis, S.E.; Varvarigou, A.; Spraul, M.; Spyroulias, G.A. Metabolic changes in early neonatal life: NMR analysis of the neonatal metabolic profile to monitor postnatal metabolic adaptations. Metabolomics 2020, 16, 58. [Google Scholar] [CrossRef] [PubMed]
- Atzori, L.; Antonucci, R.; Barberini, L.; Locci, E.; Marincola, F.C.; Scano, P.; Cortesi, P.; Agostiniani, R.; Defraia, R.; Weljie, A.; et al. 1H NMR-based metabolomic analysis of urine from preterm and term neonates. Front. Biosci. 2011, 3, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Moltu, S.J.; Sachse, D.; Blakstad, E.W.; Strømmen, K.; Nakstad, B.; Almaas, A.N.; Westerberg, A.C.; Rønnestad, A.; Brække, K.; Veierød, M.B.; et al. Urinary Metabolite Profiles in Premature Infants Show Early Postnatal Metabolic Adaptation and Maturation. Nutrients 2014, 6, 1913–1930. [Google Scholar] [CrossRef]
- Buck, A.; Kayacelebi, A.A.; Chobanyan-Jürgens, K.; Illsinger, S.; Bohnhorst, B.; Beckmann, B.; Hanff, E.; Das, A.M.; Tsikas, D.; Lücke, T. Comprehensive analysis of the l-arginine/l-homoarginine/nitric oxide pathway in preterm neonates: Potential roles for homoarginine and asymmetric dimethylarginine in foetal growth. Amino Acids 2017, 49, 783–794. [Google Scholar] [CrossRef]
- Hao, H.; Li, S.; Zhou, W.; Wang, H.; Liu, M.; Shi, C.; Chen, J.; Xiao, X. Metabolic products in urine of preterm infants characterized via gas chromatography-mass spectrometry. Int. J. Clin. Exp. Med. 2015, 8, 16454–16462. [Google Scholar]
- Ledo, A.; Arduini, A.; Asensi, M.A.; Sastre, J.; Escrig, R.; Brugada, M.; Aguar, M.; Saenz, P.; Vento, M. Human milk enhances antioxidant defenses against hydroxyl radical aggression in preterm infants. Am. J. Clin. Nutr. 2008, 89, 210–215. [Google Scholar] [CrossRef]
- Muñoz-Hoyos, A.; Molina-Carballo, A.; Macías, M.; Rodríguez-Cabezas, T.; Martín-Medina, E.; Narbona-López, E.; Valenzuela-Ruiz, A.; Acuña-Castroviejo, D. Comparison between tryptophan methoxyindole and kynurenine metabolic pathways in normal and preterm neonates and in neonates with acute fetal distress. Eur. J. Endocrinol. 1998, 139, 89–95. [Google Scholar] [CrossRef]
- Shoji, H.; Taka, H.; Kaga, N.; Ikeda, N.; Hisata, K.; Miura, Y.; Shimizu, T. Choline-related metabolites influenced by feeding patterns in preterm and term infants. J. Matern. Fetal Neonatal Med. 2020, 33, 230–235. [Google Scholar] [CrossRef]
- Shoji, H.; Shimizu, T.; Shinohara, K.; Oguchi, S.; Shiga, S.; Yamashiro, Y. Suppressive effects of breast milk on oxidative DNA damage in very low birthweight infants. Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, F136–F138. [Google Scholar] [CrossRef] [PubMed]
- Diaz, S.O.; Pinto, J.; Barros, A.S.; Morais, E.; Duarte, D.; Negrão, F.; Pita, C.; Almeida, M.D.C.; Carreira, I.M.; Spraul, M.; et al. Newborn Urinary Metabolic Signatures of Prematurity and Other Disorders: A Case Control Study. J. Proteome Res. 2015, 15, 311–325. [Google Scholar] [CrossRef]
- Farkouh, C.R.; Merrill, J.D.; Ballard, P.L.; Ballard, R.A.; Ischiropoulos, H.; Lorch, S.A. Urinary Metabolites of Oxidative Stress and Nitric Oxide in Preterm and Term Infants. Neonatology 2006, 90, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Sauerwald, U.; Keicher, U.; Saule, H.; Wawatschek, S.; Böhles, H.; Bervoets, K.; Fleith, M.; Crozier-Willi, G. Fatty acid profiles, antioxidant status, and growth of preterm infants fed diets without or with long-chain polyunsaturated fatty acids. Eur. J. Nutr. 2003, 42, 243–253. [Google Scholar] [CrossRef]
- Giribaldi, M.; Peila, C.; Coscia, A.; Cavallarin, L.; Antoniazzi, S.; Corbu, S.; Maiocco, G.; Sottemano, S.; Cresi, F.; Bertino, G.M.E.; et al. Urinary Metabolomic Profile of Preterm Infants Receiving Human Milk with Either Bovine or Donkey Milk-Based Fortifiers. Nutrients 2020, 12, 2247. [Google Scholar] [CrossRef] [PubMed]
- Morniroli, D.; Dessì, A.; Giannì, M.L.; Roggero, P.; Noto, A.; Atzori, L.; Lussu, M.; Fanos, V.; Mosca, F. Is the body composition development in premature infants associated with a distinctive nuclear magnetic resonance metabolomic profiling of urine? J. Matern. Fetal Neonatal Med. 2019, 32, 2310–2318. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.K.; Paolacci, S.; Calogero, A.E.; Cannarella, R.; Di Renzo, G.C.; Gerli, S.; Della Morte, C.; Busetto, G.M.; De Berardinis, E.; Del Giudice, F.; et al. From Myo-inositol to D-chiro-inositol molecular pathways. Eur. Rev. Med. Pharm. Sci. 2021, 25, 2390–2402. [Google Scholar]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Varkey, A.; Devi, S.; Mukhopadhyay, A.; Kamat, N.G.; Pauline, M.; Dharmar, M.; Holt, R.R.; Allen, L.H.; Thomas, T.; Keen, C.L.; et al. Metabolome and microbiome alterations related to short-term feeding of a micronutrient-fortified, high-quality legume protein-based food product to stunted school age children: A randomized controlled pilot trial. Clin. Nutr. 2020, 39, 3251–3261. [Google Scholar] [CrossRef]
- Koletzko, B.; Chourdakis, M.; Grote, V.; Hellmuth, C.; Prell, C.; Rzehak, P.; Uhl, O.; Weber, M. Regulation of Early Human Growth: Impact on Long-Term Health. Ann. Nutr. Metab. 2014, 65, 101–109. [Google Scholar] [CrossRef]
- Stevens, A.; Bonshek, C.; Whatmore, A.; Butcher, I.; Hanson, D.; De Leonibus, C.; Shaikh, G.; Brown, M.; O’Shea, E.; Victor, S.; et al. Insights into the pathophysiology of catch-up compared with non-catch-up growth in children born small for gestational age: An integrated analysis of metabolic and transcriptomic data. Pharm. J. 2014, 14, 376–384. [Google Scholar] [CrossRef]
- Dessì, A.; Atzori, L.; Noto, A.; Visser, G.H.A.; Gazzolo, D.; Zanardo, V.; Barberini, L.; Puddu, M.; Ottonello, G.; Atzei, A.; et al. Metabolomics in newborns with intrauterine growth retardation (IUGR): Urine reveals markers of metabolic syndrome. J. Matern. Neonatal Med. 2011, 24, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Dessì, A.; Marincola, F.C.; Pattumelli, M.G.; Ciccarelli, S.; Corbu, S.; Ossicini, C.; Fanos, V. Investigation of the 1H-NMR based urine metabolomic profiles of IUGR, LGA and AGA newborns on the first day of life. J. Matern. Fetal Neonatal Med. 2014, 27 (Suppl. 2), 13–19. [Google Scholar] [CrossRef] [PubMed]
- Marincola, F.C.; Dessì, A.; Pattumelli, M.G.; Corbu, S.; Ossicini, C.; Ciccarelli, S.; Agostino, R.; Mussap, M.; Fanos, V. (1)H NMR-based urine metabolic profile of IUGR, LGA, and AGA newborns in the first week of life. Clin. Chim. Acta Int. J. Clin. Chem. 2015, 451 Pt A, 28–34. [Google Scholar] [CrossRef]
- Barberini, L.; Noto, A.; Fattuoni, C.; Grapov, D.; Casanova, A.; Fenu, G.; Gaviano, M.; Carboni, R.; Ottonello, G.; Crisafulli, M.; et al. Urinary metabolomics (GC-MS) reveals that low and high birth weight infants share elevated inositol concentrations at birth. J. Matern. Neonatal Med. 2014, 27, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.X.; Li, X.W.; Fu, W.; Zhang, W.Q. Metabolomic Research on Newborn Infants With Intrauterine Growth Restriction. Medicine 2016, 95, e3564. [Google Scholar] [CrossRef]
- Irving, S.Y.; Ravishankar, C.; Miller, M.; Chittams, J.; Stallings, V.; Medoff-Cooper, B. Anthropometry Based Growth and Body Composition in Infants with Complex Congenital Heart Disease. Clin. Nurs. Res. 2022, 31, 931–940. [Google Scholar] [CrossRef]
- Young, A.; Brown, L.K.; Ennis, S.; Beattie, R.M.; Johnson, M.J. Total body water in full-term and preterm newborns: Systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 542–548. [Google Scholar] [CrossRef]
- Ueland, P.M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 2010, 34, 3–15. [Google Scholar] [CrossRef]
- Scalabre, A.; Jobard, E.; Demède, D.; Gaillard, S.; Pontoizeau, C.; Mouriquand, P.; Elena-Herrmann, B.; Mure, P.-Y. Evolution of Newborns’ Urinary Metabolomic Profiles According to Age and Growth. J. Proteome Res. 2017, 16, 3732–3740. [Google Scholar] [CrossRef]
- Cesare Marincola, F.; Corbu, S.; Lussu, M.; Noto, A.; Dessì, A.; Longo, S.; Civardi, E.; Garofoli, F.; Grenci, B.; Mongini, E.; et al. Impact of Early Postnatal Nutrition on the NMR Urinary Metabolic Profile of Infant. J. Proteome Res. 2016, 15, 3712–3723. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).