Pandanus amaryllifolius Exhibits In Vitro Anti-Amyloidogenic Activity and Promotes Neuroprotective Effects in Amyloid-β-Induced SH-SY5Y Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction of the Crude Extracts
2.3. Thioflavin T (ThT) Assay
2.4. High-Throughput Screening of Multimer Detection System (MDS-HTS) Assay
2.5. Cell Culture
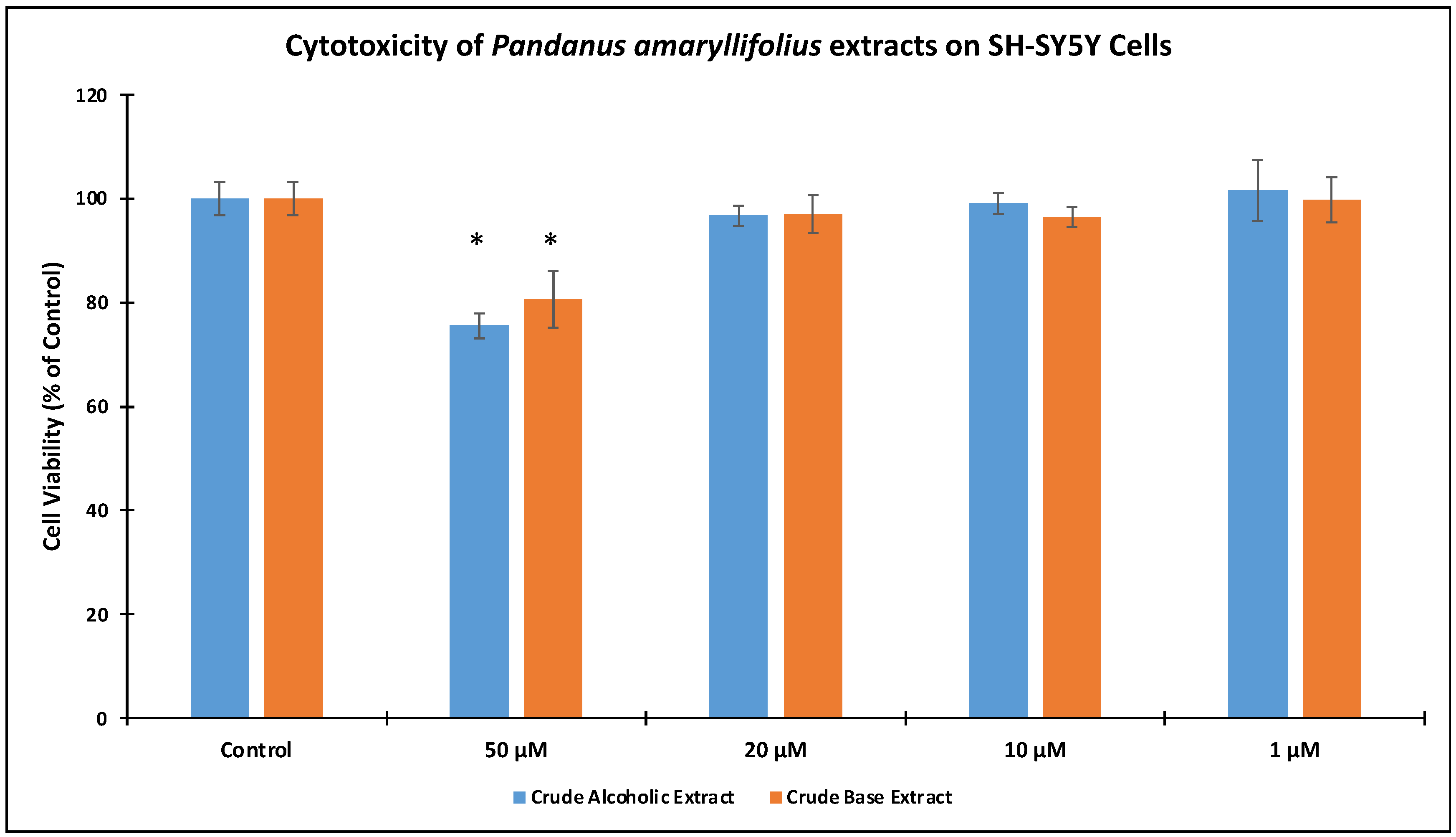
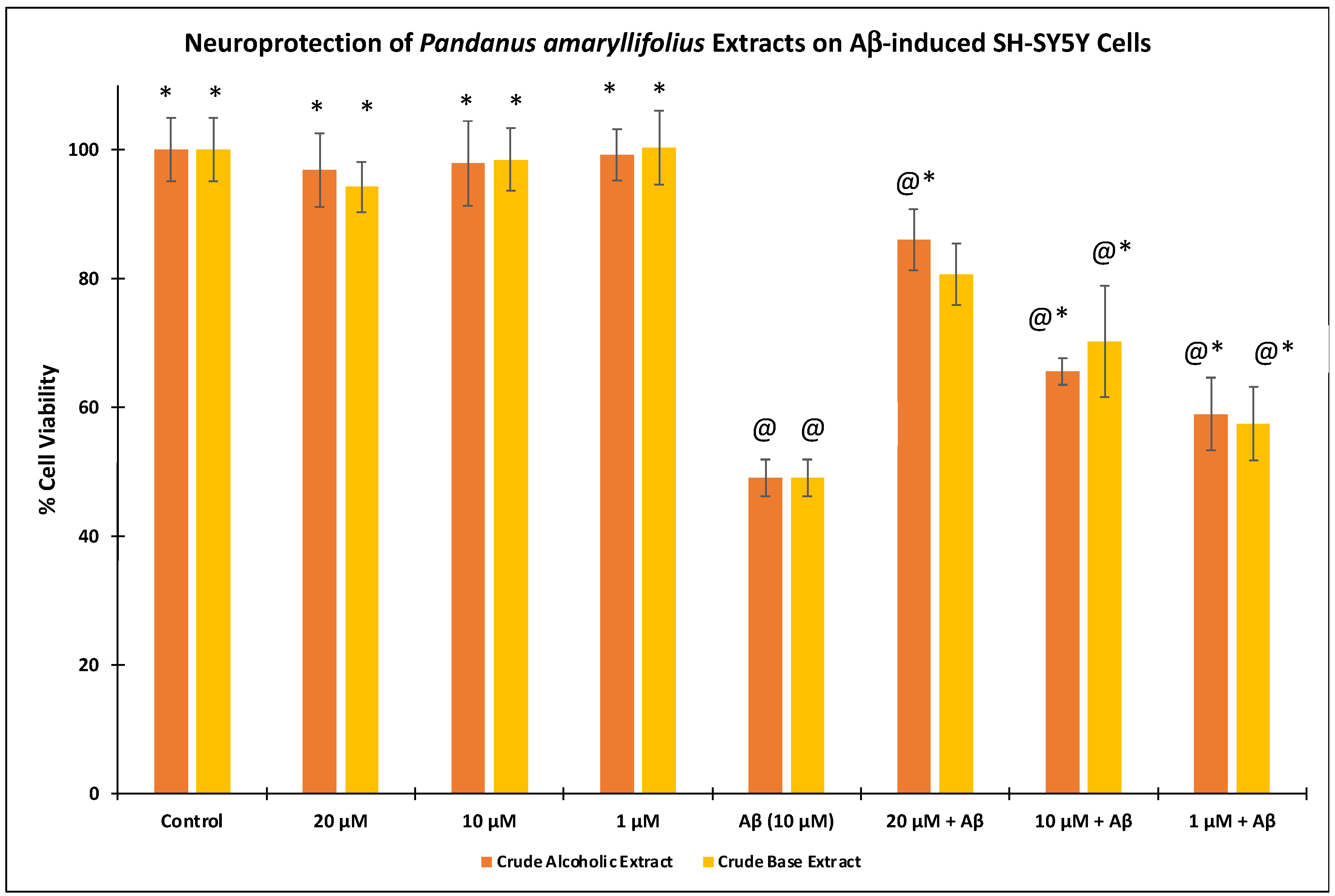
2.6. Cell Cytotoxicity and Neuroprotection Assay
2.7. Determination of Intracellular Reactive Oxygen Species (ROS)
2.8. Mitochondrial Membrane Potential (∆Ψm) Assay
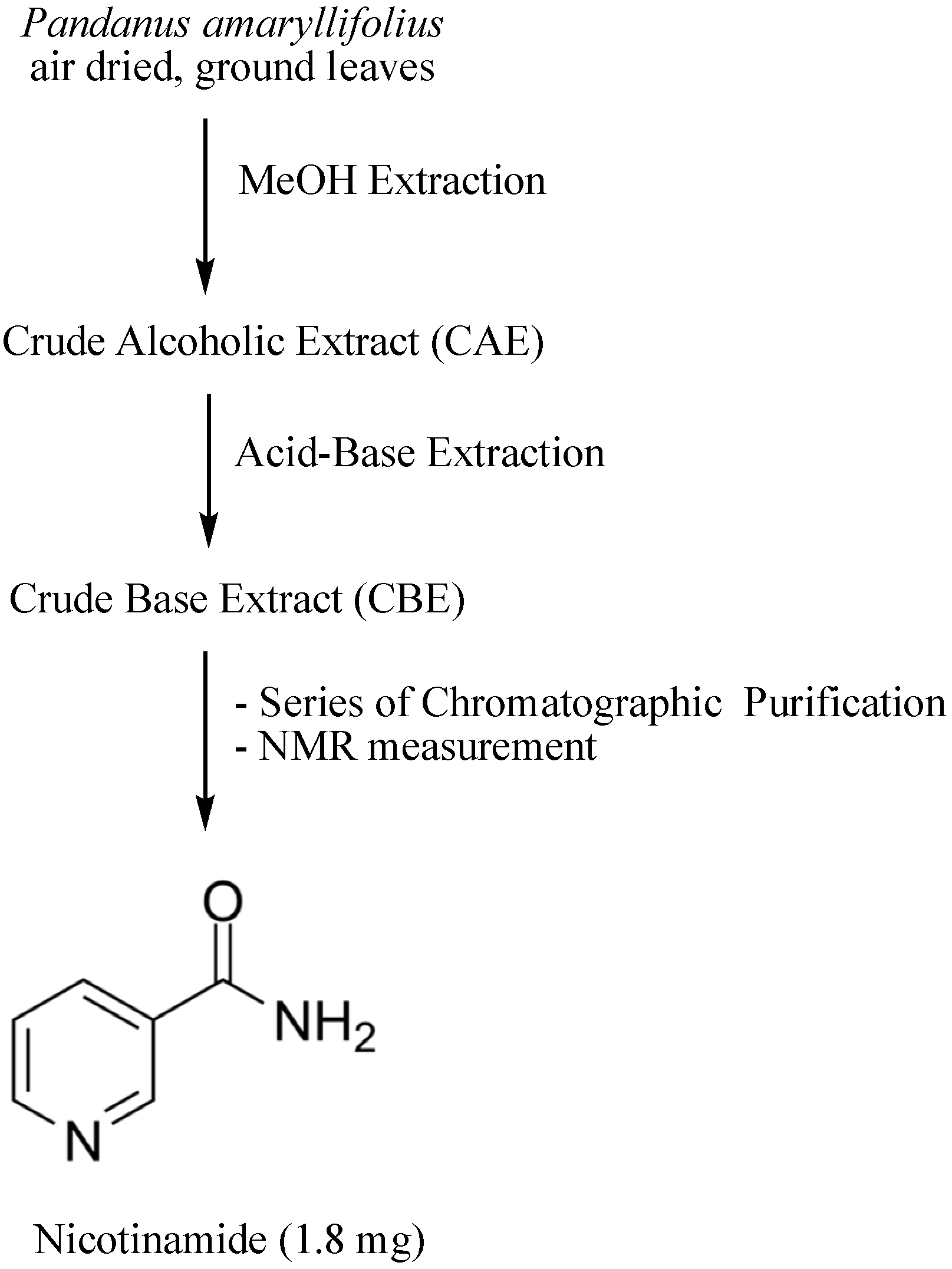
2.9. Isolation of Nicotinamide (1)
2.10. General Considerations
2.11. Statistical Analysis
3. Results
3.1. Inhibitory Effect on the Aggregation and Oligomerization of Amyloid-Beta
3.2. Cell Cytotoxicity and Neuroprotective effects of P. amaryllifolius Extracts
3.3. Level of Intracellular Reactive Oxygen Species (ROS)
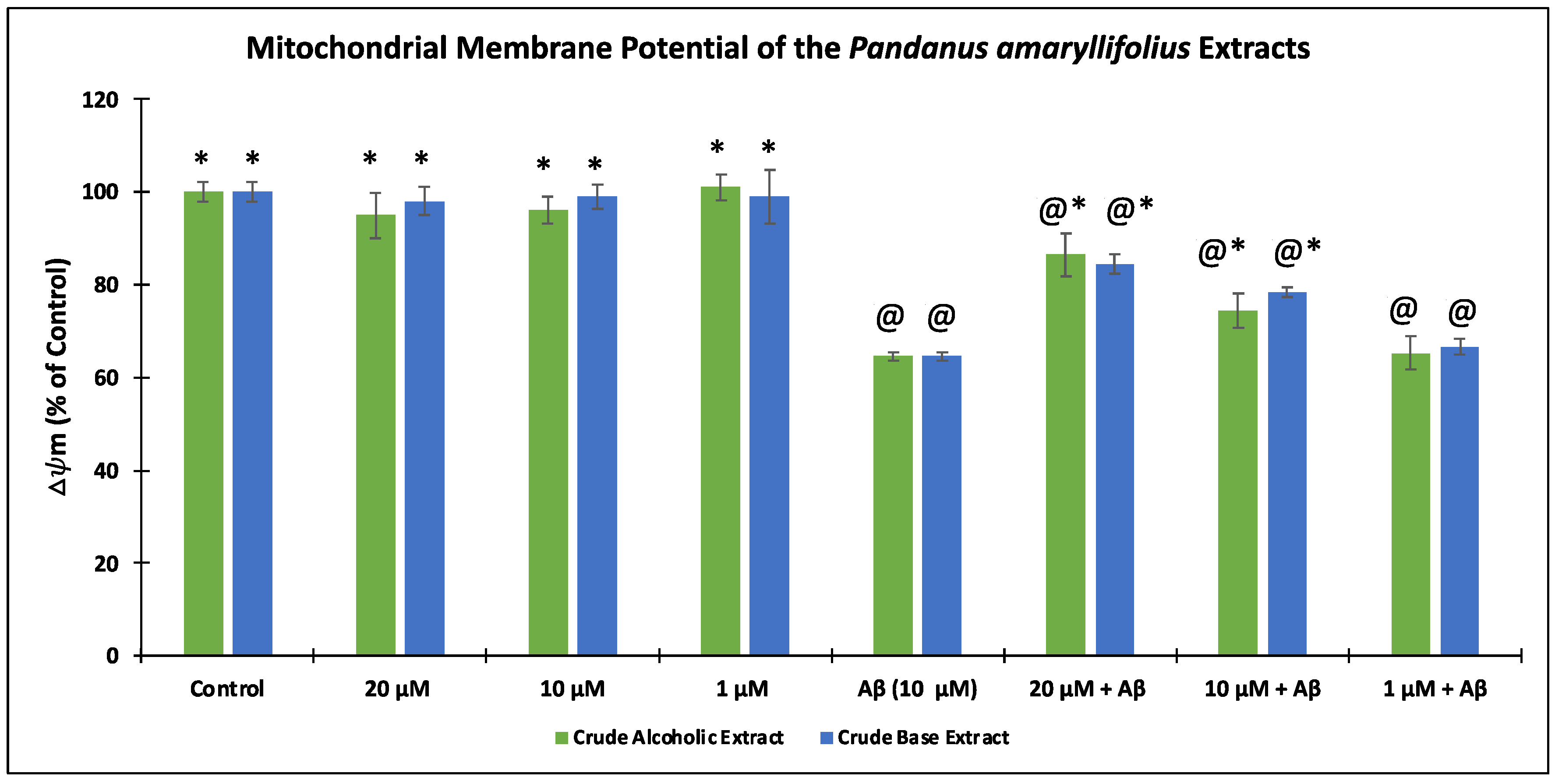
3.4. Effect of the P. amaryllifolius Extracts on the Mitochondrial Membrane Potential (Δψm)
3.5. Isolation and Identification of Nicotinamide from the Crude Base Extract
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association Report. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022, 18, 700–789. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The amyloid-β pathway in Alzheimer’s disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-M.; Huang, F.-I.; Yang, C.-R. Moscatilin ameliorates tau phosphorylation and cognitive deficits in Alzheimer’s disease models. J. Nat. Prod 2019, 82, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Woloshin, H.; Kesselheim, A.S. What to know about the Alzheimer drug Aducanumab (Aduhelm). JAMA Intern. Med. 2022, 182, 892. [Google Scholar] [CrossRef] [PubMed]
- Ruangritchankul, S.; Chantharit, P.; Srisuma, S.; Gray, L.C. Adverse drug reactions of acetylcholinesterase inhibitors in older people living with dementia: A comprehensive literature review. Ther. Clin. Risk Manag. 2021, 17, 927–949. [Google Scholar] [CrossRef]
- Sinha, P.; Barocas, J.A. Cost effectiveness of aducanumab to prevent Alzheimer’s disease progression at current list price. Alzheimers Dement. 2022, 8, e12256. [Google Scholar] [CrossRef]
- John, O.O.; Amarachi, I.S.; Chinazom, A.P.; Adaeze, E.; Kale, M.B.; Umare, M.D.; Upaganlawar, A.B. Phytotherapy: A promising approach for the treatment of Alzheimer’s disease. Pharmacol. Res. Mod. Chin. Med. 2022, 2, 100030. [Google Scholar] [CrossRef]
- Noori, T.; Dehpour, A.R.; Sureda, A.; Sobarzo-Sanchez, E.; Shirooie, S. Role of natural products for the treatment of Alzheimer’s disease. Eur. J. Pharmacol. 2021, 898, 173974. [Google Scholar] [CrossRef]
- Roy, A. Role of medicinal plants against Alzheimer’s disease. Int. J. Complement. Altern. Med. 2018, 11, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S. Potential role of medicinal plants against Alzheimer’s disease. Int. J. Complement. Altern. Med. 2021, 14, 138–140. [Google Scholar] [CrossRef]
- Wakte, K.V.; Nadaf, A.B.; Thengane, R.J.; Jawali, N. Pandanus amaryllifolius Roxb. cultivated as spice in coastal regions of India. Genet. Resour. Crop Evol. 2009, 56, 735–740. [Google Scholar] [CrossRef]
- Ordas, J.A.D.; Nonato, M.G.; Moran, C.B. Ethnobotanical uses of Pandanaceae species in selected rural communities in the Philippines. Econ. Bot. 2021, 74, 411–428. [Google Scholar] [CrossRef]
- Surojanametakul, V.; Boonbumrung, S.; Tungtrakul, P.; Varanyanoid, W.; Themtakul, K.; Yoshihashi, T. Encapsulation of natural flavor from Pandanus amaryllifolius Roxb. in rice starch aggregates. Food Sci. Technol. Res. 2019, 25, 577–585. [Google Scholar] [CrossRef]
- Routray, W.; Rayaguru, K. Chemical constituents and post-harvest prospects of Pandanus amaryllifolius leaves: A review. Food Rev. Int. 2010, 26, 230–245. [Google Scholar] [CrossRef]
- Tan, M.A.; Takayama, H. Recent progress in the chemistry of Pandanus alkaloids. In The Alkaloids; Knölker, H.J., Ed.; Academic Press: London, UK, 2019; Volume 82, pp. 1–28. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E. Profiling of phenolic compounds and their antioxidant and anticancer activities in pandan (Pandanus amaryllifolius Roxb.) extracts from different locations of Malaysia. BMC Complement. Altern. Med. 2013, 13, 341. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Kan, S.; Sugita, Y.; Shirataki, Y. p-Coumaroyl malate derivatives of the Pandanus amaryllifolius leaf and their isomerization. Chem. Pharm. Bull. 2017, 65, 1191–1194. [Google Scholar] [CrossRef]
- Tan, M.A.; An, S.S.A. Neuroprotective potential of the oxindole alkaloids isomitraphylline and mitraphylline in human neuroblastoma SH-SY5Y cells. 3 Biotech 2020, 10, 517. [Google Scholar] [CrossRef]
- Tan, M.A.; An, S.S.A. Potential therapeutic agents from Philippine medicinal plants against Alzheimer’s disease. Alzheimers Dement. 2020, 16, e038909. [Google Scholar] [CrossRef]
- Tan, M.A.; Gonzalez, S.J.B.; Alejandro, G.J.D.; An, S.S.A. Neuroprotective effects of vomifoliol, isolated from Tarenna obtusifolia Merr. (Rubiaceae), against amyoid-beta1-42-treated neuroblastoma SH-SY5Y cells. 3 Biotech 2020, 10, 424. [Google Scholar] [CrossRef]
- Tan, M.A.; Tan, B.L.U.; Nonato, M.G.; An, S.S.A. Neuroprotective effects on amyloid-beta induced cytotoxicity of Pandanus clementis Merr. 3 Biotech 2021, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.A.; Zacharova, E.; An, S.S.A. Diaportheone A analogues instigate a neuroprotective effect by protecting neuroblastoma SH-SH5Y cells from oxidative stress. Biology 2021, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- An, S.S.A.; Shim, K.H.; Kang, S.; Kim, Y.K.; Subedi, L.; Cho, H.; Hong, S.M.; Tan, M.A.; Jeon, R.; Chang, K.A.; et al. The potential anti-amyloidogenic candidate, SPA1413, for Alzheimer’s disease. Br. J. Pharmacol. 2022, 179, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- An, S.S.A.; Lee, B.-S.; Yu, J.S.; Lim, K.; Kim, G.J.; Lee, R.; Kim, S.; Kang, S.; Park, Y.H.; Wang, M.J.; et al. Dynamic changes of oligomeric amyloid β levels in plasma induced by spiked synthetic Aβ42. Alzheimer’s Res. Ther. 2017, 9, 86. [Google Scholar] [CrossRef]
- Wang, M.J.; Yi, S.; Han, J.-Y.; Park, S.Y.; Jang, J.-W.; Chun, I.K.; Kim, S.E.; Lee, B.S.; Kim, G.J.; Yu, J.S.; et al. Oligomeric forms of amyloid- β protein in plasma as a potential blood-based biomarker for Alzheimer’s disease. Alzheimer’s Res. Ther. 2017, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta 2010, 1804, 1405–1412. [Google Scholar] [CrossRef]
- Fujioka, S.; Sakurai, A.; Yamaguchi, I.; Murofushi, N.; Takahashi, N.; Kaihara, S.; Takimoto, A. Isolation and identification of L-pipecolic acid and nicotinamide as flower-inducing substances in Lemna. Plant Cell Physiol. 1987, 28, 995–1003. [Google Scholar] [CrossRef]
- Yamashita, N.; Sakata, K.; Ina, H.; Ina, K. Isolation of nicotinamide from Mallotus leaves as an attaching repellent against the blue mussel, Mytilus edulis. Agric. Biol. Chem. 1989, 53, 3351–3352. [Google Scholar] [CrossRef]
- Çatak, J. Determination of niacin profiles in some animal and plant based foods by high performance liquid chromatography: Association with healthy nutrition. J. Anim. Sci. Technol. 2019, 61, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Green, K.N.; Steffan, J.S.; Martinez-Coria, H.; Sun, X.; Schreiber, S.S.; Thompson, L.M.; LaFerla, F.M. Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J. Neurosci. 2008, 28, 11500–11510. [Google Scholar] [CrossRef]
- Liu, D.; Pitta, M.; Jiang, H.; Lee, J.H.; Zhang, G.; Chen, X.; Kawamoto, E.M.; Mattson, M.P. Nicotinamide forestalls pathology and cognitive decline in Alzheimer mice: Evidence for improved neuronal bioenergentics and autophagy procession. Neurobiol. Aging 2013, 34, 1564–1580. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, B.; Kim, H.S.; Cho, J.Y. Nicotinamide attenuates the decrease in dendritic spine density in hippocampal primary neurons from 5xFAD mice, an Alzheimer’s disease animal model. Mol. Brain 2020, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Alzheimers.gov. Nicotinamide as an Early Alzheimer’s Treatment (NEAT). 2022. Available online: https://www.alzheimers.gov/clinical-trials/nicotinamide-early-alzheimers-treatment-neat (accessed on 26 July 2022).
- ClinicalTrials.gov. Nicotinamide as an Early Alzheimer’s Disease Treatment (NEAT), ClinicalTrials.gov Identifier: NCT03061474. 2021. Available online: https://clinicaltrials.gov/ct2/show/record/NCT03061474 (accessed on 27 July 2022).
- Reshidan, N.H.; Muid, S.A.; Mamikutty, N. The effects of Pandanus amaryllifolius (Roxb.) leaf water extracts on fructose-induced metabolic syndrome rat model. BMC Complement. Med. Ther. 2019, 19, 232. [Google Scholar] [CrossRef] [PubMed]
- Thanebal, S.A.; Vun-Sang, S.; Iqbal, M. Hepatoprotective effects of Pandanus amaryllifolius against carbon tetrachloride (CCl4) induced toxicity: A biochemical and hispathological study. Arab. J. Chem. 2021, 14, 103390. [Google Scholar] [CrossRef]
- Chiabchalard, A.; Nooron, N. Antihyperglycemic effects of Pandanus amaryllifolius Roxb. leaf extract. Pharmacogn. Mag. 2015, 11, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Laluces, H.M.; Nakayama, A.; Nonato, M.G.; dela Cruz, T.E.; Tan, M.A. Antimicrobial alkaloids from the leaves of Pandanus amaryllifolius. J. Appl. Pharm. Sci. 2015, 5, 151–153. [Google Scholar] [CrossRef]
- Ooi, L.; Sun, S.; Ooi, V. Purification and characterization of a new antiviral protein from the leaves of Pandanus amaryllifolius (Pandanaceae). Int. J. Biochem. Cell Biol. 2004, 36, 1440–1446. [Google Scholar] [CrossRef]
- Suttisansanee, U.; Kunkeaw, T.; Thatsanasuwan, N.; Tonglim, J.; Temviriyanakul, P. The investigation on cholinesterase and BACE1 inhibitory activities in various tea infusions. Walailak J. Sci. Technol. 2019, 16, 165–174. [Google Scholar] [CrossRef]
- Sharma, N.; Tan, M.A.; An, S.S.A. Phytosterols: Potential metabolic modulators in neurodegenerative diseases. Int. J. Mol. Sci. 2021, 22, 12255. [Google Scholar] [CrossRef]
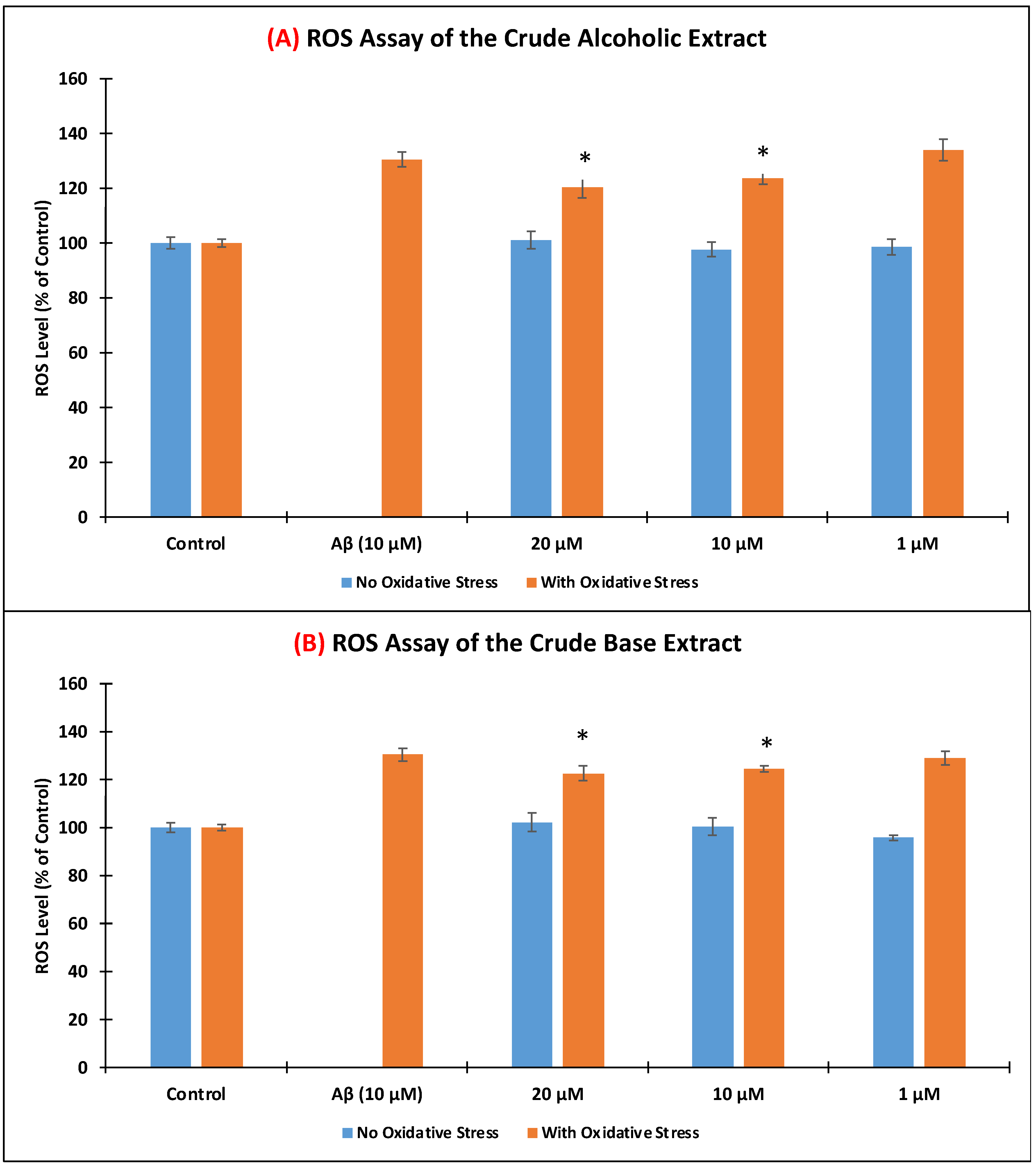
| ThT Assay a (% Inhibition) c | MDS Assay b (Optical Density) c | |
|---|---|---|
| Crude Alcoholic Extract | 73.67 ± 3.54 | 2.559 ± 0.19 ^ |
| Crude Base Extract | 89.53 ± 5.21 * | 2.027 ± 0.080 ^ |
| Phenol Red (Positive Control) | 70.06 ± 2.87 | |
| BPL-1 (Positive Control) | 1.509 ± 0.18 ^ | |
| Negative Control | 2.203 ± 0.068 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, M.A.; Ishikawa, H.; An, S.S.A. Pandanus amaryllifolius Exhibits In Vitro Anti-Amyloidogenic Activity and Promotes Neuroprotective Effects in Amyloid-β-Induced SH-SY5Y Cells. Nutrients 2022, 14, 3962. https://doi.org/10.3390/nu14193962
Tan MA, Ishikawa H, An SSA. Pandanus amaryllifolius Exhibits In Vitro Anti-Amyloidogenic Activity and Promotes Neuroprotective Effects in Amyloid-β-Induced SH-SY5Y Cells. Nutrients. 2022; 14(19):3962. https://doi.org/10.3390/nu14193962
Chicago/Turabian StyleTan, Mario A., Hayato Ishikawa, and Seong Soo A. An. 2022. "Pandanus amaryllifolius Exhibits In Vitro Anti-Amyloidogenic Activity and Promotes Neuroprotective Effects in Amyloid-β-Induced SH-SY5Y Cells" Nutrients 14, no. 19: 3962. https://doi.org/10.3390/nu14193962
APA StyleTan, M. A., Ishikawa, H., & An, S. S. A. (2022). Pandanus amaryllifolius Exhibits In Vitro Anti-Amyloidogenic Activity and Promotes Neuroprotective Effects in Amyloid-β-Induced SH-SY5Y Cells. Nutrients, 14(19), 3962. https://doi.org/10.3390/nu14193962








