Morphological Adaptation in the Jejunal Mucosa after Iso-Caloric High-Fat versus High-Carbohydrate Diets in Healthy Volunteers: Data from a Randomized Crossover Study
Abstract
:1. Introduction
2. Material and Methods
2.1. The Clinical Study
2.2. Enteroscopy and Specimen Preparation
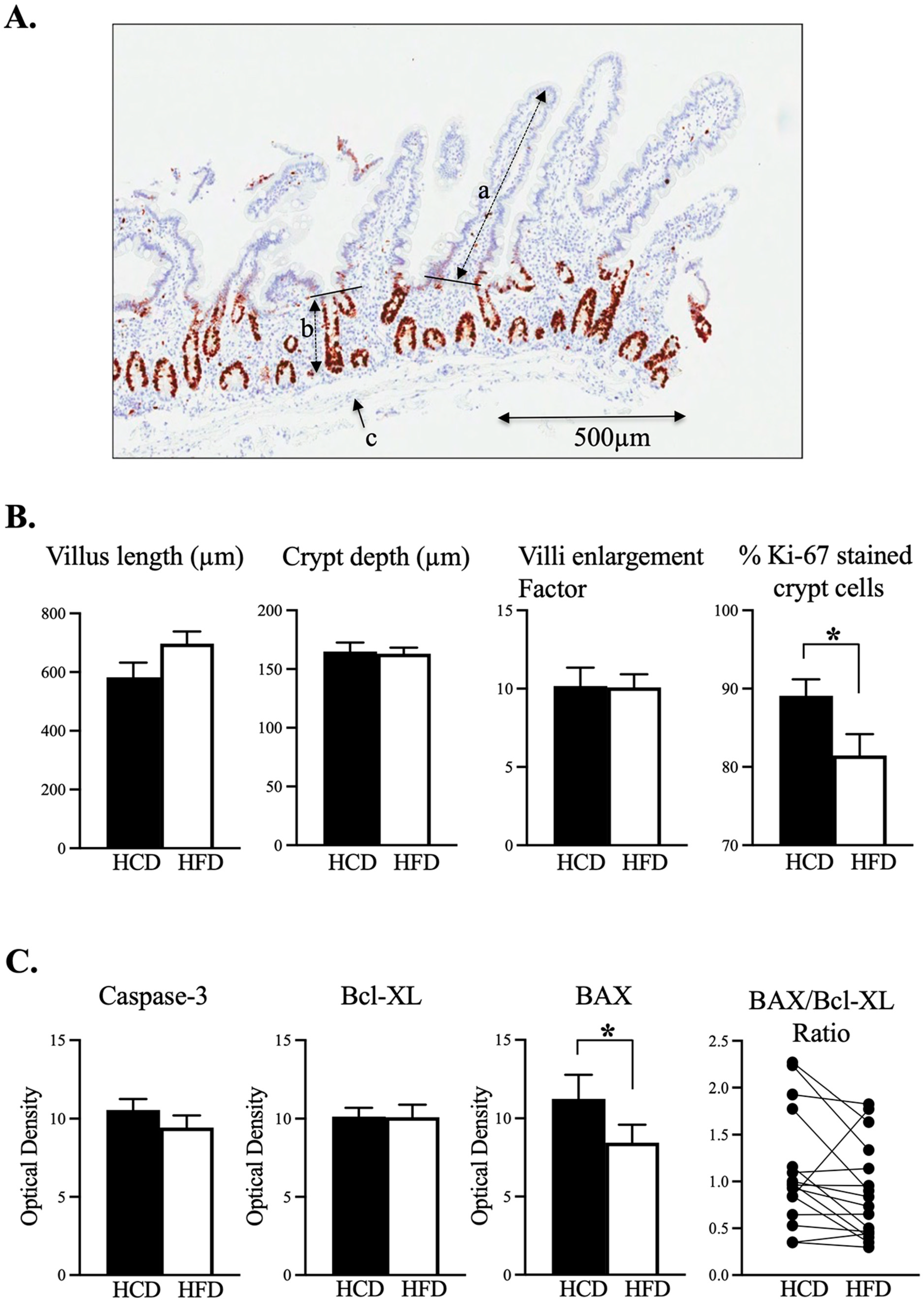
2.3. Morphometry and Cell Proliferation
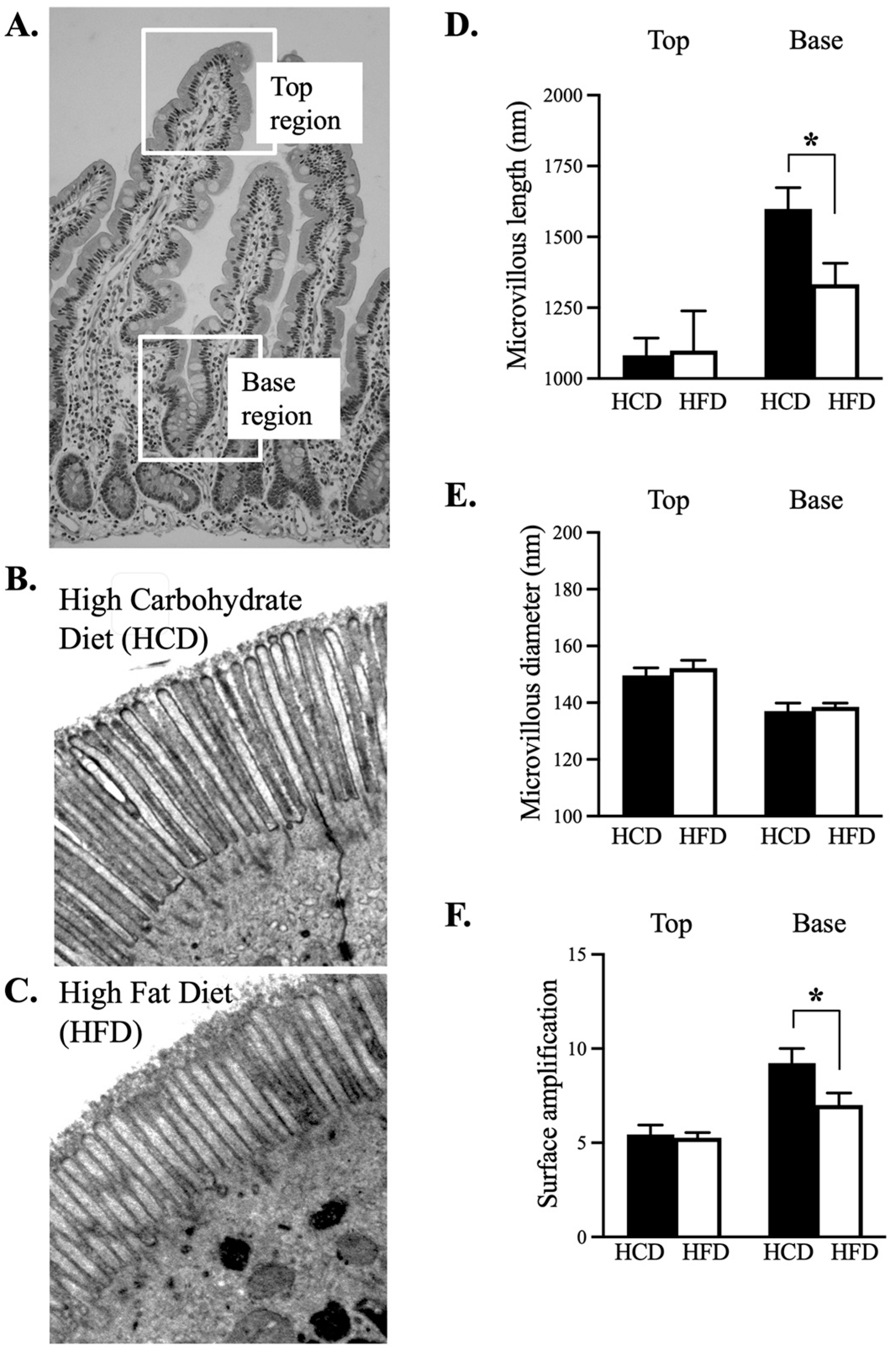
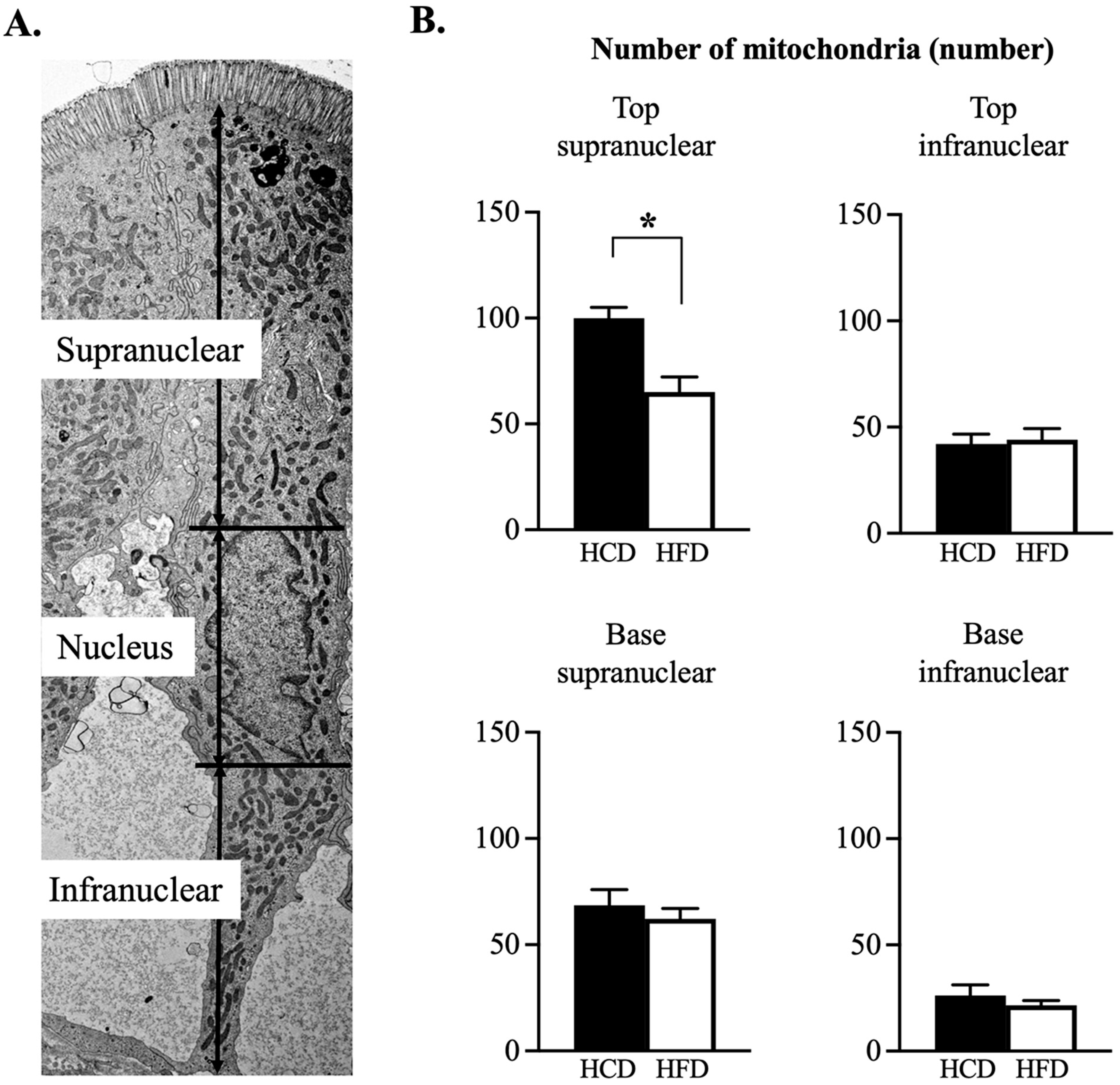
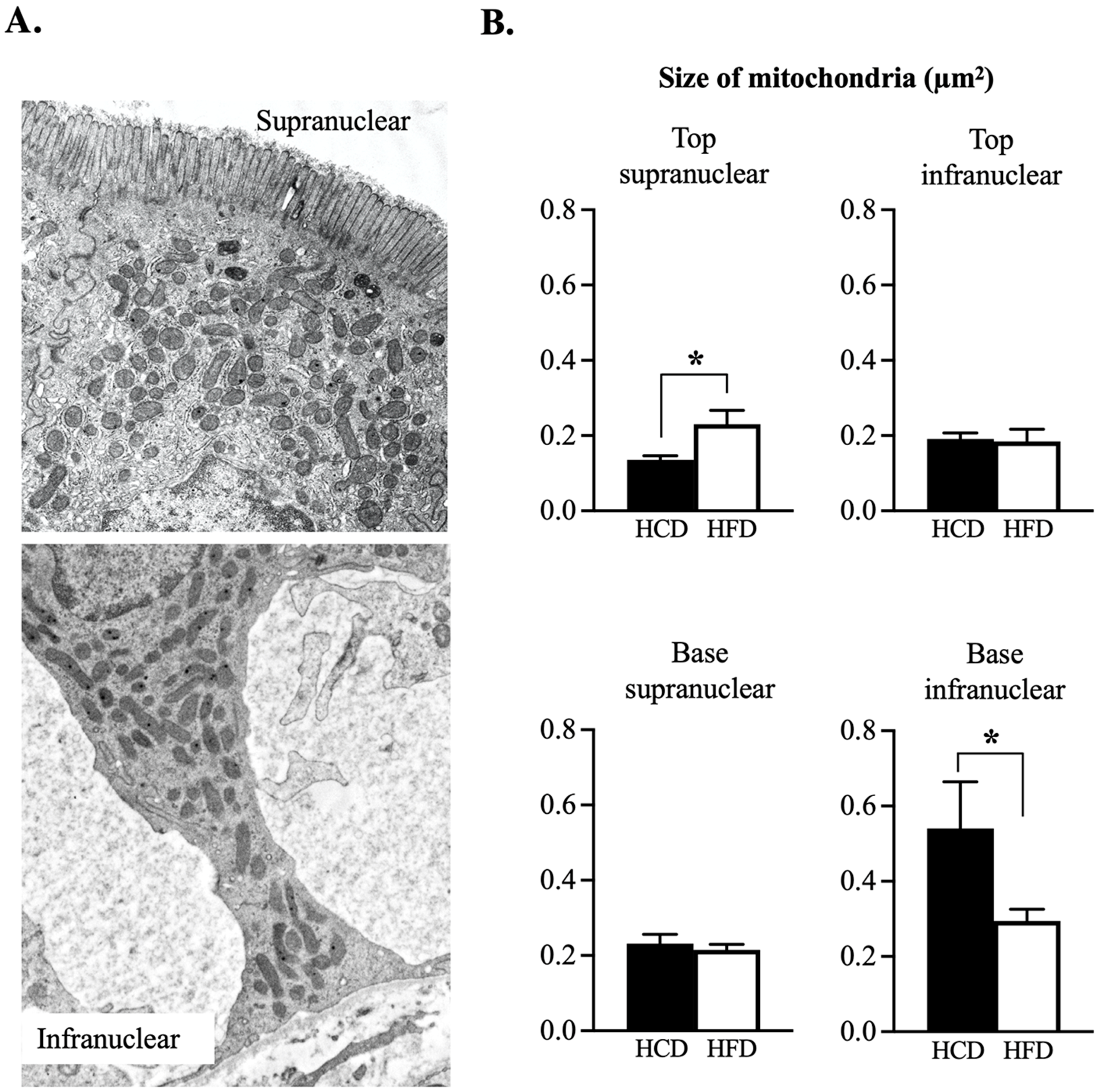
2.4. Transmission Electron Microscopy (TEM)
2.5. Confocal Microscopy
2.6. Statistical Analyses
3. Results
3.1. Intraindividual Comparison between HCD and HFD Did Not Reveal any Changed Mucosal Enlargement Factor
3.2. Ultrastructural Assessment of the Enterocyte Surface Revealed a Larger Surface Enhancement following HCD at The Base of the Villus
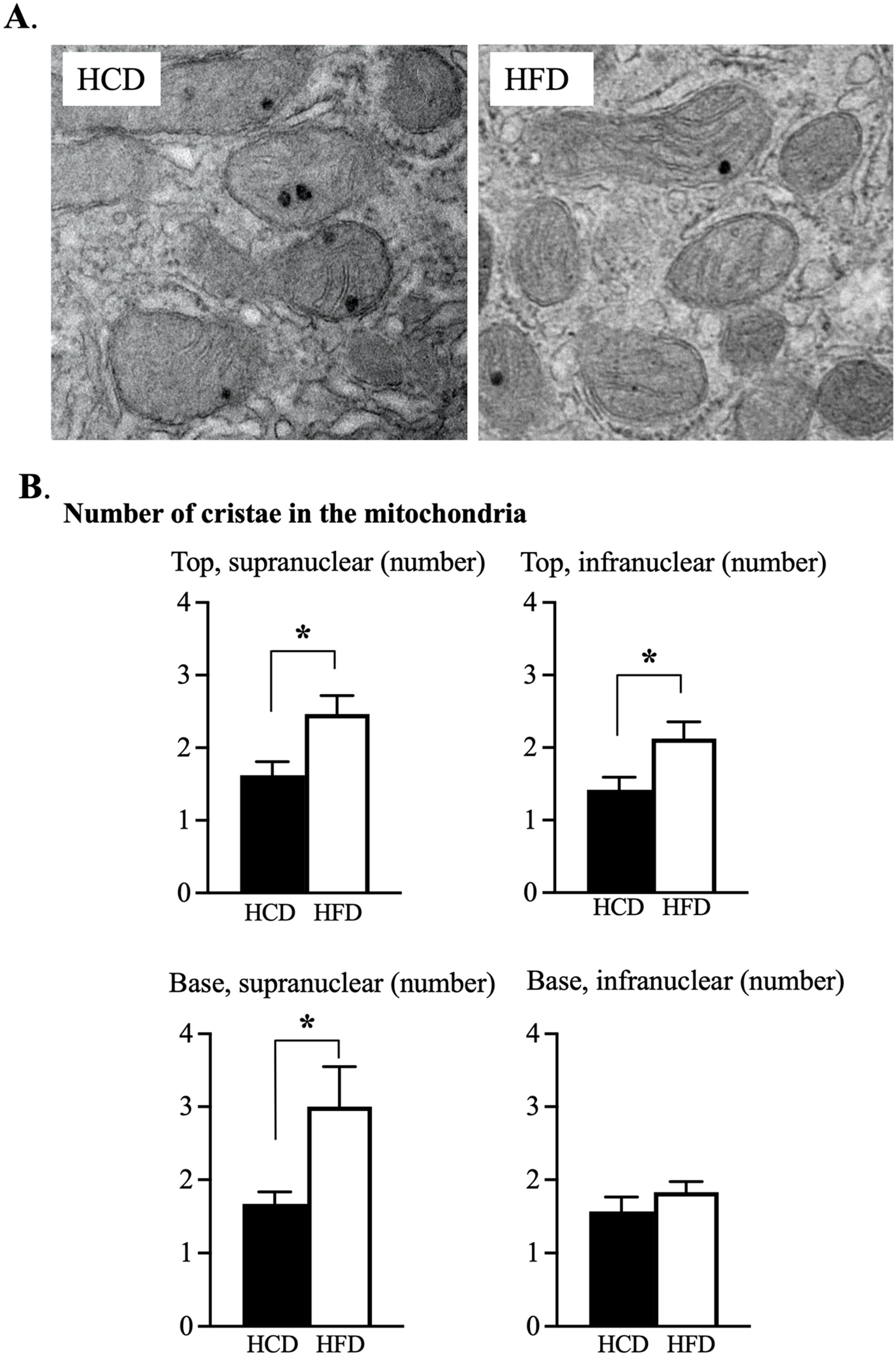
3.3. Ultrastructural Assessment of Mitochondria in the Enterocyte
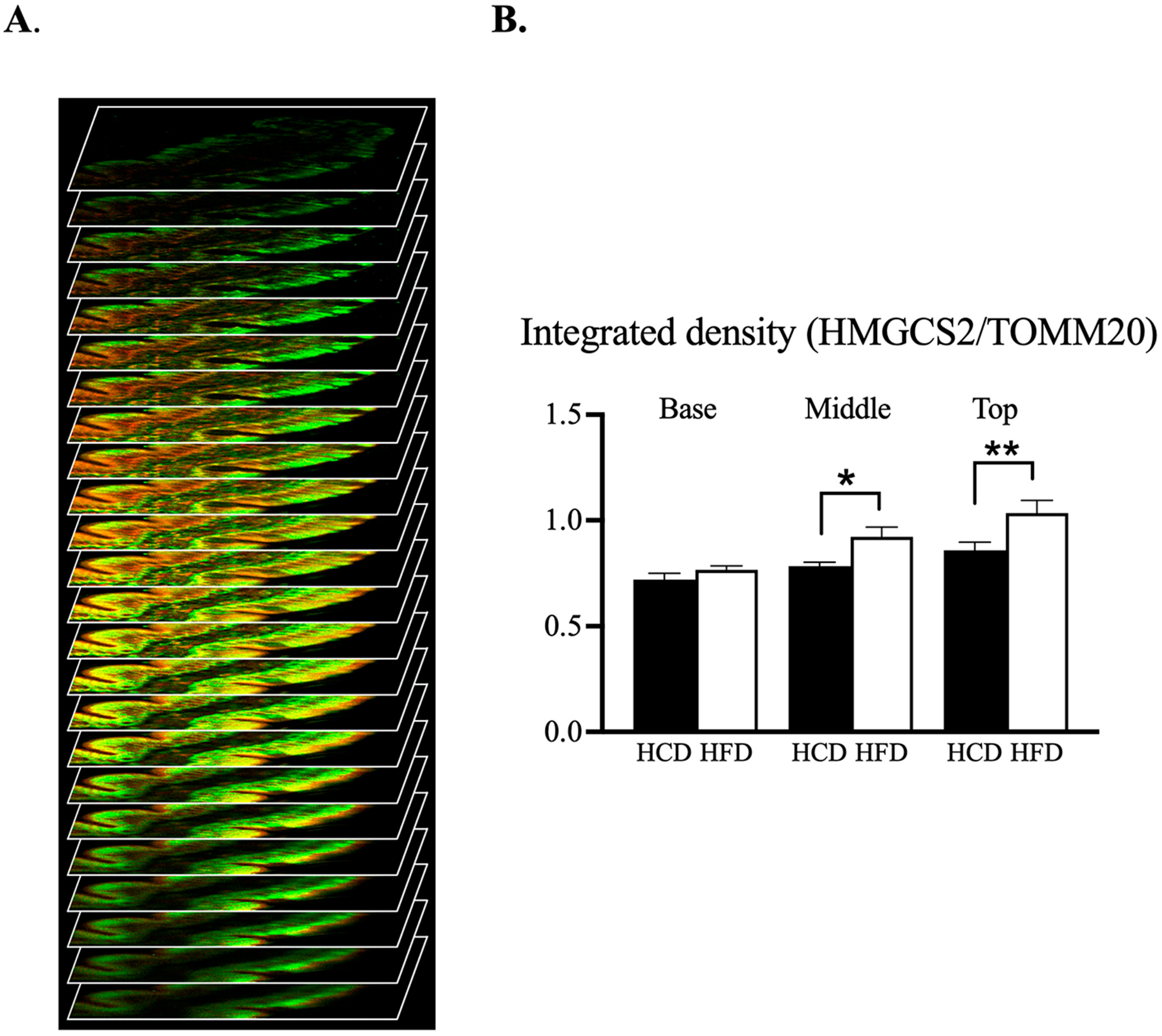
3.4. Confocal Microscopic Assessment of the Mitochondrial Phenotype along Villus-Axis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Helander, H.F.; Fändriks, L. Surface area of the digestive tract—Revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef]
- Sender, R.; Milo, R. The distribution of cellular turnover in the human body. Nat. Med. 2021, 27, 45–48. [Google Scholar] [CrossRef]
- Barker, N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014, 15, 19–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, J.C.; Gordon, J.I. The intestinal stem cell niche: There grows the neighborhood. Proc. Natl. Acad. Sci. USA 2001, 98, 12334–12336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Clara, R.; Schumacher, M.; Ramachandran, D.; Fedele, S.; Krieger, J.-P.; Langhans, W.; Mansouri, A. Metabolic Adaptation of the Small Intestine to Short- and Medium-Term High-Fat Diet Exposure. J. Cell Physiol. 2017, 232, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Wallenius, V.; Elebring, E.; Casselbrant, A.; Laurenius, A.; le Roux, C.W.; Docherty, N.G.; Biörserud, C.; Björnfot, N.; Engström, M.; Marschall, H.-U.; et al. Glycemic Control and Metabolic Adaptation in Response to High-Fat versus High-Carbohydrate Diets-Data from a Randomized Cross-Over Study in Healthy Subjects. Nutrients 2021, 13, 3322. [Google Scholar] [CrossRef] [PubMed]
- Elebring, E.; Wallenius, V.; Casselbrant, A.; Docherty, N.G.; le Roux, C.W.; Marschall, H.-U.; Fändriks, L. A Fatty Diet Induces a Jejunal Ketogenesis Which Inhibits Local SGLT1-Based Glucose Transport via an Acetylation Mechanism-Results from a Randomized Cross-Over Study between Iso-Caloric High-Fat versus High-Carbohydrate Diets in Healthy Volunteers. Nutrients 2022, 14, 1961. [Google Scholar] [CrossRef] [PubMed]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayhew, T.M. The new stereological methods for interpreting functional morphology from slices of cells and organs. Exp. Physiol. 1991, 76, 639–665. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siekevitz, P. Powerhouse of the Cell. Sci. Am. 1957, 197, 131–144. [Google Scholar] [CrossRef]
- Rath, E.; Moschetta, A.; Haller, D. Mitochondrial function—Gatekeeper of intestinal epithelial cell homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Kennady, P.K.; Ormerod, M.G.; Singh, S.; Pande, G. Variation of mitochondrial size during the cell cycle: A multiparameter flow cytometric and microscopic study. Cytometry A 2004, 62, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Jeynes, B.J.; Altmann, G.G. A region of mitochondrial division in the epithelium of the small intestine of the rat. Anat. Rec. 1975, 182, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallenius, V.; Elias, E.; Elebring, E.; Haisma, B.; Casselbrant, A.; Larraufie, P.; Spak, E.; Reimann, F.; le Roux, C.W.; Docherty, N.G.; et al. Suppression of enteroendocrine cell glucagon-like peptide (GLP)-1 release by fat-induced small intestinal ketogenesis: A mechanism targeted by Roux-en-Y gastric bypass surgery but not by preoperative very-low-calorie diet. Gut 2020, 69, 1423–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, N.; Suarez-Diaz, M.; Hooiveld, G.J.E.J.; Keijer, J.; dos Santos, V.M.; van Schothorst, E.M. A constraint-based model analysis of enterocyte mitochondrial adaptation to dietary interventions of lipid type and lipid load. Front. Physiol. 2018, 15, 749. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casselbrant, A.; Wallenius, V.; Elebring, E.; Marschall, H.-U.; Johansson, B.R.; Helander, H.F.; Fändriks, L. Morphological Adaptation in the Jejunal Mucosa after Iso-Caloric High-Fat versus High-Carbohydrate Diets in Healthy Volunteers: Data from a Randomized Crossover Study. Nutrients 2022, 14, 4123. https://doi.org/10.3390/nu14194123
Casselbrant A, Wallenius V, Elebring E, Marschall H-U, Johansson BR, Helander HF, Fändriks L. Morphological Adaptation in the Jejunal Mucosa after Iso-Caloric High-Fat versus High-Carbohydrate Diets in Healthy Volunteers: Data from a Randomized Crossover Study. Nutrients. 2022; 14(19):4123. https://doi.org/10.3390/nu14194123
Chicago/Turabian StyleCasselbrant, Anna, Ville Wallenius, Erik Elebring, Hanns-Ulrich Marschall, Bengt R. Johansson, Herbert F. Helander, and Lars Fändriks. 2022. "Morphological Adaptation in the Jejunal Mucosa after Iso-Caloric High-Fat versus High-Carbohydrate Diets in Healthy Volunteers: Data from a Randomized Crossover Study" Nutrients 14, no. 19: 4123. https://doi.org/10.3390/nu14194123
APA StyleCasselbrant, A., Wallenius, V., Elebring, E., Marschall, H.-U., Johansson, B. R., Helander, H. F., & Fändriks, L. (2022). Morphological Adaptation in the Jejunal Mucosa after Iso-Caloric High-Fat versus High-Carbohydrate Diets in Healthy Volunteers: Data from a Randomized Crossover Study. Nutrients, 14(19), 4123. https://doi.org/10.3390/nu14194123




