Characterization of Choline Nutriture among Adults and Children with Phenylketonuria
Abstract
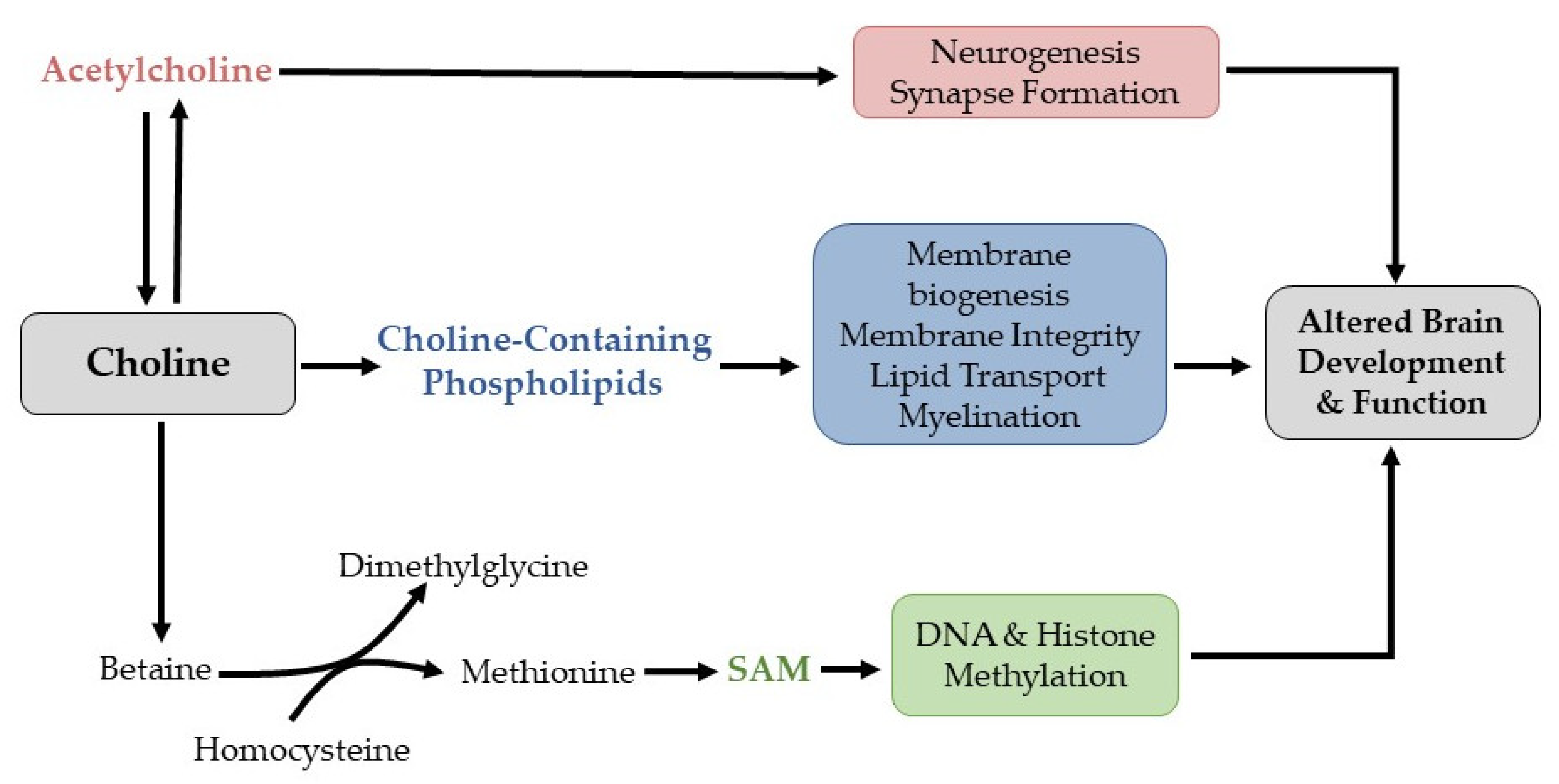
1. Introduction
2. Materials and Methods
2.1. Study Participants
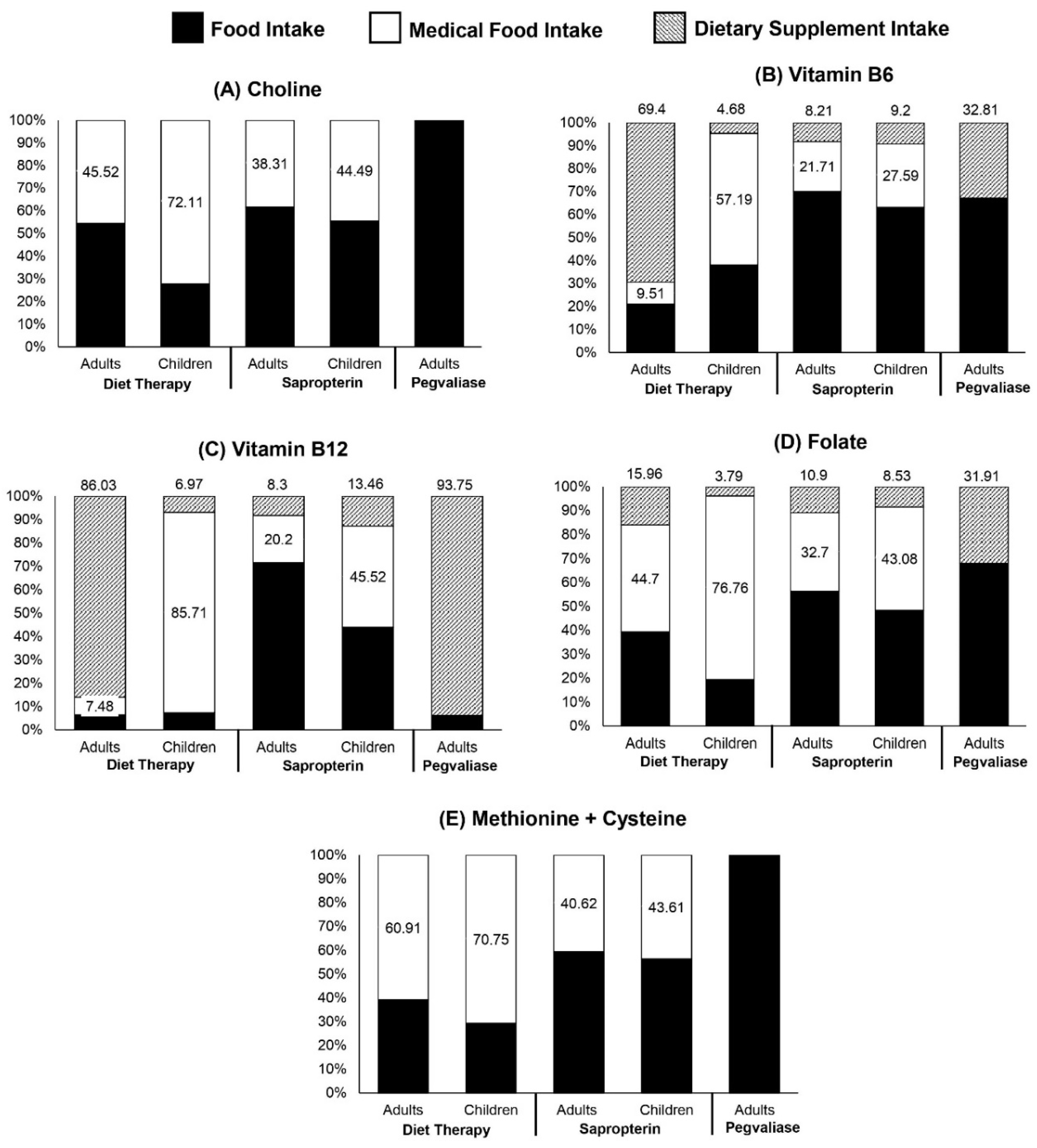
2.2. Quantification of Nutrient Intake from Food and Supplemental Sources
2.3. Estimation of Usual Intake
2.4. Estimation of Nutrient Probability of Adequacy
2.5. Statistical Analysis
3. Results
3.1. Estimated Usual Choline Intake
3.2. Mean Probability of Adequacy (MPA) for Nutrients That Affect Choline Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blau, N.; Van Spronsen, F.J.; Levy, H.L. Phenylketonuria. Lancet 2010, 376, 1417–1427. [Google Scholar] [CrossRef]
- Paine, R.S. The variability in manifestations of untreated patients with phenylketonuria (phenylpyruvic aciduria). Pediatrics 1957, 20, 290–302. [Google Scholar] [CrossRef]
- Singh, R.H.; Cunningham, A.C.; Mofidi, S.; Douglas, T.D.; Frazier, D.M.; Hook, D.G.; Jeffers, L.; McCune, H.; Moseley, K.D.; Ogata, B. Updated, web-based nutrition management guideline for PKU: An evidence and consensus based approach. Mol. Genet. Metab. 2016, 118, 72–83. [Google Scholar] [CrossRef]
- Ney, D.M.; Stroup, B.M.; Clayton, M.K.; Murali, S.G.; Rice, G.M.; Rohr, F.; Levy, H.L. Glycomacropeptide for nutritional management of phenylketonuria: A randomized, controlled, crossover trial. Am. J. Clin. Nutr. 2016, 104, 334–345. [Google Scholar] [CrossRef]
- Lammardo, A.M.; Robert, M.; Rocha, J.C.; van Rijn, M.; Ahring, K.; Bélanger-Quintana, A.; MacDonald, A.; Dokoupil, K.; Ozel, H.G.; Goyens, P.; et al. Main issues in micronutrient supplementation in phenylketonuria. Mol. Genet. Metab. 2013, 110, S1–S5. [Google Scholar] [CrossRef]
- Jans, J.J.; de Sain-van der Velden, M.G.; van Hasselt, P.M.; van den Hurk, D.T.; Vaz, F.M.; Visser, G.; Verhoeven-Duif, N.M. Supplementation with a powdered blend of PUFAs normalizes DHA and AA levels in patients with PKU. Mol. Genet. Metab. 2013, 109, 121–124. [Google Scholar] [CrossRef]
- Parra, G.A.M.; Singh, R.H.; Cetinyurek-Yavuz, A.; Kuhn, M.; MacDonald, A. Status of nutrients important in brain function in phenylketonuria: A systematic review and meta-analysis. Orphanet J. Rare Dis. 2018, 13, 101. [Google Scholar] [CrossRef]
- Stroup, B.M.; Ney, D.M.; Murali, S.G.; Rohr, F.; Gleason, S.T.; van Calcar, S.C.; Levy, H.L. Metabolomic Insights into the Nutritional Status of Adults and Adolescents with Phenylketonuria Consuming a Low-Phenylalanine Diet in Combination with Amino Acid and Glycomacropeptide Medical Foods. J. Nutr. Metab. 2017, 2017, 6859820. [Google Scholar] [CrossRef]
- Rohde, C.; von Teeffelen-Heithoff, A.; Thiele, A.G.; Arelin, M.; Mütze, U.; Kiener, C.; Gerloff, J.; Baerwald, C.; Schultz, S.; Heller, C.; et al. PKU patients on a relaxed diet may be at risk for micronutrient deficiencies. Eur. J. Clin. Nutr. 2014, 68, 119–124. [Google Scholar] [CrossRef]
- McWhorter, N.; Dhillon, J.; Hoffman, J. Preliminary Investigation of Microbiome and Dietary Differences in Patients with Phenylketonuria on Enzyme Substitution Therapy Compared to Traditional Therapies. J. Acad. Nutr. Diet. 2021, 122, 1283–1295. [Google Scholar] [CrossRef]
- Burlina, A.P.; Lachmann, R.H.; Manara, R.; Cazzorla, C.; Celato, A.; van Spronsen, F.J.; Burlina, A. The neurological and psychological phenotype of adult patients with early-treated phenylketonuria: A systematic review. J. Inherit. Metab. Dis. 2019, 42, 209–219. [Google Scholar] [CrossRef]
- Romani, C.; Manti, F.; Nardecchia, F.; Valentini, F.; Fallarino, N.; Carducci, C.; De Leo, S.; MacDonald, A.; Palermo, L.; Leuzzi, V. Adult cognitive outcomes in phenylketonuria: Explaining causes of variability beyond average Phe levels. Orphanet J. Rare Dis. 2019, 14, 273. [Google Scholar] [CrossRef]
- Tayebati, S.K.; Amenta, F. Choline-containing phospholipids: Relevance to brain functional pathways. Clin. Chem. Lab. Med. 2013, 51, 513–521. [Google Scholar] [CrossRef]
- Poly, C.; Massaro, J.M.; Seshadri, S.; Wolf, P.A.; Cho, E.; Krall, E.; Jacques, P.F.; Au, R. The relation of dietary choline to cognitive performance and white-matter hyperintensity in the Framingham Offspring Cohort. Am. J. Clin. Nutr. 2011, 94, 1584–1591. [Google Scholar] [CrossRef]
- Repetto, M.G.; Ossani, G.; Monserrat, A.J.; Boveris, A. Oxidative damage: The biochemical mechanism of cellular injury and necrosis in choline deficiency. Exp. Mol. Pathol. 2010, 88, 143–149. [Google Scholar] [CrossRef]
- Ferreira, B.K.; Rodrigues, M.T.; Streck, E.L.; Ferreira, G.C.; Schuck, P.F. White matter disturbances in phenylketonuria: Possible underlying mechanisms. J. Neurosci. Res. 2021, 99, 349–360. [Google Scholar] [CrossRef]
- Antenor-Dorsey, J.A.V.; Hershey, T.; Rutlin, J.; Shimony, J.S.; McKinstry, R.C.; Grange, D.K.; Christ, S.E.; White, D.A. White matter integrity and executive abilities in individuals with phenylketonuria. Mol. Genet. Metab. 2013, 109, 125–131. [Google Scholar] [CrossRef]
- Ribas, G.S.; Sitta, A.; Wajner, M.; Vargas, C.R. Oxidative stress in phenylketonuria: What is the evidence? Cell. Mol. Neurobiol. 2011, 31, 653. [Google Scholar] [CrossRef]
- Dobrowolski, S.F.; Lyons-Weiler, J.; Spridik, K.; Biery, A.; Breck, J.; Vockley, J.; Yatsenko, S.; Sultana, T. Altered DNA methylation in PAH deficient phenylketonuria. Mol. Genet. Metab. 2015, 115, 72–77. [Google Scholar] [CrossRef]
- Dobrowolski, S.; Lyons-Weiler, J.; Spridik, K.; Vockley, J.; Skvorak, K.; Biery, A. DNA methylation in the pathophysiology of hyperphenylalaninemia in the PAHenu2 mouse model of phenylketonuria. Mol. Genet. Metab. 2016, 119, 1–7. [Google Scholar] [CrossRef]
- Zeisel, S.H. Dietary choline: Biochemistry, physiology, and pharmacology. Annu. Rev. Nutr. 1981, 1, 95–121. [Google Scholar] [CrossRef]
- Wallace, T.C.; Fulgoni, V.L. Usual Choline Intakes Are Associated with Egg and Protein Food Consumption in the United States. Nutrients 2017, 9, 839. [Google Scholar] [CrossRef]
- Jurecki, E.; Cederbaum, S.; Kopesky, J.; Perry, K.; Rohr, F.; Sanchez-Valle, A.; Viau, K.; Sheinin, M.; Cohen-Pfeffer, J. Adherence to clinic recommendations among patients with phenylketonuria in the United States. Mol. Genet. Metab. 2017, 120, 190–197. [Google Scholar] [CrossRef]
- Niculescu, M.D.; Zeisel, S.H. Diet, methyl donors and DNA methylation: Interactions between dietary folate, methionine and choline. J. Nutr. 2002, 132, 2333s–2335s. [Google Scholar] [CrossRef]
- Kim, Y.I.; Miller, J.W.; da Costa, K.A.; Nadeau, M.; Smith, D.; Selhub, J.; Zeisel, S.H.; Mason, J.B. Severe folate deficiency causes secondary depletion of choline and phosphocholine in rat liver. J. Nutr. 1994, 124, 2197–2203. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Zola, T.; daCosta, K.A.; Pomfret, E.A. Effect of choline deficiency on S-adenosylmethionine and methionine concentrations in rat liver. Biochem. J. 1989, 259, 725–729. [Google Scholar] [CrossRef]
- Chen, T.-C.; Parker, J.D.; Clark, J.; Shin, H.-C.; Rammon, J.R.; Burt, V.L. National health and nutrition examination survey: Estimation procedures 2011–2014. Vital Health Stat. 2 2018, 177, 1–26. [Google Scholar]
- Sievert, Y.A.; Schakel, S.F.; Buzzard, I.M. Maintenance of a nutrient database for clinical trials. Control Clin. Trials 1989, 10, 416–425. [Google Scholar] [CrossRef]
- Camp, K.M.; Lloyd-Puryear, M.A.; Huntington, K.L. Nutritional treatment for inborn errors of metabolism: Indications, regulations, and availability of medical foods and dietary supplements using phenylketonuria as an example. Mol. Genet. Metab. 2012, 107, 3–9. [Google Scholar] [CrossRef]
- Nutrition Coordinating Center. Nutrition Data System for Research User Manual, Appendix 8; Regenets of the University of Minnesota: Minneapolis, MN, USA, 2020; p. A8.1. [Google Scholar]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef]
- Raper, N.; Perloff, B.; Ingwersen, L.; Steinfeldt, L.; Anand, J. An overview of USDA's dietary intake data system. J. Food Compos. Anal. 2004, 17, 545–555. [Google Scholar] [CrossRef]
- Huang, K.; Zhao, L.; Guo, Q.; Yu, D.; Yang, Y.; Cao, Q.; Yuan, X.; Ju, L.; Li, S.; Cheng, X. Comparison of the 24 h Dietary Recall of Two Consecutive Days, Two Non-Consecutive Days, Three Consecutive Days, and Three Non-Consecutive Days for Estimating Dietary Intake of Chinese Adult. Nutrients 2022, 14, 1960. [Google Scholar] [CrossRef]
- Tooze, J.A.; Midthune, D.; Dodd, K.W.; Freedman, L.S.; Krebs-Smith, S.M.; Subar, A.F.; Guenther, P.M.; Carroll, R.J.; Kipnis, V. A new statistical method for estimating the usual intake of episodically consumed foods with application to their distribution. J. Am. Diet. Assoc. 2006, 106, 1575–1587. [Google Scholar] [CrossRef]
- Tooze, J.A.; Kipnis, V.; Buckman, D.W.; Carroll, R.J.; Freedman, L.S.; Guenther, P.M.; Krebs-Smith, S.M.; Subar, A.F.; Dodd, K.W. A mixed-effects model approach for estimating the distribution of usual intake of nutrients: The NCI method. Stat. Med. 2010, 29, 2857–2868. [Google Scholar] [CrossRef]
- Bailey, R.L.; Dodd, K.W.; Gahche, J.J.; Dwyer, J.T.; Cowan, A.E.; Jun, S.; Eicher-Miller, H.A.; Guenther, P.M.; Bhadra, A.; Thomas, P.R. Best practices for dietary supplement assessment and estimation of total usual nutrient intakes in population-level research and monitoring. J. Nutr. 2019, 149, 181–197. [Google Scholar] [CrossRef]
- Institute of Medicine Subcommittee on Interpretation and Uses of Dietary Reference Intakes. DRI Dietary Reference Intakes: Applications in Dietary Assessment; The National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- Luo, H.; Dodd, K.W.; Arnold, C.D.; Engle-Stone, R. Introduction to the SIMPLE Macro, a Tool to Increase the Accessibility of 24-Hour Dietary Recall Analysis and Modeling. J. Nutr. 2021, 151, 1329–1340. [Google Scholar] [CrossRef]
- Wallace, T.C.; Fulgoni, V.L., 3rd. Assessment of Total Choline Intakes in the United States. J. Am. Coll. Nutr. 2016, 35, 108–112. [Google Scholar] [CrossRef]
- Zeisel, S.H. Nutritional importance of choline for brain development. J. Am. Coll. Nutr. 2004, 23, 621S–626S. [Google Scholar] [CrossRef]
- McCann, J.C.; Hudes, M.; Ames, B.N. An overview of evidence for a causal relationship between dietary availability of choline during development and cognitive function in offspring. Neurosci. Biobehav. Rev. 2006, 30, 696–712. [Google Scholar] [CrossRef]
- Blusztajn, J.K.; Slack, B.E.; Mellott, T.J. Neuroprotective actions of dietary choline. Nutrients 2017, 9, 815. [Google Scholar] [CrossRef]
- Wallace, T.C.; McBurney, M.; Fulgoni III, V.L. Multivitamin/mineral supplement contribution to micronutrient intakes in the United States, 2007–2010. J. Am. Coll. Nutr. 2014, 33, 94–102. [Google Scholar] [CrossRef]
- Viau, K.; Wessel, A.; Martell, L.; Sacharow, S.; Rohr, F. Nutrition status of adults with phenylketonuria treated with pegvaliase. Mol. Genet. Metab. 2021, 133, 345–351. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Sidell, N. Modulation by phenylacetate of early estrogen-mediated events in MCF-7 breast cancer cells. Cancer Chemother. Pharmacol. 2007, 59, 217–225. [Google Scholar] [CrossRef]
- Fischer, L.M.; da Costa, K.-A.; Kwock, L.; Galanko, J.; Zeisel, S.H. Dietary choline requirements of women: Effects of estrogen and genetic variation. Am. J. Clin. Nutr. 2010, 92, 1113–1119. [Google Scholar] [CrossRef]
- Horita, D.A.; Hwang, S.; Stegall, J.M.; Friday, W.B.; Kirchner, D.R.; Zeisel, S.H. Two methods for assessment of choline status in a randomized crossover study with varying dietary choline intake in people: Isotope dilution MS of plasma and in vivo single-voxel magnetic resonance spectroscopy of liver. Am. J. Clin. Nutr. 2021, 113, 1670–1678. [Google Scholar] [CrossRef]
- Fischer, L.M.; DaCosta, K.A.; Kwock, L.; Stewart, P.W.; Lu, T.-S.; Stabler, S.P.; Allen, R.H.; Zeisel, S.H. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am. J. Clin. Nutr. 2007, 85, 1275–1285. [Google Scholar] [CrossRef]
- Slow, S.; Lever, M.; Chambers, S.T.; George, P.M. Plasma dependent and independent accumulation of betaine in male and female rat tissues. Physiol. Res. 2009, 58, 403–410. [Google Scholar] [CrossRef]
- Hillert, A.; Anikster, Y.; Belanger-Quintana, A.; Burlina, A.; Burton, B.K.; Carducci, C.; Chiesa, A.E.; Christodoulou, J.; Đorđević, M.; Desviat, L.R.; et al. The Genetic Landscape and Epidemiology of Phenylketonuria. Am. J. Hum. Genet. 2020, 107, 234–250. [Google Scholar] [CrossRef]
| PKU | NHANES | |||
|---|---|---|---|---|
| Diet Therapy | Sapropterin Dihydrochloride | Pegvaliase | ||
| Adults (≥18 years) | n = 17 | n = 21 | n = 33 | n = 7267 |
| Age, years 2 | 21 | 27 | 36 | 43 |
| (18, 26) | (22, 34) | (32, 44) | (30, 56) | |
| Age range, years | 18–50 | 18–45 | 22–61 | 18–70 |
| Female, n (%) 1 | 12 (70.6) | 13 (61.9) | 20 (60.6) | 3589 (50.6) |
| Caucasian, n (%) 1 | 16 (94.1) | 21 (100.0) | 33 (100.0) | 2191 (61.3) |
| Weight status, n (%) 1 | ||||
| Overweight | 7 (41.2) | 5 (23.8) | 11 (33.3) | 2040 (30.0) |
| Obese | 4 (23.5) | 8 (38.1) | 16 (48.5) | 2939 (41.5) |
| Plasma phe (µmol/L) 2 | 842.0 | 362.0 | 23.0 | -------- |
| (613.2, 951.1) | (261.0, 533.2) | (3.0, 218.0) | ||
| Taking medical food, n (%) | 17 (100.0) | 12 (57.1) | 0 (0.0) | -------- |
| Children (<18 years) | n = 32 | n = 17 | n = 0 | n = 3414 |
| Age, years 2 | 14 | 10 | -------- | 10 |
| (10, 16) | (8, 13) | (7, 14) | ||
| Age range, years | 4–17 | 6–16 | -------- | 4–17 |
| Female, n (%) 1 | 23 (71.9) | 8 (47.1) | -------- | 1666 (49.1) |
| Caucasian, n (%) 1 | 31 (96.9) | 16 (94.1) | -------- | 999 (49.6) |
| Weight status, n (%) 1 | -------- | |||
| Overweight | 4 (12.5) | 4 (23.5) | -------- | 526 (15.8) |
| Obese | 4 (12.5) | 4 (23.5) | -------- | 719 (21.2) |
| Plasma phe (µmol/L) 2 | 626.0 | 302.0 | -------- | -------- |
| (323.5, 1082.0) | (185.0, 397.0) | |||
| Taking medical food, n (%) | 32 (100.0) | 13 (76.5) | -------- | -------- |
| Percentile | ||||||
|---|---|---|---|---|---|---|
| Subgroup | n 1 | Mean (SE) | 25th (SE) | 50th (SE) | 75th (SE) | % >AI |
| NHANES | 7267 | 341.3 (3.6) | 257.9 (3.6) | 327.5 (3.5) | 409.0 (4.7) | 9.5 |
| PKU diet therapy | 17 | |||||
| With MF 2 | 203.6 (34.5) | 116.4 (18.0) | 175.7 (24.0) | 263.9 (32.5) | 5.6 | |
| Without MF 2 | 115.3 (13.3) | 73.3 (11.0) | 101.5 (13.2) | 145.6 (17.2) | 0.2 | |
| PKU sapropterin | 21 | |||||
| With MF 2 | 299.4 (37.0) | 179.2 (22.2) | 268.3 (27.3) | 390.6 (37.4) | 14.2 | |
| Without MF 2 | 176.6 (21.4) | 114.2 (17.6) | 160.4 (20.7) | 222.1 (26.7) | 0.8 | |
| PKU pegvaliase | 33 | 302.3 (28.0) | 185.1 (21.8) | 273.4 (26.2) | 389.5 (35.3) | 14.8 |
| Percentile | ||||||
|---|---|---|---|---|---|---|
| Subgroup | n 1 | Mean (SE) | 25 (SE) | 50 (SE) | 75 (SE) | % >AI |
| NHANES | 3414 | 261.2 (2.8) | 191.5 (2.9) | 249.3 (2.9) | 318.2 (3.6) | 22.0 |
| PKU diet therapy | 32 | |||||
| With MF 2 | 221.0 (16.0) | 148.2 (12.7) | 211.2 (15.0) | 287.0 (24.2) | 12.3 | |
| Without MF 2 | 61.6 (5.93) | 45.4 (6.0) | 58.9 (5.9) | 75.6 (7.4) | 0 | |
| PKU sapropterin | 17 | |||||
| With MF 2 | 174.8 (15.1) | 105.3 (15.2) | 167.4 (15.8) | 232.4 (22.4) | 6.4 | |
| Without MF 2 | 96.7 (8.8) | 75.6 (9.6) | 94.6 (8.9) | 115.1 (9.9) | 0 | |
| Adults | Children | |||||
|---|---|---|---|---|---|---|
| n 2 | MPA3 | MPAm 4 | n 2 | MPA 3 | MPAm 4 | |
| NHANES | 7267 | 3414 | ||||
| With DS 5 | 100 | ----- | 100 | ----- | ||
| (100, 100) | (100, 100) | |||||
| Without DS 5 | 100 | ----- | 100 | ----- | ||
| (100, 100) | (100, 100) | |||||
| PKU diet therapy | 17 | 32 | ||||
| With MF + DS 5 | 100 | 100 | 100 (100, 100) | 100 | ||
| (100, 100) | (100, 100) | (100, 100) | ||||
| Without MF + DS 5 | 65.1 | 48.8 | 37.2 | 28.1 | ||
| (26.7, 96.3) | (25.2, 72.6) | (1.6, 64.8) | (3.2, 51.8) | |||
| PKU sapropterin | 21 | 17 | ||||
| With MF + DS 5 | 100 | 100 | 100 | 98.9 | ||
| (95.7, 100) | (83.9, 100) | (94.7, 100) | (88.1, 100) | |||
| Without MF + DS 5 | 77.4 | 74.4 | 66.7 | 73.3 | ||
| (66.7, 100) | (52.6, 98.5) | (31.9, 97.3) | (25.4, 97.3) | |||
| PKU pegvaliase | 33 | 0 | ||||
| With DS 5 | 100 | 100 | ----- | ----- | ||
| (96.1, 100) | (97.1, 100) | |||||
| Without DS 5 | 99.8 | 99.8 | ----- | ----- | ||
| (80.0, 100) | (85.0, 100) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoen, M.S.; Ramakrishnan, U.; Alvarez, J.A.; Ziegler, T.R.; Cui, X.; Singh, R.H. Characterization of Choline Nutriture among Adults and Children with Phenylketonuria. Nutrients 2022, 14, 4056. https://doi.org/10.3390/nu14194056
Schoen MS, Ramakrishnan U, Alvarez JA, Ziegler TR, Cui X, Singh RH. Characterization of Choline Nutriture among Adults and Children with Phenylketonuria. Nutrients. 2022; 14(19):4056. https://doi.org/10.3390/nu14194056
Chicago/Turabian StyleSchoen, Meriah S., Usha Ramakrishnan, Jessica A. Alvarez, Thomas R. Ziegler, Xiangqin Cui, and Rani H. Singh. 2022. "Characterization of Choline Nutriture among Adults and Children with Phenylketonuria" Nutrients 14, no. 19: 4056. https://doi.org/10.3390/nu14194056
APA StyleSchoen, M. S., Ramakrishnan, U., Alvarez, J. A., Ziegler, T. R., Cui, X., & Singh, R. H. (2022). Characterization of Choline Nutriture among Adults and Children with Phenylketonuria. Nutrients, 14(19), 4056. https://doi.org/10.3390/nu14194056





