Supplementation with Vitis vinifera Jingzaojing Leaf and Shoot Extract Improves Exercise Endurance in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of JLSE
2.2. High-Performance Liquid Chromatography (HPLC) Analysis
2.3. Animals and Ethical Statements
2.4. Treadmill Running and Grip Strength Measurement
2.5. Statistical Analysis
2.6. Additional Methods
3. Results
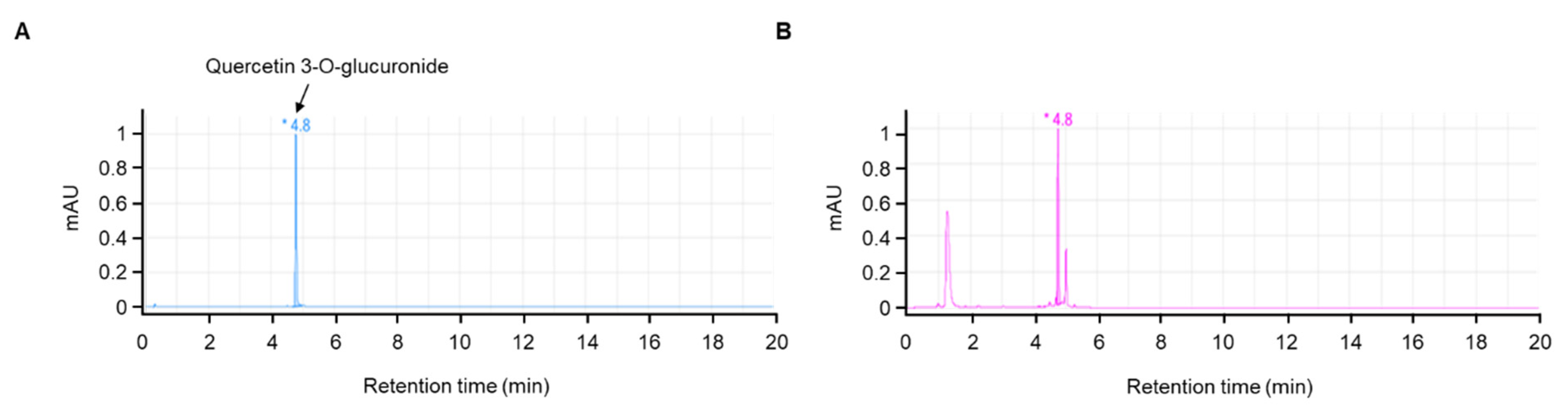
3.1. HPLC Analysis of JLSE
3.2. JLSE Supplementation Enhances Endurance Exercise Performance in Mice
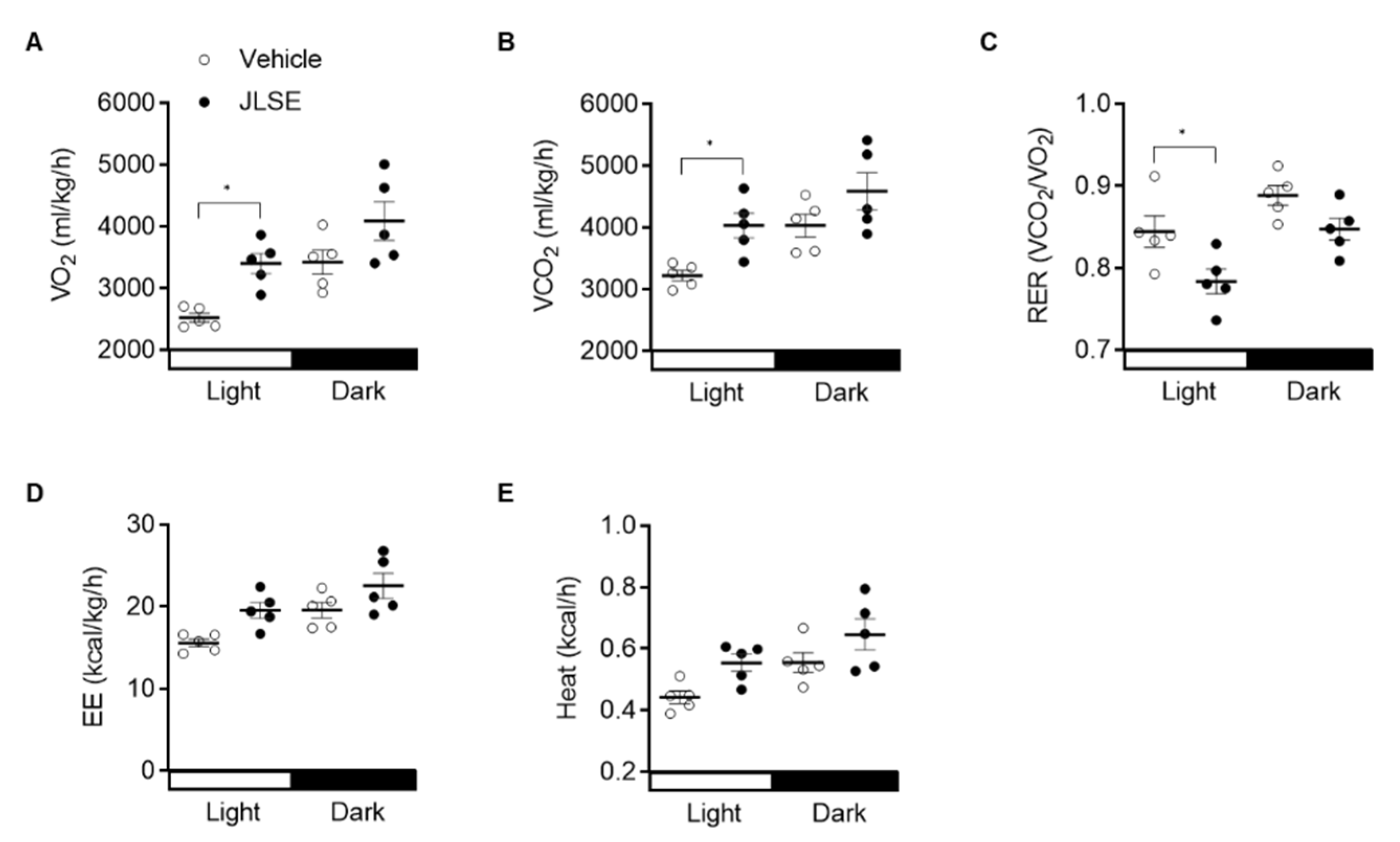
3.3. JLSE Supplementation Increases Mitochondrial Oxidative Capacity in Mice
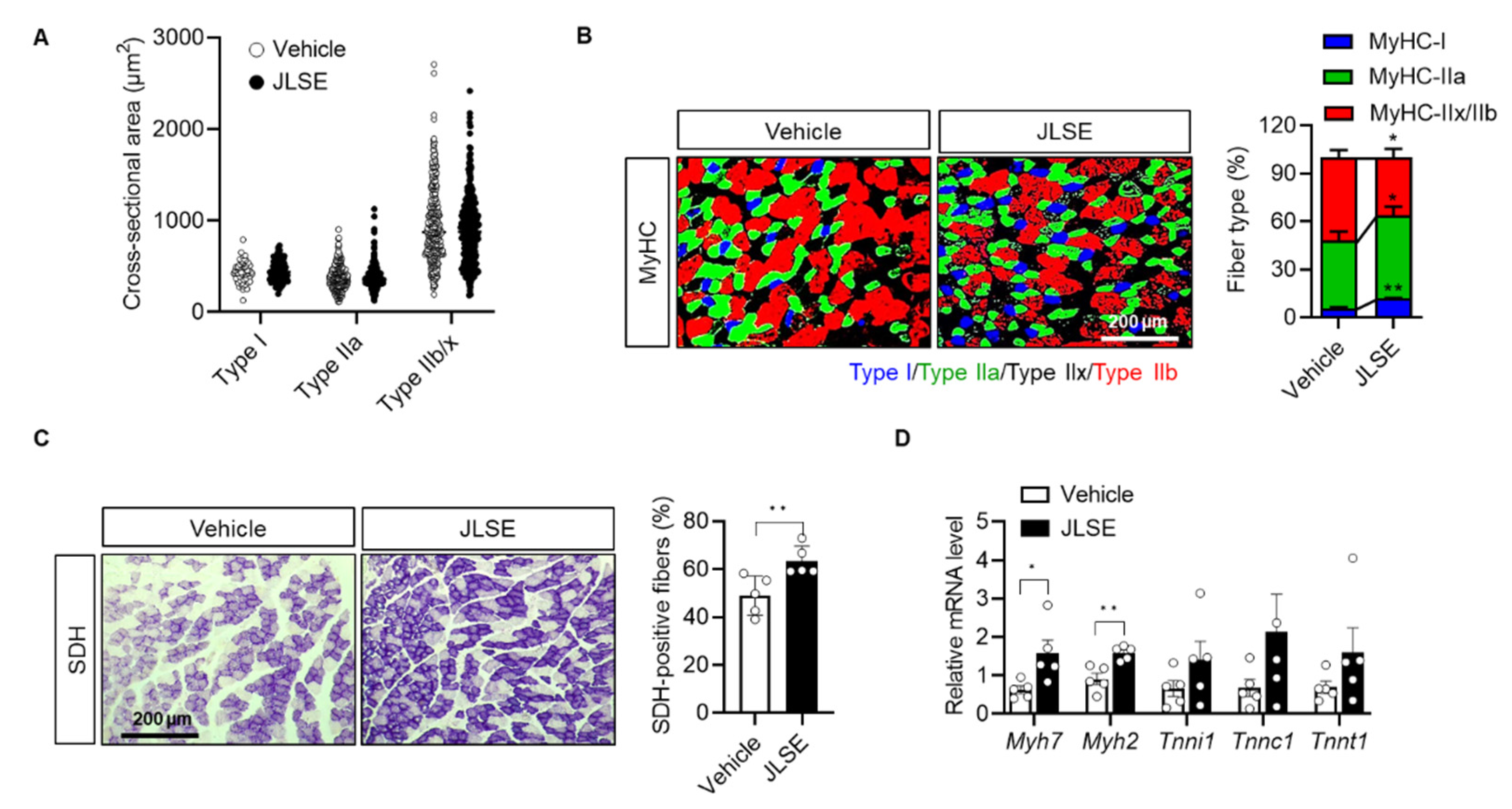
3.4. JLSE Supplementation Increases the Proportion of Slow Muscle Fibers in Gastrocnemius Muscles
3.5. JLSE Supplementation Enhances Mitochondrial Biogenesis by Upregulating Sirt6
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.C.R.; Semenova, E.A.; Bondareva, E.A.; Borisov, O.V.; Andryushchenko, O.N.; Andryushchenko, L.B.; Zmijewski, P.; Generozov, E.V.; Ahmetov, I.I. Association of muscle fiber composition with health and exercise-related traits in athletes and untrained subjects. Biol. Sport 2021, 38, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, U.R.; Agergaard, J.; Couppe, C.; Grosset, J.F.; Karlsen, A.; Magnusson, S.P.; Schjerling, P.; Kjaer, M.; Mackey, A.L. Skeletal muscle morphology and regulatory signalling in endurance-trained and sedentary individuals: The influence of ageing. Exp. Gerontol. 2017, 93, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Oberbach, A.; Bossenz, Y.; Lehmann, S.; Niebauer, J.; Adams, V.; Paschke, R.; Schon, M.R.; Bluher, M.; Punkt, K. Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care 2006, 29, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.J.; Le, A.M.; Zhang, L.; Kahn, M.; Samuel, V.T.; Shulman, G.I.; Bennett, A.M. MAPK phosphatase-1 facilitates the loss of oxidative myofibers associated with obesity in mice. J. Clin. Invest. 2009, 119, 3817–3829. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, F.; Tan, B.; Yao, K.; Yin, Y. Metabolic control of myofibers: Promising therapeutic target for obesity and type 2 diabetes. Obes. Rev. 2017, 18, 647–659. [Google Scholar] [CrossRef]
- Whytock, K.L.; Parry, S.A.; Turner, M.C.; Woods, R.M.; James, L.J.; Ferguson, R.A.; Stahlman, M.; Boren, J.; Strauss, J.A.; Cocks, M.; et al. A 7-day high-fat, high-calorie diet induces fibre-specific increases in intramuscular triglyceride and perilipin protein expression in human skeletal muscle. J. Physiol. 2020, 598, 1151–1167. [Google Scholar] [CrossRef]
- Stuart, C.A.; McCurry, M.P.; Marino, A.; South, M.A.; Howell, M.E.; Layne, A.S.; Ramsey, M.W.; Stone, M.H. Slow-twitch fiber proportion in skeletal muscle correlates with insulin responsiveness. J. Clin. Endocrinol. Metab. 2013, 98, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N.; et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Linden, M.A.; Fuller, S.E.; Goldsmith, F.R.; Simon, J.; Batdorf, H.M.; Scott, M.C.; Essajee, N.M.; Brown, J.M.; Noland, R.C. Combined effects of a ketogenic diet and exercise training alter mitochondrial and peroxisomal substrate oxidative capacity in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E1053–E1067. [Google Scholar] [CrossRef]
- Roberts, J.D.; Roberts, M.G.; Tarpey, M.D.; Weekes, J.C.; Thomas, C.H. The effect of a decaffeinated green tea extract formula on fat oxidation, body composition and exercise performance. J. Int. Soc. Sports Nutr. 2015, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Kan, N.W.; Lee, M.C.; Tung, Y.T.; Chiu, C.C.; Huang, C.C.; Huang, W.C. The synergistic effects of resveratrol combined with resistant training on exercise performance and physiological adaption. Nutrients 2018, 10, 1360. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Hsu, Y.J.; Ho, H.H.; Kuo, Y.W.; Lin, W.Y.; Tsai, S.Y.; Chen, W.L.; Lin, C.L.; Huang, C.C. Effectiveness of human-origin Lactobacillus plantarum PL-02 in improving muscle mass, exercise performance and anti-fatigue. Sci. Rep. 2021, 11, 19469. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Pan, C.H.; Wei, C.C.; Huang, H.Y. Lactobacillus plantarum PS128 improves physiological adaptation and performance in triathletes through gut microbiota modulation. Nutrients 2020, 12, 2315. [Google Scholar] [CrossRef] [PubMed]
- Di Meglio, F.; Sacco, A.M.; Belviso, I.; Romano, V.; Sirico, F.; Loiacono, C.; Palermi, S.; Pempinello, C.; Montagnani, S.; Nurzynska, D.; et al. Influence of supplements and drugs used for the treatment of musculoskeletal disorders on adult human tendon-derived stem cells. Muscles Ligaments Tendons J. 2020, 10, 376–384. [Google Scholar] [CrossRef]
- Munoz-Bernal, O.A.; Coria-Oliveros, A.J.; de la Rosa, L.A.; Rodrigo-Garcia, J.; Del Rocio Martinez-Ruiz, N.; Sayago-Ayerdi, S.G.; Alvarez-Parrilla, E. Cardioprotective effect of red wine and grape pomace. Food Res. Int. 2021, 140, 110069. [Google Scholar] [CrossRef] [PubMed]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Summo, C.; Caponio, F. Bioactive compounds from vine shoots, grape stalks, and wine lees: Their potential use in agro-food chains. Foods 2021, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Cho, B.O.; Jang, S.I. Muscat Bailey A grape stalk extract ameliorates high-fat diet induced obesity by downregulating PPARγ and C/EPBα in mice. Int. J. Mol. Med. 2019, 43, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Che, D.N.; Kang, H.J.; Cho, B.O.; Shin, J.Y.; Jang, S.I. Combined effects of Diospyros lotus leaf and grape stalk extract in high-fat-diet-induced obesity in mice. Food Sci. Biotechnol. 2019, 28, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Bujor, O.C.; Ginies, C.; Popa, V.I.; Dufour, C. Phenolic compounds and antioxidant activity of lingonberry (Vaccinium vitis-idaea L.) leaf, stem and fruit at different harvest periods. Food Chem. 2018, 252, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Amen, Y.; Sherif, A.E.; Shawky, N.M.; Abdelrahman, R.S.; Wink, M.; Sobeh, M. Grape-leaf extract attenuates alcohol-induced liver injury via interference with NF-κB signaling pathway. Biomolecules 2020, 10, 558. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Hong, M.H.; Yoon, J.J.; Kim, D.S.; Na, S.W.; Jang, Y.J.; Lee, Y.J.; Kang, D.G.; Lee, H.S. Protective effect of Vitis labrusca leaves extract on cardiovascular dysfunction through HMGB1-TLR4-NFκB signaling in spontaneously hypertensive rats. Nutrients 2020, 12, 3096. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Chen, C.R.; Wu, W.H.; Wen, C.L.; Chang, C.I.; Hou, W.C. Anti-α-glucosidase and anti-dipeptidyl peptidase-IV activities of extracts and purified compounds from Vitis thunbergii var. taiwaniana. J. Agric. Food Chem. 2015, 63, 6393–6401. [Google Scholar] [CrossRef]
- Meng, L.; Jiao, Y.; Zhou, X.; Liang, C.; Yan, K.; Zhao, Y.; Deng, X.; Han, X.; Yang, Y.; Liu, H.; et al. Leaf extract from Vitis vinifera L. reduces high fat diet-induced obesity in mice. Food Funct. 2021, 12, 6452–6463. [Google Scholar] [CrossRef]
- Minegishi, Y.; Haramizu, S.; Hase, T.; Murase, T. Red grape leaf extract improves endurance capacity by facilitating fatty acid utilization in skeletal muscle in mice. Eur. J. Appl. Physiol. 2011, 111, 1983–1989. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondrial dynamics and its involvement in disease. Annu. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef]
- Song, M.Y.; Han, C.Y.; Moon, Y.J.; Lee, J.H.; Bae, E.J.; Park, B.H. Sirt6 reprograms myofibers to oxidative type through CREB-dependent Sox6 suppression. Nat. Commun. 2022, 13, 1808. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, D.; Zhao, W.; Wang, D.; Liu, T.; Liu, Y.; Yang, Y.; Liu, Y.; Mu, J.; Li, B.; et al. Obesity-induced overexpression of miR-802 impairs insulin transcription and secretion. Nat. Commun. 2020, 11, 1822. [Google Scholar] [CrossRef]
- Spriet, L.L.; Watt, M.J. Regulatory mechanisms in the interaction between carbohydrate and lipid oxidation during exercise. Acta. Physiol. Scand. 2003, 178, 443–452. [Google Scholar] [CrossRef]
- van Loon, L.J.; Greenhaff, P.L.; Constantin-Teodosiu, D.; Saris, W.H.; Wagenmakers, A.J. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001, 536, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Purdom, T.; Kravitz, L.; Dokladny, K.; Mermier, C. Understanding the factors that effect maximal fat oxidation. J. Int. Soc. Sports Nutr. 2018, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Lagerwaard, B.; Keijer, J.; McCully, K.K.; de Boer, V.C.J.; Nieuwenhuizen, A.G. In vivo assessment of muscle mitochondrial function in healthy, young males in relation to parameters of aerobic fitness. Eur. J. Appl. Physiol. 2019, 119, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liang, D.; Huang, Z.; Jia, G.; Zhao, H.; Liu, G. Quercetin regulates skeletal muscle fiber type switching via adiponectin signaling. Food Funct. 2021, 12, 2693–2702. [Google Scholar] [CrossRef]
- Davis, J.M.; Carlstedt, C.J.; Chen, S.; Carmichael, M.D.; Murphy, E.A. The dietary flavonoid quercetin increases VO2max and endurance capacity. Int. J. Sport. Nutr. Exerc. Metab. 2010, 20, 56–62. [Google Scholar] [CrossRef]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Davis, B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1071–R1077. [Google Scholar] [CrossRef]
- Nieman, D.C.; Williams, A.S.; Shanely, R.A.; Jin, F.; McAnulty, S.R.; Triplett, N.T.; Austin, M.D.; Henson, D.A. Quercetin’s influence on exercise performance and muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2010, 42, 338–345. [Google Scholar] [CrossRef]
- Koshinaka, K.; Honda, A.; Masuda, H.; Sato, A. Effect of quercetin treatment on mitochondrial biogenesis and exercise-induced AMP-activated protein kinase activation in rat skeletal muscle. Nutrients 2020, 12, 729. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.G.; Woo, H.; Choi, C.; Ryoo, G.-H.; Chung, Y.-J.; Lee, J.-H.; Jung, S.-J.; Chae, S.-W.; Bae, E.J.; Park, B.-H. Supplementation with Vitis vinifera Jingzaojing Leaf and Shoot Extract Improves Exercise Endurance in Mice. Nutrients 2022, 14, 4033. https://doi.org/10.3390/nu14194033
Lee YG, Woo H, Choi C, Ryoo G-H, Chung Y-J, Lee J-H, Jung S-J, Chae S-W, Bae EJ, Park B-H. Supplementation with Vitis vinifera Jingzaojing Leaf and Shoot Extract Improves Exercise Endurance in Mice. Nutrients. 2022; 14(19):4033. https://doi.org/10.3390/nu14194033
Chicago/Turabian StyleLee, Yong Gyun, Hayoung Woo, Chul Choi, Ga-Hee Ryoo, Yun-Jo Chung, Ju-Hyung Lee, Su-Jin Jung, Soo-Wan Chae, Eun Ju Bae, and Byung-Hyun Park. 2022. "Supplementation with Vitis vinifera Jingzaojing Leaf and Shoot Extract Improves Exercise Endurance in Mice" Nutrients 14, no. 19: 4033. https://doi.org/10.3390/nu14194033
APA StyleLee, Y. G., Woo, H., Choi, C., Ryoo, G.-H., Chung, Y.-J., Lee, J.-H., Jung, S.-J., Chae, S.-W., Bae, E. J., & Park, B.-H. (2022). Supplementation with Vitis vinifera Jingzaojing Leaf and Shoot Extract Improves Exercise Endurance in Mice. Nutrients, 14(19), 4033. https://doi.org/10.3390/nu14194033






