Prediction of Iron Deficiency Anemia in Third Trimester of Pregnancy Based on Data in the First Trimester: A Prospective Cohort Study in a High-Income Country
Abstract
:1. Introduction
2. Materials and Methods
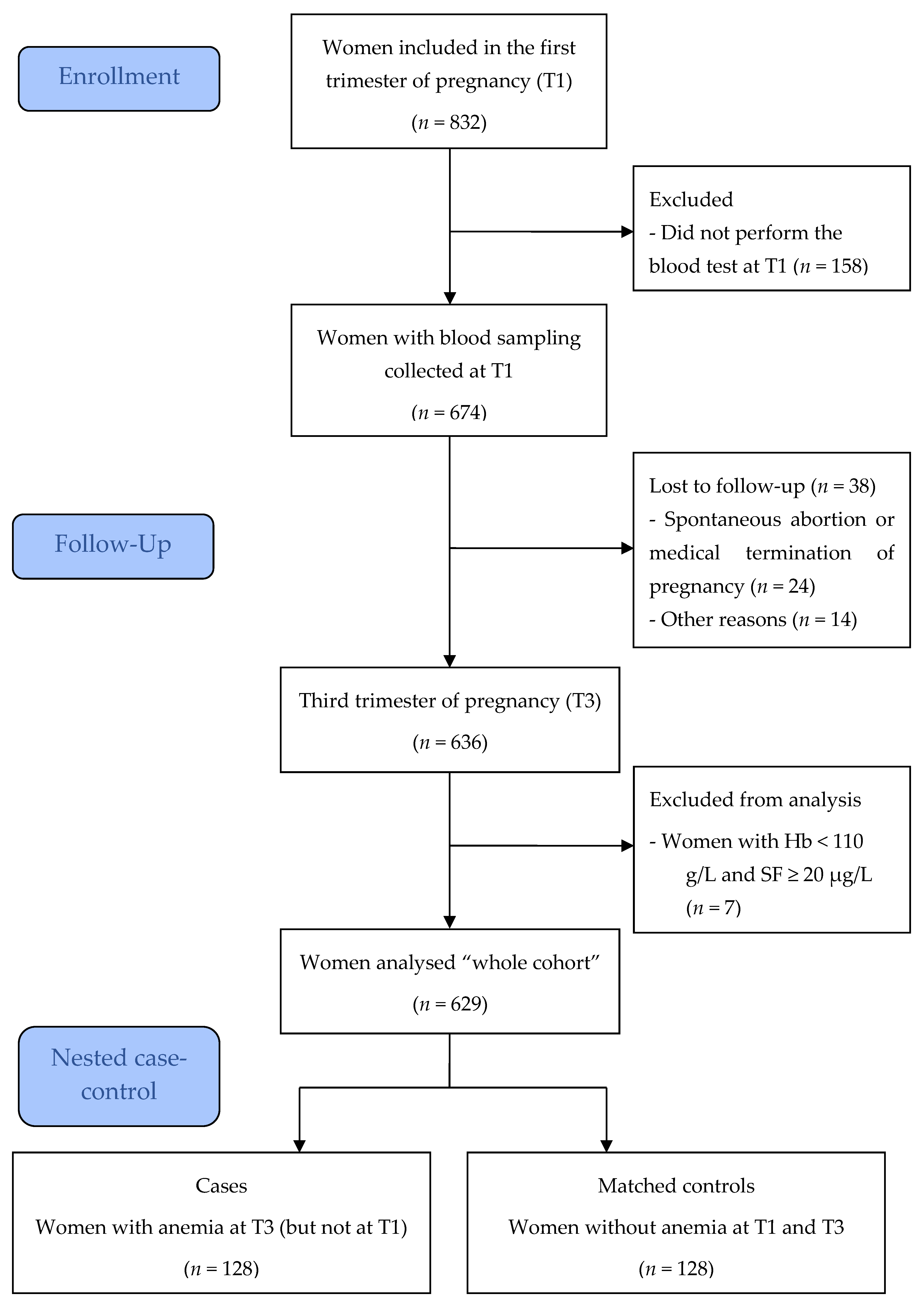
2.1. Study Design, Setting, and Participants
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pavord, S.; Daru, J.; Prasannan, N.; Robinson, S.; Stanworth, S.; Girling, J.; BSH Committe. UK guidelines on the management of iron deficiency in pregnancy. Br. J. Haematol. 2020, 188, 819–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgieff, M.K. Iron deficiency in pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 516–524. [Google Scholar] [CrossRef]
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M.; Nutrition Impact Model Study Group (Anaemia). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non- pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef] [Green Version]
- Peña-Rosas, J.P.; De-Regil, L.M.; Garcia-Casal, M.N.; Dowswell, T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst. Rev. 2015, 22, CD004736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, M.F.; Oaks, B.M.; Tandon, S.; Martorell, R.; Dewey, K.G.; Wendt, A.S. Maternal hemoglobin concentrations across pregnancy and maternal and child health: A systematic review and meta-analysis. Ann. N. Y. Acad. Sci. 2019, 1450, 47–68. [Google Scholar] [CrossRef] [Green Version]
- Camaschella, C. Iron-Deficiency Anemia. N. Engl. J. Med. 2015, 373, 485–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, A.L.; Nemeth, E. Iron homeostasis during pregnancy. Am. J. Clin. Nutr. 2017, 106, 1567S–1574S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- Haute Autorité de Santé—Suivi et Orientation des Femmes Enceintes en Fonction des Situations à Risque Identifiées. Available online: https://www.has-sante.fr/upload/docs/application/pdf/suivi_des_femmes_enceintes_-_recommandations_23-04-2008.pdf (accessed on 15 September 2022).
- Lecorguillé, M.; Camier, A.; Kadawathagedara, M. Recommandations Pour la Pratique Clinique: “Interventions Pendant la Période Périnatale”. Chapitre 3: Variations de Poids, Apports Nutritionnels Essentiels et Contaminants, Supplémentation Chez les Femmes Enceintes et les Femmes en Âge de Procréer. [Rapport de Recherche] Collège National des Sages-Femmes de France. 2021. Available online: https://hal.uca.fr/hal-03283264/document (accessed on 15 September 2022).
- Leplège, A.; Ecosse, E.; Verdier, A.; Perneger, T.V. The French SF-36 Health Survey: Translation, cultural adaptation and preliminary psychometric evaluation. J. Clin. Epidemiol. 1998, 51, 1013–1023. [Google Scholar] [CrossRef]
- Labbe, E.; Blanquet, M.; Gerbaud, L.; Poirier, G.; Sass, C.; Vendittelli, F.; Moulin, J.J. A new reliable index to measure individual deprivation: The EPICES score. Eur. J. Public Health 2015, 25, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Grondin, M.A.; Ruivard, M.; Perrève, A.; Derumeaux-Burel, H.; Perthus, I.; Roblin, J.; Thiollières, F.; Gerbaud, L. Prevalence of Iron Deficiency and Health-related Quality of Life among Female Students. J. Am. Coll. Nutr. 2008, 27, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Noshiro, K.; Umazume, T.; Hattori, R.; Kataoka, S.; Yamada, T.; Watari, H. Hemoglobin Concentration during Early Pregnancy as an Accurate Predictor of Anemia during Late Pregnancy. Nutrients 2022, 14, 839. [Google Scholar] [CrossRef] [PubMed]
- Mayasari, N.R.; Bai, C.H.; Hu, T.Y.; Chao, J.C.J.; Chen, Y.C.; Huang, Y.L.; Wang, F.F.; Tinkov, A.A.; Skalny, A.V.; Chang, J.S. Associations of Food and Nutrient Intake with Serum Hepcidin and the Risk of Gestational Iron-Deficiency Anemia among Pregnant Women: A Population-Based Study. Nutrients 2021, 13, 3501. [Google Scholar] [CrossRef]
- Galan, P.; Yoon, H.C.; Preziosi, P.; Viteri, F.; Valeix, P.; Fieux, B.; Briançon, S.; Malvy, D.; Roussel, A.M.; Favier, A.; et al. Determining factors in the iron status of adult women in the SU.VI.MAX study. SUpplementation en VItamines et Minéraux AntioXydants. Eur. J. Clin. Nutr. 1998, 52, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Milman, N.; Taylor, C.L.; Merkel, J.; Brannon, P.M. Iron status in pregnant women and women of reproductive age in Europe. Am. J. Clin. Nutr. 2017, 106, 1655S–1662S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, G.; Lausman, A.; Abdulrehman, J.; Petrucci, J.; Nisenbaum, R.; Hicks, L.K.; Sholzberg, M. Prevalence of iron deficiency and iron deficiency anemia during pregnancy: A single centre Canadian study. Blood 2019, 134, 3389. [Google Scholar] [CrossRef]
- Oh, C.; Keats, E.C.; Bhutta, Z.A. Vitamin and Mineral Supplementation During Pregnancy on Maternal, Birth, Child Health and Development Outcomes in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achebe, M.M.; Gafter-Gvili, A. How I treat anemia in pregnancy: Iron, cobalamin, and folate. Blood 2017, 129, 940–949. [Google Scholar] [CrossRef] [PubMed]
| Characteristics at T1 | Whole Cohort | Women with Anemia at T3 (Cases) | Matched Women without Anemia at T3 (Controls) | p-Value |
|---|---|---|---|---|
| No. of women | 629 | 128 | 128 | |
| Age, year, median (IQR) | 30.0 (27.0–33.0) | 29.0 (25.5–33.5) | 30.0 (27.0–33.0) | 0.071 |
| Age, year, n (%) | 0.655 | |||
| <20 | 12 (1.9) | 3 (2.3) | 2 (1.6) | |
| 20–34 | 491 (78.1) | 99 (77.3) | 101 (78.9) | |
| ≥35 | 126 (20.0) | 26 (20.3) | 25 (19.5) | |
| Education level, n (%) | 0.121 | |||
| Lower than high school | 139 (25.2) | 33 (31.1) | 22 (20.8) | |
| High school | 106 (19.2) | 19 (17.9) | 19 (17.9) | |
| Higher education | 307 (55.6) | 54 (50.9) | 65 (61.3) | |
| Life status, n (%) | 0.039 | |||
| Live alone | 45 (7.9) | 10 (9.1) | 2 (1.8) | |
| Live in couple | 523 (92.1) | 100 (90.9) | 108 (98.2) | |
| Professional activity, n (%) | 374 (65.8) | 63 (56.8) | 76 (68.5) | 0.105 |
| Current smoking, n (%) | 113 (20.2) | 18 (17.0) | 21 (19.8) | 0.711 |
| BMI before pregnancy, kg/m2, median (IQR) | 0.152 | |||
| Underweight (<18.5) | 52 (9.0) | 13 (11.3) | 8 (7.0) | |
| Normal weight (18.5 to <25) | 346 (59.6) | 64 (55.7) | 75 (65.2) | |
| Overweight (≥25) | 183 (31.5) | 38 (33.0) | 32 (27.8) | |
| Parity, n (%) | 0.279 | |||
| 0 | 108 (18.6) | 23 (19.7) | 19 (16.2) | |
| 1 | 272 (46.7) | 49 (41.9) | 61 (52.1) | |
| 2 | 132 (22.7) | 31 (26.5) | 27 (23.1) | |
| ≥3 | 70 (12.0) | 14 (12.0) | 10 (8.5) | |
| Twin pregnancy, n (%) | 8 (1.4) | 2 (1.7) | 2 (1.7) | 1.000 |
| EPICES score 1, median (IQR) | 14.8 (7.1–29.0) | 18.3 (13.6–38.5) | 14.8 (6.5–26.0) | 0.024 |
| Deprivation 2, n (%) | 84 (23.5) | 13 (35.1) | 8 (21.6) | 0.332 |
| Very low incomes (under CMU), n (%) | 92 (16.0) | 25 (21.9) | 10 (8.8) | 0.011 |
| MOS SF-36 scores 3, median (IQR) | ||||
| Physical functioning | 90.0 (70.7–95.0) | 85.0 (65.0–100) | 90.0 (75.0–95.0) | 0.643 |
| Role physical | 62.5 (43.8–81.3) | 62.5 (43.8–81.3) | 62.5 (50.0–75.0) | 0.847 |
| Bodily pain | 67.5 (45.0–90.0) | 57.5 (45.0–90.0) | 77.5 (57.5–90.0) | 0.070 |
| Vitality | 40.0 (25.0–50.0) | 40.0 (25.0–50.0) | 40.0 (25.0–50.0) | 0.360 |
| Mental health | 68.0 (56.0–80.0) | 68.0 (52.0–80.0) | 72.0 (56.0–84.0) | 0.315 |
| Role emotional | 83.3 (58.3–100) | 83.3 (58.3–100) | 83.3 (58.3–100) | 0.833 |
| Social functioning | 75.0 (62.5–87.5) | 75.0 (62.5–87.5) | 75.0 (62.5–100) | 0.907 |
| General health | 70.0 (60.0–80.0) | 70.0 (60.0–80.0) | 70.0 (55.0–85.0) | 0.820 |
| Clinical Characteristics and Biomarkers at T3 | Whole Cohort | Women with Anemia at T3 (Cases) | Matched Women without Anemia at T3 (Controls) | p-Value |
|---|---|---|---|---|
| No. of women | 629 | 128 | 128 | |
| Systolic blood pressure, mm Hg, median (IQR) | 120 (111–130) | 120 (111–128) | 121 (113–133) | 0.094 |
| Diastolic blood pressure, mm Hg, median (IQR) | 75 (67–82) | 74 (65–81) | 77 (69–86) | 0.022 |
| Hypertension, n (%) | 0.317 | |||
| No | 520 (97.7) | 94 (95.9) | 96 (98.0) | |
| Chronic isolated | 1 (0.2) | 0 | 0 | |
| Gestational (without proteinuria) | 1 (0.2) | 0 | 0 | |
| Moderate pre-eclampsia | 8 (1.5) | 3 (3.1) | 2 (2.0) | |
| Severe pre-eclampsia | 2 (0.4) | 1 (1.0) | 0 | |
| Hb, g/L, median (IQR) | 118 (111–126) | 104 (100–107) | 121 (115–130) | <0.001 |
| MCV, fL, median (IQR) | 87.0 (84.0–90.0) | 84.0 (81.0–87.0) | 88.0 (84.0–91.0) | <0.001 |
| MCHC, pg, median (IQR) | 29.4 (27.7–30.6) | 27.6 (26.2–29.3) | 29.7 (28.1–30.9) | <0.001 |
| Term of delivery, weeks of amenorrhea, n (%) | 0.876 | |||
| <37 | 31 (5.3) | 10 (8.5) | 6 (5.1) | |
| 37–41 | 453 (77.4) | 90 (76.9) | 92 (78.6) | |
| >41 | 101 (17.3) | 17 (14.5) | 19 (16.2) | |
| Birth weight of newborns, g, n (%) | 0.157 | |||
| <2500 | 41 (6.9) | 9 (7.7) | 7 (6.0) | |
| 2500–3999 | 522 (88.2) | 96 (82.0) | 104 (88.9) | |
| ≥4000 | 29 (4.9) | 12 (10.3) | 6 (5.1) | |
| Small for gestational age *, n (%) | 37 (6.3) | 5 (4.3) | 4 (3.4) | 1.000 |
| Biomarkers at T1 | Whole Cohort | Women with Anemia at T3 (cases) | Matched Women without Anemia at T3 (controls) | p-Value |
|---|---|---|---|---|
| No. of women | 629 | 128 | 128 | |
| Hb, g/L | 127 (120–133) | 123 (117–129) | 129 (124–135) | <0.001 |
| MCV, fL | 86.0 (83.3–88.5) | 85.5 (82.5–87.8) | 86.3 (83.4–89.2) | 0.049 |
| MCHC, pg | 29.8 (28.7–30.8) | 29.4 (28.5–30.4) | 30.1 (28.8–31.1) | 0.010 |
| RDW, %, | 12.8 (12.3–13.2) | 12.9 (12.3–13.4) | 12.7 (12.4–13.2) | 0.143 |
| RHC, pg | 34.1 (32.5–35.8) | 33.9 (32.1–35.4) | 34.1 (32.5–36.2) | 0.030 |
| SF, µg/L | - | 27.0 (11.9–49.9) | 43.6 (25.1–70.3) | <0.001 |
| Hepc, µg/L | - | 15.6 (3.8–24.4) | 18.4 (10.9–24.0) | 0.089 |
| STfR, nmol/L | - | 14.1 (12.2–17.4) | 13.5 (12.0–15.3) | 0.030 |
| Ratio Hepc/SF | - | 0.32 (0.18–0.67) | 0.33 (0.20–0.53) | 0.314 |
| Ratio SF/STfR | - | 1.9 (0.8–3.9) | 3.3 (1.7–5.7) | <0.001 |
| Model | Biological Factors at T1 | Unadjusted Standardized | Adjusted Standardized * | ||||
|---|---|---|---|---|---|---|---|
| Without Selection Method | With Forward Selection Method | ||||||
| OR | p-Value | OR | p-Value | OR | p-Value | ||
| Model 1 | Hb | 0.63 | <0.001 | 0.56 | <0.001 | 0.66 | <0.001 |
| MCV | 0.85 | 0.043 | 0.70 | 0.128 | |||
| MCHC | 0.85 | 0.025 | 1.39 | 0.133 | |||
| RDW | 1.12 | 0.099 | 0.75 | 0.027 | |||
| RHC | 0.83 | 0.017 | 0.79 | 0.134 | |||
| SF | 0.76 | 0.002 | 0.73 | 0.009 | |||
| Hepc | 0.87 | 0.061 | 1.28 | 0.083 | |||
| STfR | 1.18 | 0.032 | 1.38 | 0.031 | |||
| Model 2 | Hb | - | - | 0.61 | <0.001 | 0.66 | <0.001 |
| MCV | - | - | 0.70 | 0.118 | |||
| MCHC | - | - | 1.27 | 0.236 | |||
| RDW | - | - | 0.83 | 0.094 | |||
| RHC | - | - | 0.80 | 0.151 | |||
| Hepc | - | - | 1.15 | 0.257 | |||
| Ratio SF/STfR | 0.77 | 0.002 | 0.73 | 0.010 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resseguier, A.-S.; Guiguet-Auclair, C.; Debost-Legrand, A.; Serre-Sapin, A.-F.; Gerbaud, L.; Vendittelli, F.; Ruivard, M. Prediction of Iron Deficiency Anemia in Third Trimester of Pregnancy Based on Data in the First Trimester: A Prospective Cohort Study in a High-Income Country. Nutrients 2022, 14, 4091. https://doi.org/10.3390/nu14194091
Resseguier A-S, Guiguet-Auclair C, Debost-Legrand A, Serre-Sapin A-F, Gerbaud L, Vendittelli F, Ruivard M. Prediction of Iron Deficiency Anemia in Third Trimester of Pregnancy Based on Data in the First Trimester: A Prospective Cohort Study in a High-Income Country. Nutrients. 2022; 14(19):4091. https://doi.org/10.3390/nu14194091
Chicago/Turabian StyleResseguier, Anne-Sophie, Candy Guiguet-Auclair, Anne Debost-Legrand, Anne-Françoise Serre-Sapin, Laurent Gerbaud, Françoise Vendittelli, and Marc Ruivard. 2022. "Prediction of Iron Deficiency Anemia in Third Trimester of Pregnancy Based on Data in the First Trimester: A Prospective Cohort Study in a High-Income Country" Nutrients 14, no. 19: 4091. https://doi.org/10.3390/nu14194091






