Bifidobacterium longum 070103 Fermented Milk Improve Glucose and Lipid Metabolism Disorders by Regulating Gut Microbiota in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Probiotic Fermented Milk
2.2. Heterologous Expression and Purification of Glucokinase
2.3. In Vitro Glucokinase Enzymatic Assays
2.4. Animal Experiments
2.5. Oral Glucose Tolerance Test (OGTT)
2.6. Determination of Serum Metabolites and Intestinal Barrier Index
2.7. Histopathological Examination
2.8. Metabolome Analysis of Fecal Metabolites
2.9. Gut Microbiota Analysis
2.10. Detection Colonization Ability of B.longum 070103 in Mouse Feces by Quantitative Real-Time PCR
2.11. Statistical Analysis
3. Results
3.1. B. longum 070103 Fermented Milk Increased Glucokinase Activity
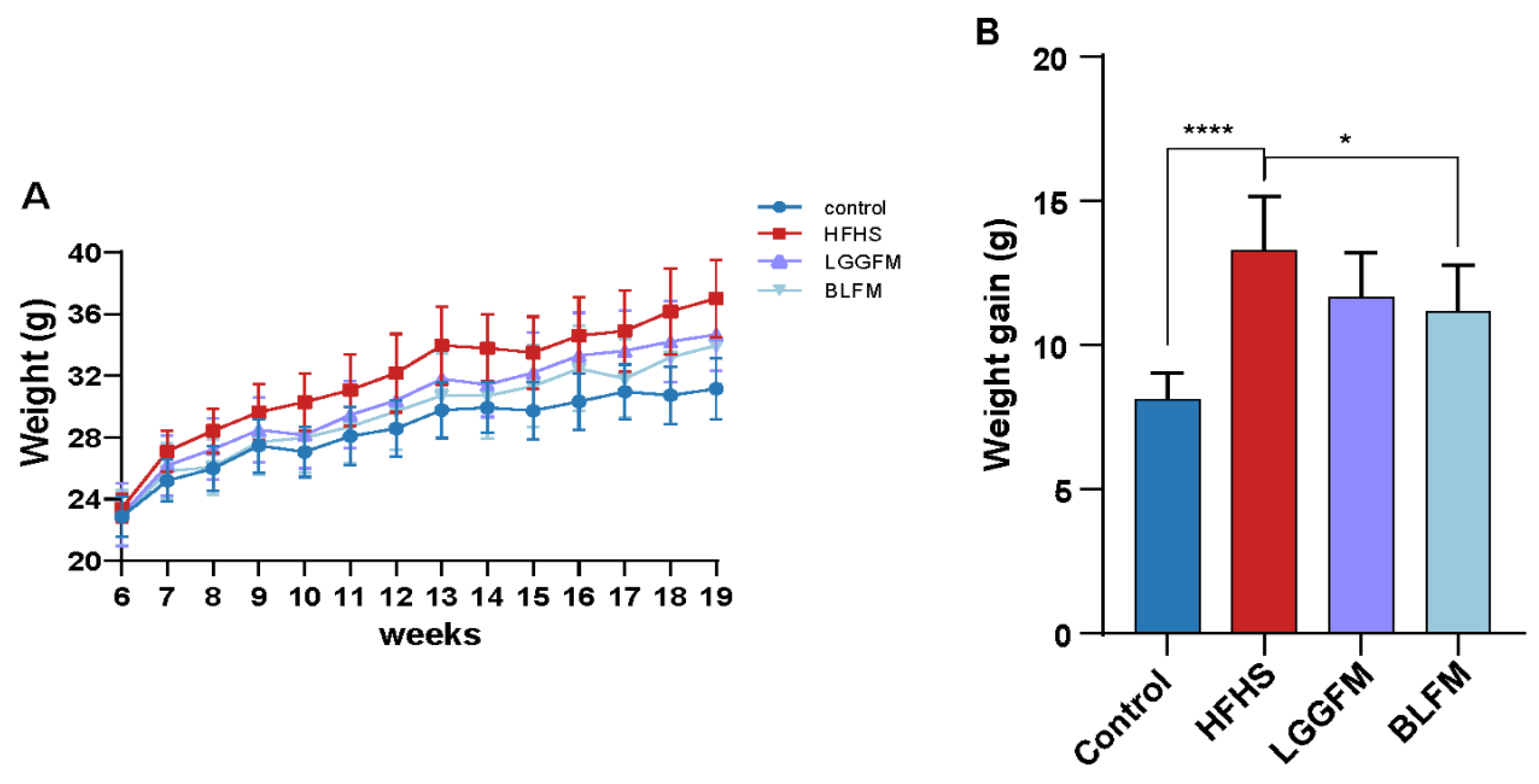
3.2. B. longum Fermented Milk (BLFM) Improves Obesity Caused by HFHS
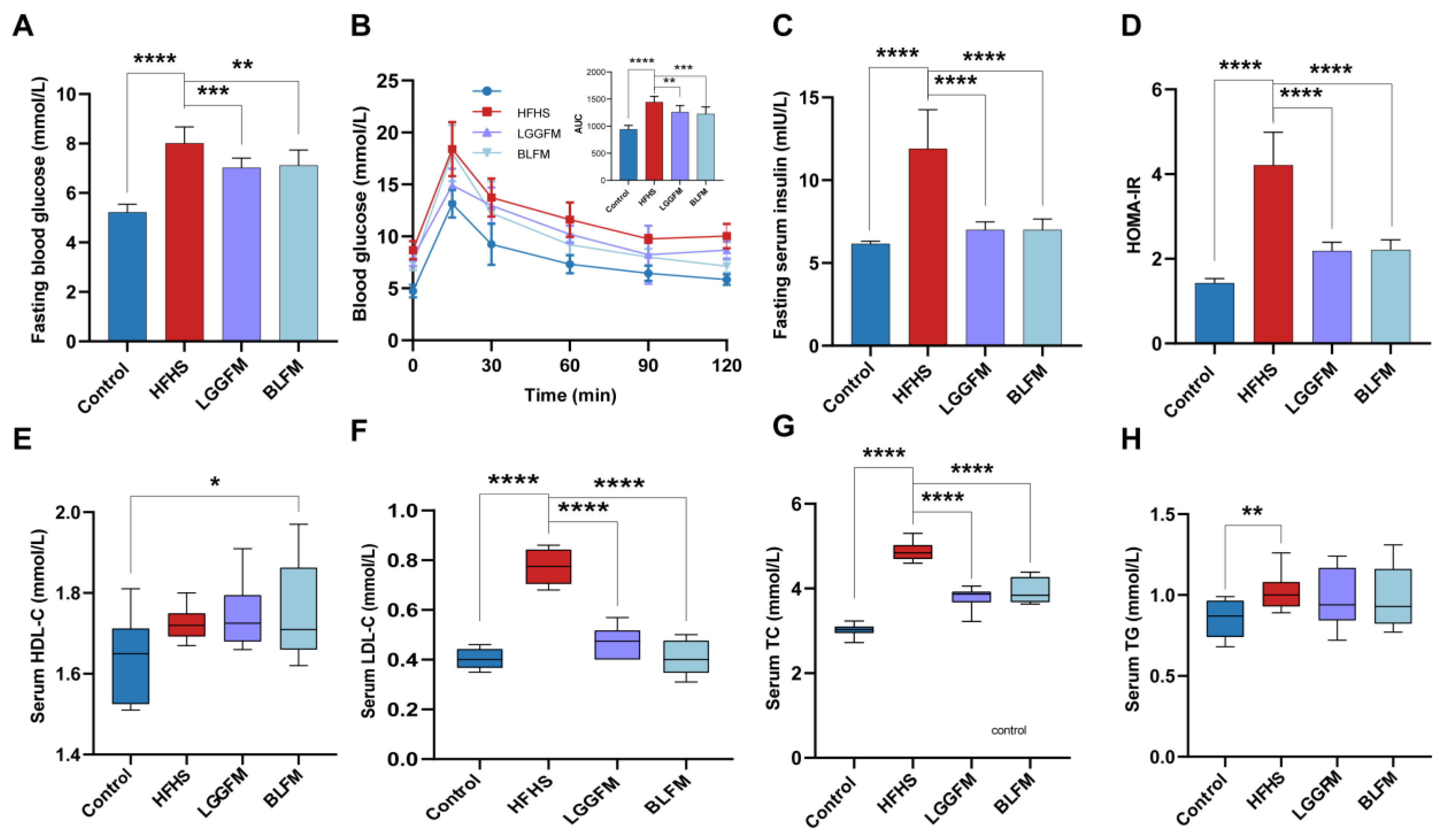
3.3. BLFM Improves Hyperglycemia and Dyslipidemia in HFHS-Fed Mice
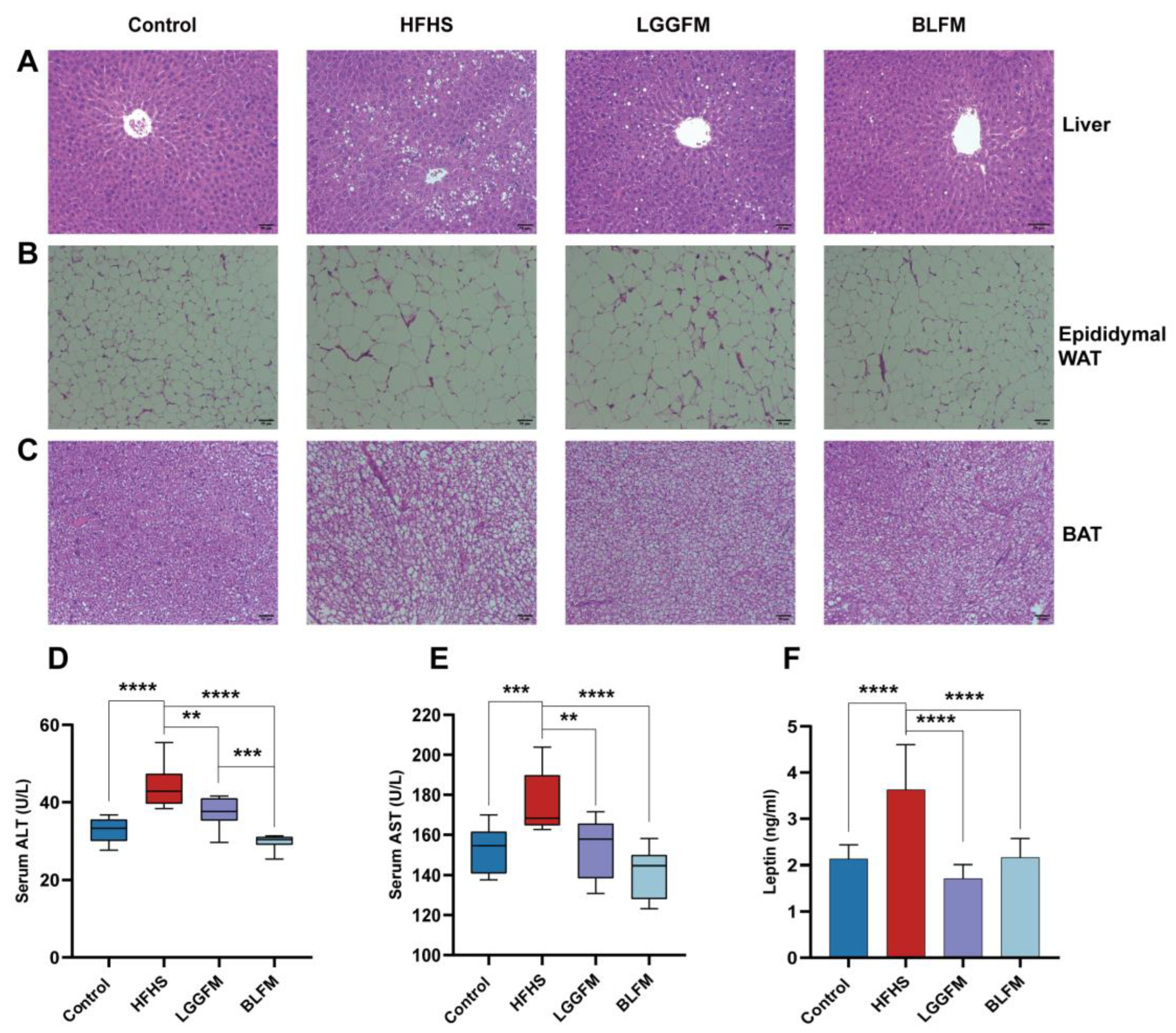
3.4. BLFM Improves HFHS Induced Liver Steatosis, Inhibits White Adipose Tissue (WAT) Proliferation
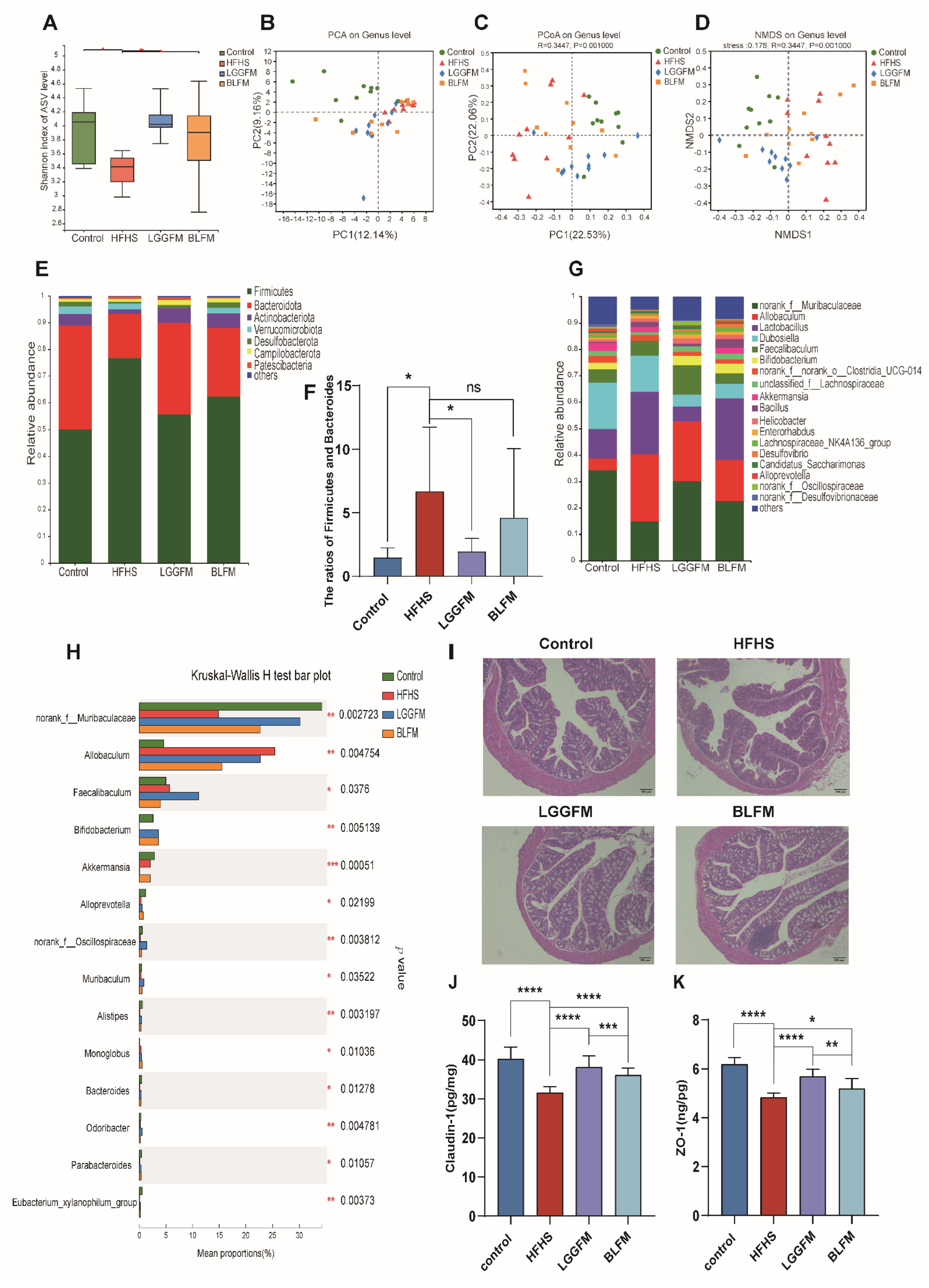
3.5. BLFM Modulates Gut Microbiota
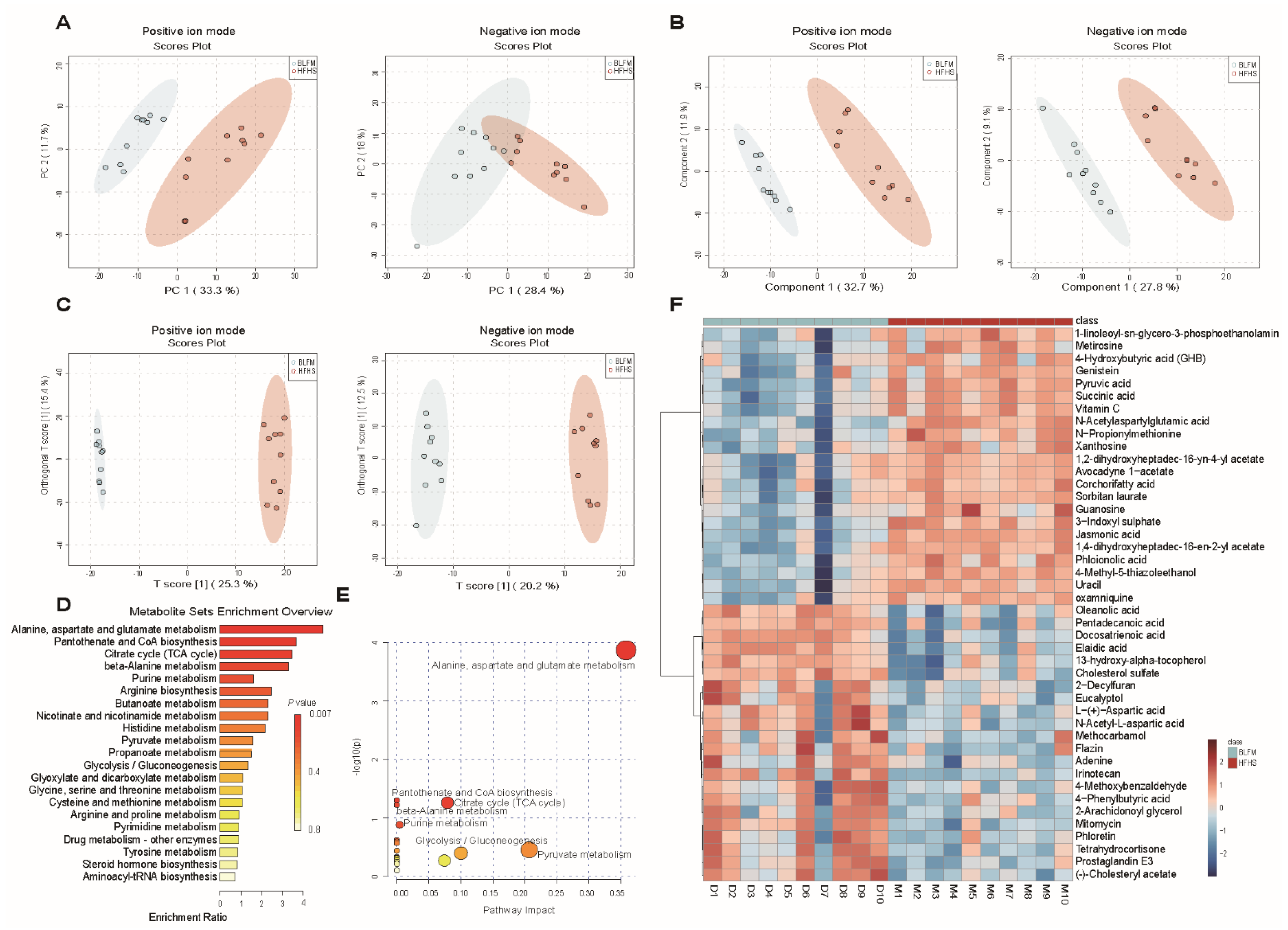
3.6. BLFM Improves HFHS Induced Fecal Metabolites in Mice
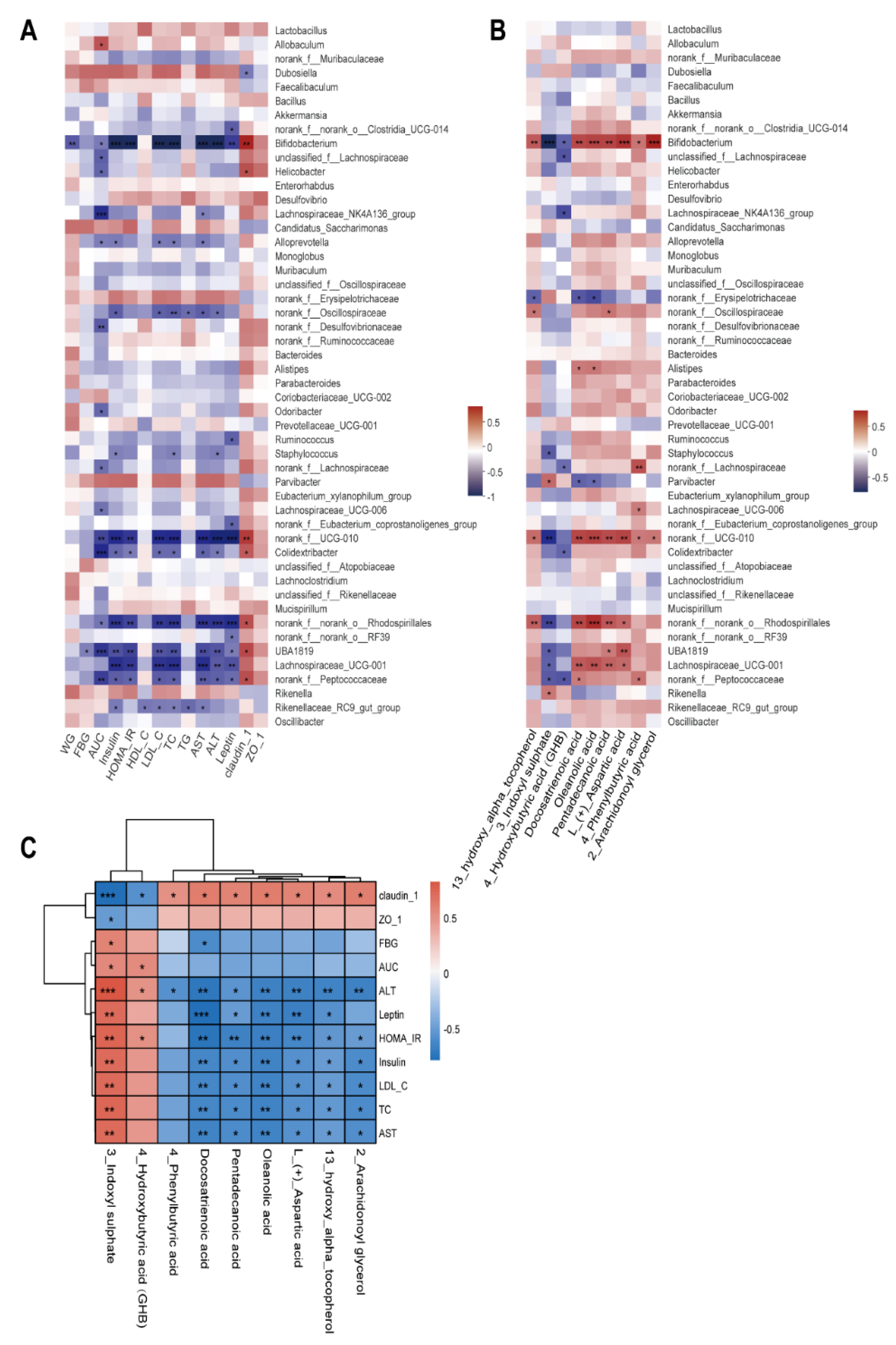
3.7. Correlation among Gut Microbiota, Fecal Metabolites, and Pathological Indicators
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Shock, T.; Badang, L.; Ferguson, B.; Martinez-Guryn, K. The interplay between diet, gut microbes, and host epigenetics in health and disease. J. Nutr. Biochem. 2021, 95, 108631. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Xu, J.; Li, G.; Liu, T.; Guo, X.; Wang, H.; Luo, L. Ethanol extract of propolis prevents high-fat diet-induced insulin resistance and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 2020, 130, 108939. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.T.; Chaves-Filho, A.B.; Yoshinaga, M.Y.; Paiva, N.C.N.; Carneiro, C.M.; Miyamoto, S.; Festuccia, W.T.; Guerra-Sa, R. Liver lipidome signature and metabolic pathways in nonalcoholic fatty liver disease induced by a high-sugar diet. J. Nutr. Biochem. 2021, 87, 108519. [Google Scholar] [CrossRef] [PubMed]
- Bisanz, J.E.; Upadhyay, V.; Turnbaugh, J.A.; Ly, K.; Turnbaugh, P.J. Meta-analysis reveals reproducible gut microbiome alterations in response to a high-fat diet. Cell Host Microbe 2019, 26, 265–272.e264. [Google Scholar] [CrossRef] [PubMed]
- Anhe, F.F.; Nachbar, R.T.; Varin, T.V.; Trottier, J.; Dudonne, S.; Le Barz, M.; Feutry, P.; Pilon, G.; Barbier, O.; Desjardins, Y.; et al. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut 2019, 68, 453–464. [Google Scholar] [CrossRef]
- Mazloom, K.; Siddiqi, I.; Covasa, M. Probiotics: How effective are they in the fight against obesity? Nutrients 2019, 11, 258. [Google Scholar] [CrossRef]
- Chen, J.; Wang, R.; Li, X.F.; Wang, R.L. Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome. Br. J. Nutr. 2012, 107, 1429–1434. [Google Scholar] [CrossRef]
- Monk, J.M.; Wu, W.; Lepp, D.; Wellings, H.R.; Hutchinson, A.L.; Liddle, D.M.; Graf, D.; Pauls, K.P.; Robinson, L.E.; Power, K.A. Navy bean supplemented high-fat diet improves intestinal health, epithelial barrier integrity and critical aspects of the obese inflammatory phenotype. J. Nutr. Biochem. 2019, 70, 91–104. [Google Scholar] [CrossRef]
- Li, B.; Hong, Y.; Gu, Y.; Ye, S.; Hu, K.; Yao, J.; Ding, K.; Zhao, A.; Jia, W.; Li, H. Functional metabolomics reveals that astragalus polysaccharides improve lipids metabolism through microbial metabolite 2-hydroxybutyric acid in obese mice. Engineering 2020, 9, 111–122. [Google Scholar] [CrossRef]
- Wang, G.; Si, Q.; Yang, S.; Jiao, T.; Zhu, H.; Tian, P.; Wang, L.; Li, X.; Gong, L.; Zhao, J.; et al. Lactic acid bacteria reduce diabetes symptoms in mice by alleviating gut microbiota dysbiosis and inflammation in different manners. Food Funct. 2020, 11, 5898–5914. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Zare, Z.; Shakeri, H.; Sabihi, S.S.; Esmaillzadeh, A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann. Nutr. Metab. 2013, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bernini, L.J.; Simao, A.N.C.; de Souza, C.H.B.; Alfieri, D.F.; Segura, L.G.; Costa, G.N.; Dichi, I. Effect of Bifidobacterium lactis HN019 on inflammatory markers and oxidative stress in subjects with and without the metabolic syndrome. Br. J. Nutr. 2018, 120, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, J.; Zhang, H.; Xie, Y.; Jin, J. Bifidobacterium from breastfed infant faeces prevent high-fat-diet-induced glucose tolerance impairment, mediated by the modulation of glucose intake and the incretin hormone secretion axis. J. Sci. Food Agric. 2020, 100, 3308–3318. [Google Scholar] [CrossRef] [PubMed]
- Velho, G.; Froguel, P. Genetic, metabolic and clinical characteristics of maturity onset diabetes of the young. Eur. J. Endocrinol. 1998, 138, 233–239. [Google Scholar] [CrossRef]
- Lei, L.; Liu, S.; Li, Y.; Song, H.; He, L.; Liu, Q.; Sun, S.; Li, Y.; Feng, Z.; Shen, Z. The potential role of glucokinase activator SHP289-04 in anti-diabetes and hepatic protection. Eur. J. Pharmacol. 2018, 826, 17–23. [Google Scholar] [CrossRef]
- Shirakawa, J.; Togashi, Y.; Sakamoto, E.; Kaji, M.; Tajima, K.; Orime, K.; Inoue, H.; Kubota, N.; Kadowaki, T.; Terauchi, Y. Glucokinase Activation Ameliorates ER Stress–Induced Apoptosis in Pancreatic β-Cells. Diabetes 2013, 62, 3448–3458. [Google Scholar] [CrossRef]
- Sakandar, H.A.; Zhang, H. Trends in Probiotic(s)-Fermented milks and their in vivo functionality: A review. Trends Food Sci. Technol. 2021, 110, 55–65. [Google Scholar] [CrossRef]
- Segers, M.E.; Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG-host interactions. Microb. Cell Fact. 2014, 13 (Suppl. S1), S7. [Google Scholar] [CrossRef]
- Xu, H.; Wang, J.; Cai, J.; Feng, W.; Wang, Y.; Liu, Q.; Cai, L. Protective Effect of Lactobacillus rhamnosus GG and its Supernatant against Myocardial Dysfunction in Obese Mice Exposed to Intermittent Hypoxia is Associated with the Activation of Nrf2 Pathway. Int. J. Biol. Sci. 2019, 15, 2471–2483. [Google Scholar] [CrossRef]
- Wang, J.; Tang, H.; Zhang, C.; Zhao, Y.; Derrien, M.; Rocher, E.; van-Hylckama Vlieg, J.E.; Strissel, K.; Zhao, L.; Obin, M.; et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jangra, S.; Sharma, R.K.; Pothuraju, R.; Bhakri, G. Milk fermented with Lactobacillus casei NCDC19 improves high fat and sucrose diet alters gene expression in obese mice. Int. Dairy J. 2019, 90, 15–22. [Google Scholar] [CrossRef]
- Yang, S.; Xie, X.; Ma, J.; He, X.; Li, Y.; Du, M.; Li, L.; Yang, L.; Wu, Q.; Chen, W.; et al. Selective Isolation of Bifidobacterium from Human Faeces Using Pangenomics, Metagenomics, and Enzymology. Front. Microbiol. 2021, 12, 649698. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Cane, D.E. Stereospecific formation of Z-trisubstituted double bonds by the successive action of ketoreductase and dehydratase domains from trans-AT polyketide synthases. Biochemistry 2018, 57, 3126–3129. [Google Scholar] [CrossRef]
- Lei, L.; Liu, Q.; Liu, S.; Huan, Y.; Sun, S.; Chen, Z.; Li, L.; Feng, Z.; Li, Y.; Shen, Z. Antidiabetic potential of a novel dual-target activator of glucokinase and peroxisome proliferator activated receptor-gamma. Metabolism 2015, 64, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, S.; Zhang, B.; Xiao, C.; Chen, Z.; Si, F.; Fu, J.; Lin, X.; Zheng, G.; Yu, G.; et al. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging 2020, 12, 8583–8604. [Google Scholar] [CrossRef]
- Tan, R.; Dong, H.; Chen, Z.; Jin, M.; Yin, J.; Li, H.; Shi, D.; Shao, Y.; Wang, H.; Chen, T.; et al. Intestinal microbiota mediates high-fructose and high-fat diets to induce chronic intestinal inflammation. Front. Cell. Infect. Microbiol. 2021, 11, 654074. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.C.; Xiao, J.; Zhang, P.P.; Chen, L.L.; Chen, X.X.; Wang, S.M. Longitudinal Changes in Liver Aminotransferases Predict Metabolic Syndrome in Chinese Patients with Nonviral Hepatitis. Biomed. Environ. Sci. 2016, 29, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Amacher, D.E. A toxicologist’s guide to biomarkers of hepatic response. Hum. Exp. Toxicol. 2002, 21, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Amacher, D.E. Serum transaminase elevations as indicators of hepatic injury following the administration of drugs. Regul. Toxicol. Pharmacol. 1998, 27, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.; Halaas, J.; Ravussin, E.; Pratley, R.E.; Lee, G.H.; Zhang, Y.; Fei, H.; Kim, S.; Lallone, R.; Ranganathan, S.; et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1995, 1, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Guo, J.; Su, Z. Advances in understanding the interrelations between leptin resistance and obesity. Physiol. Behav. 2014, 130, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Manzanarez-Quin, C.G.; Beltran-Barrientos, L.M.; Hernandez-Mendoza, A.; Gonzalez-Cordova, A.F.; Vallejo-Cordoba, B. Invited review: Potential antiobesity effect of fermented dairy products. J. Dairy Sci. 2021, 104, 3766–3778. [Google Scholar] [CrossRef]
- Sternisha, S.M.; Miller, B.G. Molecular and cellular regulation of human glucokinase. Arch. Biochem. Biophys. 2019, 663, 199–213. [Google Scholar] [CrossRef]
- Pothuraju, R.; Sharma, R.K.; Kavadi, P.K.; Chagalamarri, J.; Jangra, S.; Bhakri, G.; De, S. Anti-obesity effect of milk fermented by Lactobacillus plantarum NCDC 625 alone and in combination with herbs on high fat diet fed C57BL/6J mice. Benef. Microbes 2016, 7, 375–385. [Google Scholar] [CrossRef]
- Alihosseini, N.; Moahboob, S.A.; Farrin, N.; Mobasseri, M.; Taghizadeh, A.; Ostadrahimi, A.R. Effect of probiotic fermented milk (Kefir) on serum level of insulin and homocysteine in type 2 diabetes patients. Acta Endocrinol. 2017, 13, 431–436. [Google Scholar] [CrossRef]
- Naito, E.; Yoshida, Y.; Kunihiro, S.; Makino, K.; Kasahara, K.; Kounoshi, Y.; Aida, M.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Effect of Lactobacillus casei strain Shirota-fermented milk on metabolic abnormalities in obese prediabetic Japanese men: A randomised, double-blind, placebo-controlled trial. Biosci. Microbiota Food Health 2018, 37, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Dey, D.K.; Vij, R.; Meena, S.; Kapila, R.; Kapila, S. Evaluation of anti-diabetic attributes of Lactobacillus rhamnosus MTCC: 5957, Lactobacillus rhamnosus MTCC: 5897 and Lactobacillus fermentum MTCC: 5898 in streptozotocin induced diabetic rats. Microb. Pathog. 2018, 125, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Hosainzadegn, H.; Hosainzadegan, M. Traditional probiotic (Kefir) effects on glycated hemoglobin a level and weight of an indexed diabetic patient. Int. J. Prev. Med. 2021, 12, 139. [Google Scholar] [PubMed]
- Mu, J.; Zhang, J.; Zhou, X.; Zalan, Z.; Hegyi, F.; Takács, K.; Ibrahim, A.; Awad, S.; Wu, Y.; Zhao, X.; et al. Effect of Lactobacillus plantarum KFY02 isolated from naturally fermented yogurt on the weight loss in mice with high-fat diet-induced obesity via PPAR-α/γ signaling pathway. J. Funct. Foods 2020, 75, 104264. [Google Scholar] [CrossRef]
- Zhou, A.L.; Ward, R.E. Milk polar lipids modulate lipid metabolism, gut permeability, and systemic inflammation in high-fat-fed C57BL/6J ob/ob mice, a model of severe obesity. J. Dairy Sci. 2019, 102, 4816–4831. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.H.; Xie, Q.S.; Chen, G.C.; Huang, C.L.; Yu, T.; Chen, Q.K.; Li, J.Y. Impaired intestinal barrier function in type 2 diabetic patients measured by serum LPS, Zonulin, and IFABP. J. Diabetes Its Complicat. 2021, 35, 107766. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chen, J.; Yi, K.; Peng, L.; Xie, J.; Gou, X.; Peng, T.; Tang, L. Phlorizin ameliorates obesity-associated endotoxemia and insulin resistance in high-fat diet-fed mice by targeting the gut microbiota and intestinal barrier integrity. Gut Microbes 2020, 12, 1–18. [Google Scholar] [CrossRef]
- Bahlouli, W.; Breton, J.; Lelouard, M.; L’Huillier, C.; Tirelle, P.; Salameh, E.; Amamou, A.; Atmani, K.; Goichon, A.; Bole-Feysot, C.; et al. Stress-induced intestinal barrier dysfunction is exacerbated during diet-induced obesity. J. Nutr. Biochem. 2020, 81, 108382. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Fact. 2020, 19, 23. [Google Scholar] [CrossRef] [Green Version]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 2017, 15, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Zandani, G.; Anavi-Cohen, S.; Tsybina-Shimshilashvili, N.; Sela, N.; Nyska, A.; Madar, Z. Broccoli florets supplementation improves insulin sensitivity and alters gut microbiome population-a steatosis mice model induced by high-fat diet. Front. Nutr. 2021, 8, 680241. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Salazar, N.; Dewulf, E.M.; Neyrinck, A.M.; Bindels, L.B.; Cani, P.D.; Mahillon, J.; de Vos, W.M.; Thissen, J.P.; Gueimonde, M.; de Los Reyes-Gavilan, C.G.; et al. Inulin-type fructans modulate intestinal Bifidobacterium species populations and decrease fecal short-chain fatty acids in obese women. Clin. Nutr. 2015, 34, 501–507. [Google Scholar] [CrossRef]
- Requena, T.; Song, Y.; Peláez, C.; Martínez-Cuesta, M.C. Modulation and metabolism of obesity-associated microbiota in a dynamic simulator of the human gut microbiota. LWT 2021, 141, 110921. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Li, D.; Hu, X.; Chen, F. Beneficial effects of ginger on prevention of obesity through modulation of gut microbiota in mice. Eur. J. Nutr. 2020, 59, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Kwon, S.J.; Cho, K.M.; Seo, S.H.; Kim, E.J.; Unno, T.; Bok, S.H.; Park, D.H.; Son, H.S. Intervention with kimchi microbial community ameliorates obesity by regulating gut microbiota. J. Microbiol. 2020, 58, 859–867. [Google Scholar] [CrossRef]
- Hou, D.; Zhao, Q.; Yousaf, L.; Khan, J.; Xue, Y.; Shen, Q. Consumption of mung bean (Vigna radiata L.) attenuates obesity, ameliorates lipid metabolic disorders and modifies the gut microbiota composition in mice fed a high-fat diet. J. Funct. Foods 2020, 64, 103687. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 2019, 26, 222–235.e225. [Google Scholar] [CrossRef]
- Maya-Lucas, O.; Murugesan, S.; Nirmalkar, K.; Alcaraz, L.D.; Hoyo-Vadillo, C.; Pizano-Zarate, M.L.; Garcia-Mena, J. The gut microbiome of Mexican children affected by obesity. Anaerobe 2019, 55, 11–23. [Google Scholar] [CrossRef]
- Zheng, Z.; Lyu, W.; Ren, Y.; Li, X.; Zhao, S.; Yang, H.; Xiao, Y. Allobaculum involves in the modulation of intestinal ANGPTLT4 expression in mice treated by high-fat diet. Front. Nutr. 2021, 8, 690138. [Google Scholar] [CrossRef] [PubMed]
- Zierer, J.; Jackson, M.A.; Kastenmuller, G.; Mangino, M.; Long, T.; Telenti, A.; Mohney, R.P.; Small, K.S.; Bell, J.T.; Steves, C.J.; et al. The fecal metabolome as a functional readout of the gut microbiome. Nat. Genet. 2018, 50, 790–795. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: A review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 2020, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, J.; Gui, W.; Sun, D.; Dai, H.; Xiao, L.; Chu, H.; Du, F.; Zhu, Q.; Schnabl, B.; et al. Tauroursodeoxycholic acid inhibits intestinal inflammation and barrier disruption in mice with non-alcoholic fatty liver disease. Br. J. Pharmacol. 2018, 175, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, J.; Wang, S.; Xiong, J.; Chen, Y.; Liu, Y.; Xiao, T.; Li, Y.; He, T.; Li, Y.; et al. Indoxyl sulfate induces intestinal barrier injury through IRF1-DRP1 axis-mediated mitophagy impairment. Theranostics 2020, 10, 7384–7400. [Google Scholar] [CrossRef]
- Omori, K.; Katakami, N.; Arakawa, S.; Yamamoto, Y.; Ninomiya, H.; Takahara, M.; Matsuoka, T.A.; Tsugawa, H.; Furuno, M.; Bamba, T.; et al. Identification of plasma inositol and indoxyl sulfate as novel biomarker candidates for atherosclerosis in patients with type 2 diabetes. -findings from metabolome analysis using GC/MS. J. Atheroscler. Thromb. 2020, 27, 1053–1067. [Google Scholar] [CrossRef]
- Yu, Q.; Lai, B.K.; Ahooghalandari, P.; Helander, A.; Gylfe, E.; Gilon, P.; Tengholm, A. Gamma-hydroxybutyrate does not mediate glucose inhibition of glucagon secretion. J. Biol. Chem. 2020, 295, 5419–5426. [Google Scholar] [CrossRef]
- Xue, C.; Li, Y.; Lv, H.; Zhang, L.; Bi, C.; Dong, N.; Shan, A.; Wang, J. Oleanolic acid targets the gut-liver axis to alleviate metabolic disorders and hepatic steatosis. J. Agric. Food Chem. 2021, 69, 7884–7897. [Google Scholar] [CrossRef]
- Rao, Y.; Kuang, Z.; Li, C.; Guo, S.; Xu, Y.; Zhao, D.; Hu, Y.; Song, B.; Jiang, Z.; Ge, Z.; et al. Gut Akkermansia muciniphila ameliorates metabolic dysfunction-associated fatty liver disease by regulating the metabolism of L-aspartate via gut-liver axis. Gut Microbes 2021, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of dietary odd-chain saturated fatty acid pentadecanoic acid parallels broad associated health benefits in humans: Could it be essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef] [PubMed]
- Santaren, I.D.; Watkins, S.M.; Liese, A.D.; Wagenknecht, L.E.; Rewers, M.J.; Haffner, S.M.; Lorenzo, C.; Hanley, A.J. Serum pentadecanoic acid (15:0), a short-term marker of dairy food intake, is inversely associated with incident type 2 diabetes and its underlying disorders. Am. J. Clin. Nutr. 2014, 100, 1532–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Strain Name | Strain Number | Strain Source | Activation Multiple |
|---|---|---|---|
| Bifidobacterium longum | 070103 | human feces | 1.16 ± 0.03 |
| Bifidobacterium bifidum | 070507 | human feces | 1.10 ± 0.05 |
| Bifidobacterium longum | 070106 | human feces | 0.81 ± 0.06 |
| Lactobacillus delbrueckii | R37 | fermented dairy products | 1.09 ± 0.08 |
| Lactiplantibacillusplantarum | R51 | fermented dairy products | 1.04 ± 0.08 |
| Lactiplantibacillusplantarum | R95 | fermented dairy products | 1.07 ± 0.04 |
| Lactiplantibacillusplantarum | 1–134 | fermented dairy products | 1.06 ± 0.03 |
| Levilactobacillusbrevis | SR52-2 | salted fish | 1.05 ± 0.03 |
| Lactiplantibacillusplantarum | R22 | yogurt | 0.89 ± 0.06 |
| Lactiplantibacillusplantarum | 0101127-4 | pickle | 0.93 ± 0.07 |
| Group | Control | HFHS | LGGFM | BLFM |
|---|---|---|---|---|
| Liver index | 3.90 ± 0.05 a | 4.20 ± 0.13 b | 3.78 ± 0.06 a | 3.86 ± 0.08 a |
| Renal index | 1.26 ± 0.03 a | 1.40 ± 0.03 b | 1.22 ± 0.03 a | 1.36 ± 0.02 b |
| Spleen index | 0.27 ± 0.01 a | 0.30 ± 0.01 b | 0.26 ± 0.01 a | 0.28 ± 0.01 ab |
| Perirenal fat coefficient | 0.44 ± 0.06 a | 1.35 ± 0.18 bc | 1.53 ± 0.21 c | 1.01 ± 0.09 b |
| Epididymal fat coefficient | 1.45 ± 0.16 a | 4.31 ± 0.35 c | 4.10 ± 0.43 c | 3.14 ± 0.30 b |
| Brown fat coefficient | 0.39 ± 0.03 a | 0.43 ± 0.03 a | 0.36 ± 0.02 a | 0.36 ± 0.04 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Li, Y.; Li, L.; Liang, T.; Du, M.; Yang, L.; Yang, J.; Yang, R.; Zhao, H.; Chen, M.; et al. Bifidobacterium longum 070103 Fermented Milk Improve Glucose and Lipid Metabolism Disorders by Regulating Gut Microbiota in Mice. Nutrients 2022, 14, 4050. https://doi.org/10.3390/nu14194050
Jiang T, Li Y, Li L, Liang T, Du M, Yang L, Yang J, Yang R, Zhao H, Chen M, et al. Bifidobacterium longum 070103 Fermented Milk Improve Glucose and Lipid Metabolism Disorders by Regulating Gut Microbiota in Mice. Nutrients. 2022; 14(19):4050. https://doi.org/10.3390/nu14194050
Chicago/Turabian StyleJiang, Tong, Ying Li, Longyan Li, Tingting Liang, Mingzhu Du, Lingshuang Yang, Juan Yang, Runshi Yang, Hui Zhao, Moutong Chen, and et al. 2022. "Bifidobacterium longum 070103 Fermented Milk Improve Glucose and Lipid Metabolism Disorders by Regulating Gut Microbiota in Mice" Nutrients 14, no. 19: 4050. https://doi.org/10.3390/nu14194050






