Association between Urinary Advanced Glycation End Products and Subclinical Inflammation in Children and Adolescents: Results from the Italian I.Family Cohort
Abstract
1. Introduction
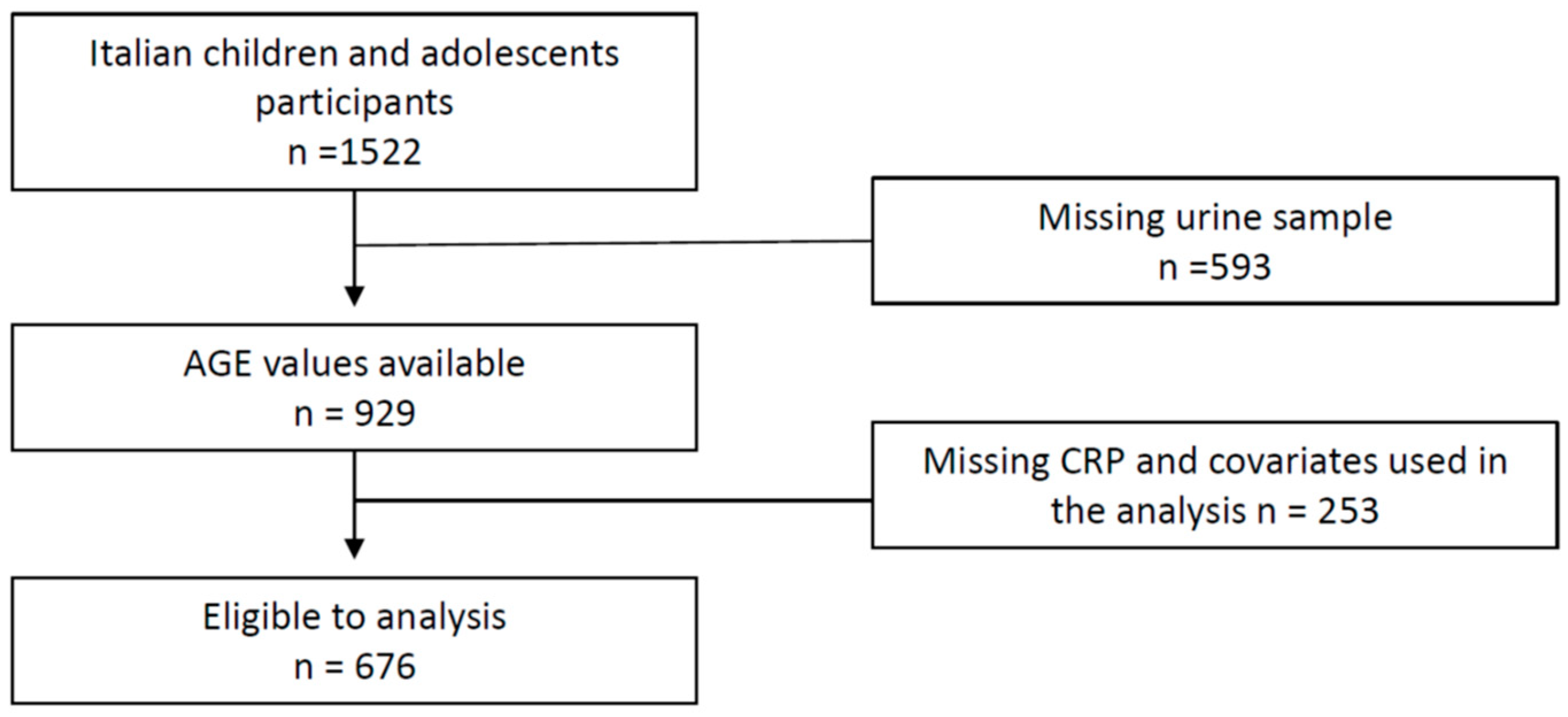
2. Materials and Methods
2.1. Experimental Design and Cohort
2.2. Sample Processing and Analytical Procedures
2.3. Inflammatory Biomarkers
2.4. Fluorescence
2.5. Anthropometric and Blood Pressure Measurements
2.6. Medical History and Medication Use
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ulrich, P. Protein Glycation, Diabetes, and Aging. Recent Prog. Horm. Res. 2001, 56, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Rowan, S.; Bejarano, E.; Taylor, A. Mechanistic targeting of advanced glycation end-products in age-related diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3631–3643. [Google Scholar] [CrossRef] [PubMed]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxidat. Med. Cell. Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Monnier, V.M.; Kohn, R.R.; Cerami, A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc. Natl. Acad. Sci. USA 1984, 81, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Schmitt, J.; Münch, G.; Gasic-Milencovic, J. Characterization of advanced glycation end products for biochemical studies: Side chain modifications and fluorescence characteristics. Anal. Biochem. 2005, 338, 201–215. [Google Scholar] [CrossRef]
- Kern, E.F.; Erhard, P.; Sun, W.; Genuth, S.; Weiss, M.F. Early Urinary Markers of Diabetic Kidney Disease: A Nested Case-Control Study From the Diabetes Control and Complications Trial (DCCT). Am. J. Kidney Dis. 2010, 55, 824–834. [Google Scholar] [CrossRef]
- Molinari, P.; Caldiroli, L.; Dozio, E.; Rigolini, R.; Giubbilini, P.; Romanelli, M.M.C.; Messa, P.; Vettoretti, S. AGEs and sRAGE Variations at Different Timepoints in Patients with Chronic Kidney Disease. Antioxidants 2021, 10, 1994. [Google Scholar] [CrossRef]
- Dozio, E.; Vettoretti, S.; Caldiroli, L.; Nerini-Molteni, S.; Tacchini, L.; Ambrogi, F.; Messa, P.; Romanelli, M.M.C. Advanced Glycation End Products (AGE) and Soluble Forms of AGE Receptor: Emerging Role as Mortality Risk Factors in CKD. Biomedicines 2020, 8, 638. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Yan, S.D.; Yan, S.F.; Stern, D.M. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J. Clin. Investig. 2001, 108, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Reynaert, N.L.; Gopal, P.; Rutten, E.P.; Wouters, E.F.; Schalkwijk, C.G. Advanced glycation end products and their receptor in age-related, non-communicable chronic inflammatory diseases; Overview of clinical evidence and potential contributions to disease. Int. J. Biochem. Cell Biol. 2016, 81, 403–418. [Google Scholar] [CrossRef]
- Luan, Z.-G.; Zhang, H.; Yang, P.-T.; Ma, X.-C.; Zhang, C.; Guo, R.-X. HMGB1 activates nuclear factor-κB signaling by RAGE and increases the production of TNF-α in human umbilical vein endothelial cells. Immunobiology 2010, 215, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Younessi, P.; Yoonessi, A. Advanced Glycation End-Products and Their Receptor-Mediated Roles: Inflammation and Oxidative Stress. Iran. J. Med. Sci. 2011, 36, 154–166. [Google Scholar] [PubMed]
- Kellow, N.; Coughlan, M.T. Effect of diet-derived advanced glycation end products on inflammation. Nutr. Rev. 2015, 73, 737–759. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Spinelli, R.; D’Esposito, V.; Zatterale, F.; Fiory, F.; Nigro, C.; Raciti, G.; Miele, C.; Formisano, P.; Beguinot, F.; et al. Pathologic endoplasmic reticulum stress induced by glucotoxic insults inhibits adipocyte differentiation and induces an inflammatory phenotype. Biochim. Biophys. Acta 2016, 1863, 1146–1156. [Google Scholar] [CrossRef]
- Mishra, M.; Prasad, K. AGE–RAGE Stress, Stressors, and Antistressors in Health and Disease. Int. J. Angiol. 2018, 27, 1–12. [Google Scholar] [CrossRef]
- Prasad, K. AGE–RAGE stress: A changing landscape in pathology and treatment of Alzheimer’s disease. Mol. Cell. Biochem. 2019, 459, 95–112. [Google Scholar] [CrossRef]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating Glycotoxins and Dietary Advanced Glycation Endproducts: Two Links to Inflammatory Response, Oxidative Stress, and Aging. J. Gerontol. Ser. A 2007, 62, 427–433. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T.; Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced Glycation End Products and Oxidative Stress in Type 2 Diabetes Mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef]
- Batkulwar, K.B.; Godbole, R.K.; Banarjee, R.M.; Kassaar, O.M.; Williams, R.J.; Kulkarni, M.J. Advanced Glycation End Products Modulate Amyloidogenic APP Processing and Tau Phosphorylation: A Mechanistic Link between Glycation and the Development of Alzheimer’s Disease. ACS Chem. Neurosci. 2018, 9, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Sergi, D.; Boulestin, H.; Campbell, F.M.; Williams, L.M. The Role of Dietary Advanced Glycation End Products in Metabolic Dysfunction. Mol. Nutr. Food Res. 2021, 65, e1900934. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Woodward, M.; Tripp, E.; Goldberg, L.; Pyzik, R.; Yee, K.; Tansman, L.; Chen, X.; Mani, V.; et al. Elevated Serum Advanced Glycation Endproducts in Obese Indicate Risk for the Metabolic Syndrome: A Link Between Healthy and Unhealthy Obesity? J. Clin. Endocrinol. Metab. 2015, 100, 1957–1966. [Google Scholar] [CrossRef]
- Šebeková, K.; Somoza, V.; Jarčušková, M.; Heidland, A.; Podracká, L. Plasma advanced glycation end products are decreased in obese children compared with lean controls. Pediatr. Obes. 2009, 4, 112–118. [Google Scholar] [CrossRef]
- Accacha, S.; Rosenfeld, W.; Jacobson, A.; Michel, L.; Schnurr, F.; Shelov, S.; Ten, S.; Boucher-Berry, C.; Carey, D.; Speiser, P.; et al. Plasma Advanced Glycation End Products (AGEs), Receptors for AGEs and Their Correlation with Inflammatory Markers in Middle School-Age Children. Horm. Res. Paediatr. 2013, 80, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Heier, M.; Margeirsdottir, H.D.; Torjesen, P.A.; Seljeflot, I.; Stensæth, K.H.; Gaarder, M.; Brunborg, C.; Hanssen, K.F.; Dahl-Jørgensen, K. The advanced glycation end product methylglyoxal-derived hydroimidazolone-1 and early signs of atherosclerosis in childhood diabetes. Diabetes Vasc. Dis. Res. 2015, 12, 139–145. [Google Scholar] [CrossRef]
- Garay-Sevilla, M.E.; Torres-Graciano, S.; Villegas-Rodríguez, M.E.; Rivera-Cisneros, A.E.; Wróbel, K.; Uribarri, J. Advanced glycation end products and their receptors did not show any association with body mass parameters in metabolically healthy adolescents. Acta Paediatr. 2018, 107, 2146–2151. [Google Scholar] [CrossRef]
- Corica, D.; Aversa, T.; Ruggeri, R.M.; Cristani, M.; Alibrandi, A.; Pepe, G.; De Luca, F.; Wasniewska, M. Could AGE/RAGE-Related Oxidative Homeostasis Dysregulation Enhance Susceptibility to Pathogenesis of Cardio-Metabolic Complications in Childhood Obesity? Front. Endocrinol. 2019, 10, 426. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Albers, R.; Bosco, N.; Bourdet-Sicard, R.; Haller, D.; Holgate, S.T.; Jönsson, L.S.; Latulippe, M.E.; Marcos, A.; et al. A Consideration of Biomarkers to be Used for Evaluation of Inflammation in Human Nutritional Studies. Br. J. Nutr. 2013, 109, S1–S34. [Google Scholar] [CrossRef]
- Ridker, P.M. Clinical Application of C-Reactive Protein for Cardiovascular Disease Detection and Prevention. Circulation 2003, 107, 363–369. [Google Scholar] [CrossRef]
- Choi, J.; Joseph, L.; Pilote, L. Obesity and C-reactive protein in various populations: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Nappo, A.; Iacoviello, L.; Fraterman, A.; Gonzalez-Gil, E.M.; Hadjigeorgiou, C.; Marild, S.; Molnar, D.; Moreno, L.A.; Peplies, J.; Sioen, I.; et al. High-sensitivity C-reactive Protein is a Predictive Factor of Adiposity in Children: Results of the Identification and prevention of Dietary- and lifestyle-induced health Effects in Children and InfantS (IDEFICS) Study. J. Am. Heart Assoc. 2013, 2, e000101. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-T.; Hou, F.-F.; Guo, Z.-J.; Shan, Y.-X.; Zhang, X.; Liu, Z.-Q. Advanced Glycation End Products Upregulate C-reactive Protein Synthesis by Human Hepatocytes Through Stimulation of Monocyte IL-6 and IL-1β Production. Scand. J. Immunol. 2007, 66, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.A.; Drury, S.; Fu, C.; Qu, W.; Taguchi, A.; Lu, Y.; Avila, C.; Kambham, N.; Bierhaus, A.; Nawroth, P.; et al. RAGE Mediates a Novel Proinflammatory Axis: A Central Cell Surface Receptor for S100/Calgranulin Polypeptides. Cell 1999, 97, 889–901. [Google Scholar] [CrossRef]
- McNair, E.D.; Wells, C.R.; Qureshi, A.M.; Basran, R.; Pearce, C.; Orvold, J.; Devilliers, J.; Prasad, K. Modulation of high sensitivity C-reactive protein by soluble receptor for advanced glycation end products. Mol. Cell. Biochem. 2010, 341, 135–138. [Google Scholar] [CrossRef]
- Suehiro, A.; Uchida, K.; Nakanishi, M.; Wakabayashi, I. Measurement of urinary advanced glycation end-products (AGEs) using a fluorescence assay for metabolic syndrome-related screening tests. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, S110–S113. [Google Scholar] [CrossRef]
- Steenbeke, M.; De Bruyne, S.; Van Aken, E.; Glorieux, G.; Van Biesen, W.; Himpe, J.; De Meester, G.; Speeckaert, M.; Delanghe, J. UV Fluorescence-Based Determination of Urinary Advanced Glycation End Products in Patients with Chronic Kidney Disease. Diagnostics 2020, 10, 34. [Google Scholar] [CrossRef]
- Ahrens, W.; Siani, A.; Adan, R.; De Henauw, S.; Eiben, G.; Gwozdz, W.; Hebestreit, A.; Hunsberger, M.; Kaprio, J.; Krogh, V.; et al. Cohort Profile: The transition from childhood to adolescence in European children–how I.Family extends the IDEFICS cohort. Int. J. Epidemiol. 2017, 46, 1394–1395j. [Google Scholar] [CrossRef]
- Peplies, J.; Günther, K.; Gottlieb, A.; Luebke, A.; Bammann, K.; Ahrens, W.; On Behalf of the IDEFICS and I.Family Consortia. Biological samples—Standard operating procedures for collection, shipment, storage and documentation. In Instruments for Health Surveys in Children and Adolescents; Bammann, K., Lissner, L., Pigeot, I., Ahrens, W., Eds.; Springer: Cham, Switzerland, 2019; pp. 57–76. [Google Scholar]
- Koelman, L.; Pivovarova-Ramich, O.; Pfeiffer, A.F.H.; Grune, T.; Aleksandrova, K. Cytokines for evaluation of chronic inflammatory status in ageing research: Reliability and phenotypic characterisation. Immun. Ageing 2019, 16, 11. [Google Scholar] [CrossRef]
- Nagrani, R.; Foraita, R.; Wolters, M.; De Henauw, S.; Marild, S.; Molnár, D.; Moreno, L.A.; Russo, P.; Tornaritis, M.; Veidebaum, T.; et al. Longitudinal association of inflammatory markers with markers of glycaemia and insulin resistance in European children. Diabetes Metab. Res. Rev. 2022, 38, e3511. [Google Scholar] [CrossRef]
- Bammann, K.; Peplies, J.; Mårild, S.; Molnár, D.; Suling, M.; Siani, A.; On Behalf of the IDEFICS and I.Family Consortia. Physical examinations. In Instruments for Health Surveys in Children and Adolescents; Bammann, K., Lissner, L., Pigeot, I., Ahrens, W., Eds.; Springer: Cham, Switzerland, 2019; pp. 47–55. [Google Scholar]
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Alpert, B.S. Validation of the Welch Allyn Spot Vital Signs blood pressure device according to the ANSI/AAMI SP10: 2002. Accuracy and cost-efficiency successfully combined. Blood Press. Monit. 2007, 12, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Barba, G.; Buck, C.; Bammann, K.; Hadjigeorgiou, C.; Hebestreit, A.; Mårild, S.; Molnár, D.; Russo, P.; Veidebaum, T.; Vyncke, K.; et al. Blood pressure reference values for European non-overweight school children: The IDEFICS study. Int. J. Obes. 2014, 38, S48–S56. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, K.; Makita, Z.; Shiroshita, K.; Ueda, T.; Fusegawa, T.; Kuwajima, S.; Takeuchi, M.; Koike, T. Specific fluorescence assay for advanced glycation end products in blood and urine of diabetic patients. Metabolism 1998, 47, 1348–1353. [Google Scholar] [CrossRef]
- De La Maza, M.P.; Bravo, A.; Leiva, L.; Gattas, V.; Barrera, G.; Petermann, M.; Garrido, F.; Uribarri, J.; Bunout, D.; Hirsch, S. Urinary excretion of fluorescent advanced glycation end products (AGEs) in the elderly. J. Nutr. Health Aging 2008, 12, 222–224. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018, 93, 803–813. [Google Scholar] [CrossRef]
- Bammann, K.; Lissner, L.; Pigeot, I.; Ahrens, W. (Eds.) Instruments for Health Surveys in Children and Adolescents; Springer Series on Epidemiology and Public Health: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
| Below Median (n 361) | Above Median (n 315) | p-Value | |
|---|---|---|---|
| Urinary AGEs (AU) | 230.4 ± 49.0 | 384.3 ± 123.0 | 0.0001 |
| Sex (F/M %) | 45.3/54.7 | 48.6/51.4 | 0.457 |
| Age (years) | 11.8 ± 1.6 | 11.8 ± 1.7 | 0.876 |
| Height (cm) | 151.3 ± 11.6 | 151.2 ± 11.2 | 0.232 |
| Weight (kg) | 50.5 ± 13.8 | 51.1 ± 15.0 | 0.578 |
| BMI z-score | 1.2 ± 1.0 | 1.3 ± 1.1 | 0.294 |
| SBP (mm Hg) | 108.0 ± 9.0 | 108.4 ± 9.7 | 0.565 |
| DBP (mm Hg) | 65.0 ± 6.4 | 65.4 ± 6.4 | 0.343 |
| hs-CRP (mg/dL) | 0.24 ± 0.6 | 0.44 ± 1.1 | 0.002 * |
| Inflammation Parameters | Urinary AGEs | |
|---|---|---|
| Correlation Coefficient (Spearman’s Rho) | p-Value (2 Tailed) | |
| hs-CRP | 0.258 | 0.0001 |
| IP-10 | 0.105 | 0.006 |
| IL-15 | 0.131 | 0.001 |
| IL-6 | 0.050 | 0.198 |
| IL-8 | −0.007 | 0.848 |
| TNF-α | 0.004 | 0.912 |
| IL-1Ra | 0.138 | 0.001 |
| Dependent Variable | Independent Variables | B (SE) | p-Value |
|---|---|---|---|
| hsCRP(Ln) mg/dl | Sex (m/f) | −0.023 (0.064) | 0.719 |
| Age (years) | −0.050 (0.020) | 0.011 | |
| BMI z-score | 0.175 (0.030) | 0.0001 | |
| Urinary AGEs (category) | 0.189 (0.064) | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borriello, M.; Lauria, F.; Sirangelo, I.; Aleksandrova, K.; Hebestreit, A.; Siani, A.; Russo, P. Association between Urinary Advanced Glycation End Products and Subclinical Inflammation in Children and Adolescents: Results from the Italian I.Family Cohort. Nutrients 2022, 14, 4135. https://doi.org/10.3390/nu14194135
Borriello M, Lauria F, Sirangelo I, Aleksandrova K, Hebestreit A, Siani A, Russo P. Association between Urinary Advanced Glycation End Products and Subclinical Inflammation in Children and Adolescents: Results from the Italian I.Family Cohort. Nutrients. 2022; 14(19):4135. https://doi.org/10.3390/nu14194135
Chicago/Turabian StyleBorriello, Margherita, Fabio Lauria, Ivana Sirangelo, Krasimira Aleksandrova, Antje Hebestreit, Alfonso Siani, and Paola Russo. 2022. "Association between Urinary Advanced Glycation End Products and Subclinical Inflammation in Children and Adolescents: Results from the Italian I.Family Cohort" Nutrients 14, no. 19: 4135. https://doi.org/10.3390/nu14194135
APA StyleBorriello, M., Lauria, F., Sirangelo, I., Aleksandrova, K., Hebestreit, A., Siani, A., & Russo, P. (2022). Association between Urinary Advanced Glycation End Products and Subclinical Inflammation in Children and Adolescents: Results from the Italian I.Family Cohort. Nutrients, 14(19), 4135. https://doi.org/10.3390/nu14194135








