Local Application of Krill Oil Accelerates the Healing of Artificially Created Wounds in Diabetic Mice
Abstract
:1. Introduction
2. Materials and Methods
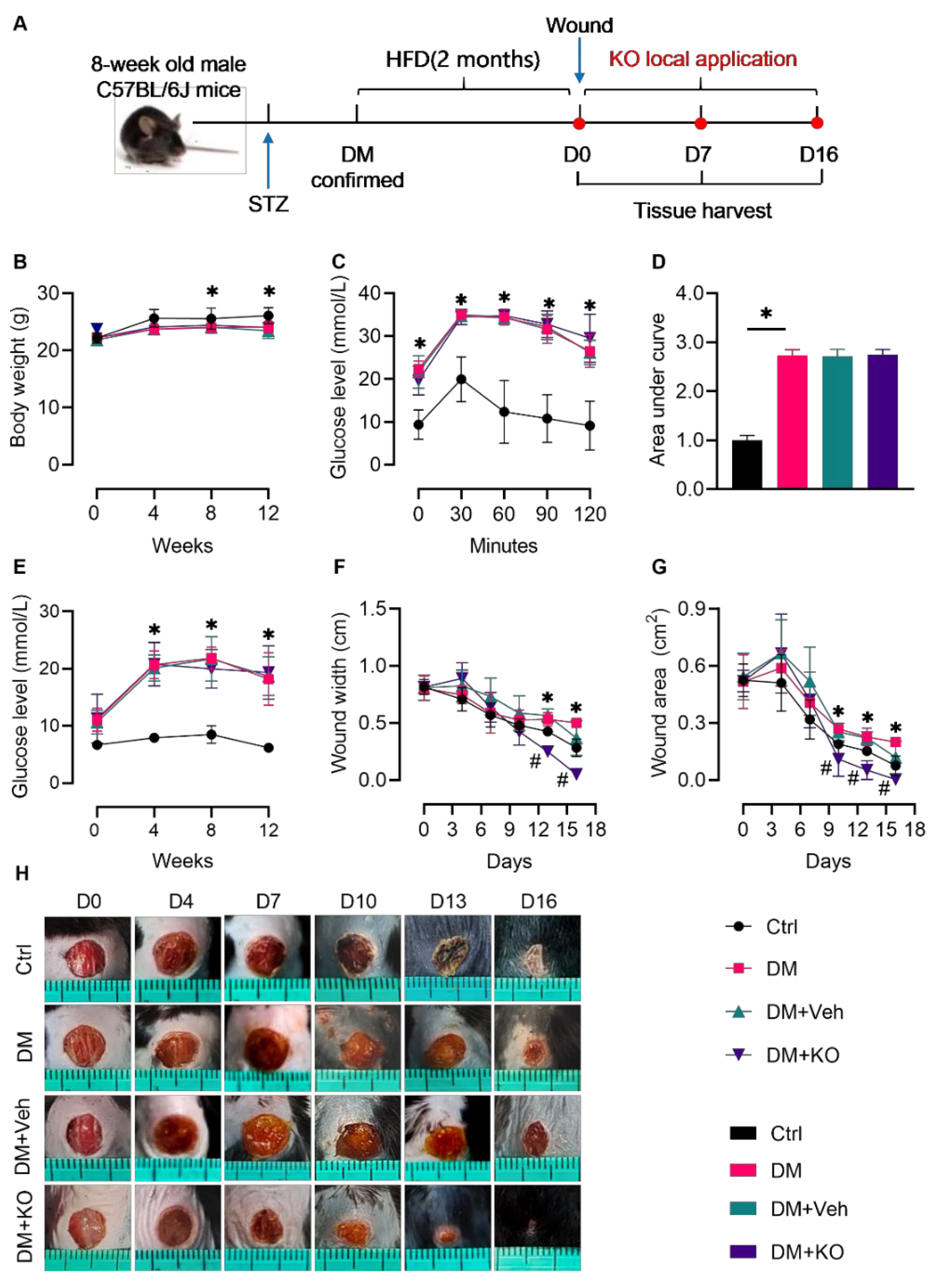
2.1. Animal Housing and Induction of DM
2.2. Skin Excision and Wound Healing Evaluation
2.3. Assessment of Cutaneous Pathology and Immunohistochemical (IHC) Staining
2.4. Cell Culture and Treatments
2.5. Cell Scratch Assay
2.6. Matrigel Tube Formation Assay
2.7. Cell Immunofluorescence
2.8. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.9. Western Blot Analysis
3. Results
3.1. KO Accelerated Wound Healing in the Diabetic Mice
3.2. KO Attenuated the DM-Induced Cutaneous Inflammation
3.3. KO Alleviated the TNF-α-Induced Macrophage Inflammation
3.4. KO Facilitated Cutaneous Collagen Deposition in the Diabetic Wounds
3.5. KO Promoted the Profibrotic Transition of TNF-α-Stimulated Fibroblasts
3.6. KO Enhanced Neoangiogenesis in Diabetic Wounds
3.7. KO Induced Angiogenesis in TNF-α-Treated ECs
4. Discussion
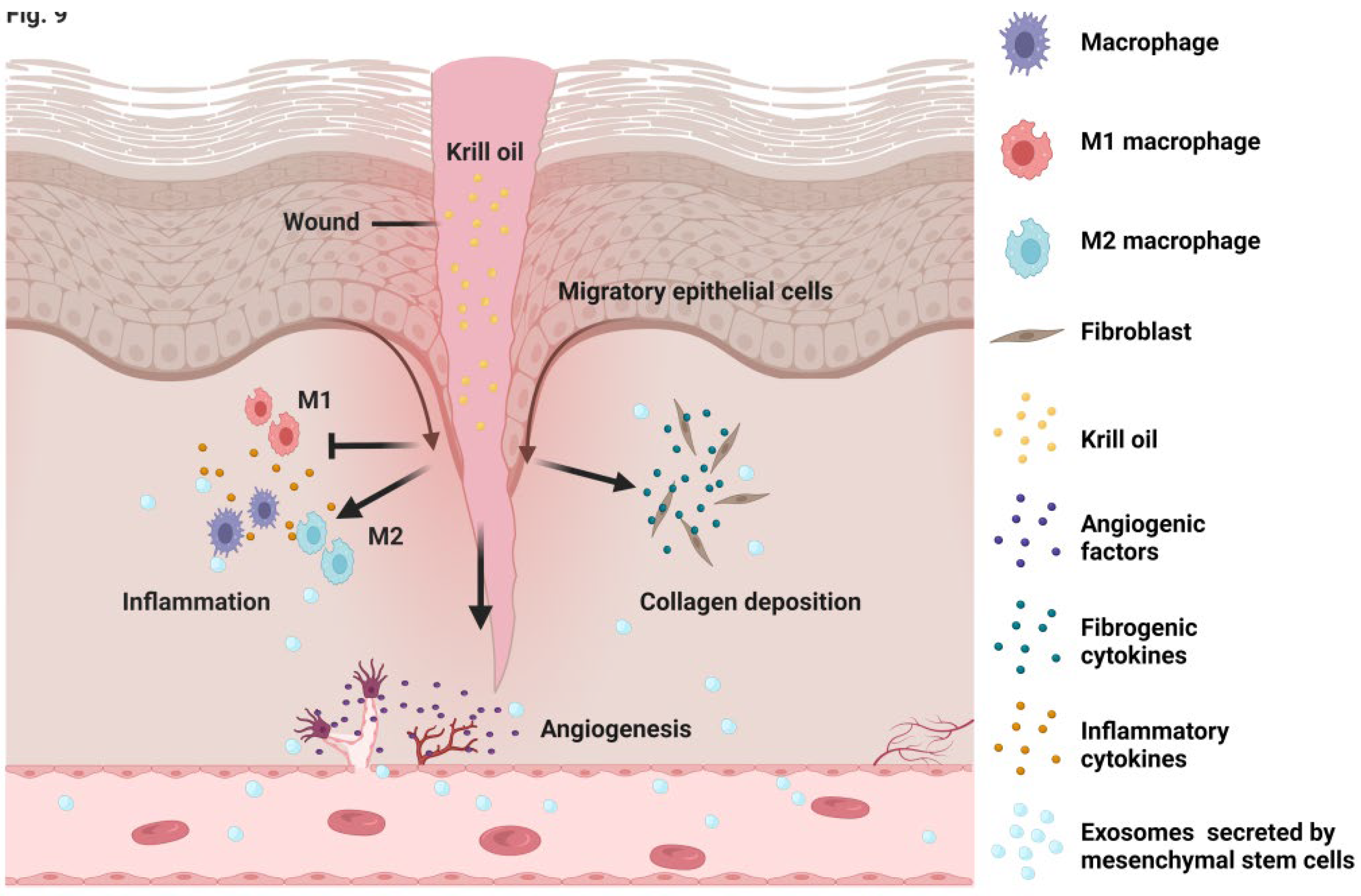
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fradkin, J.E.; Rodgers, G.P. Diabetes research: A perspective from the National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes 2013, 62, 320–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jere, S.W.; Abrahamse, H.; Houreld, N.N. The JAK/STAT signaling pathway and photobiomodulation in chronic wound healing. Cytokine Growth Factor Rev. 2017, 38, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Everett, E.; Mathioudakis, N. Update on management of diabetic foot ulcers. Ann. N. Y. Acad. Sci. 2018, 1411, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.; Schaller, M.; Joshi, A.; Davis, F.M.; denDekker, A.; Boniakowski, A.; Bermick, J.; Obi, A.; Moore, B.; Henke, P.K.; et al. Ly6C(Hi) Blood Monocyte/Macrophage Drive Chronic Inflammation and Impair Wound Healing in Diabetes Mellitus. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1102–1114. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Hou, Q.; Zhong, L.; Zhao, Y.; Fu, X. Macrophage Related Chronic Inflammation in Non-Healing Wounds. Front. Immunol. 2021, 12, 681710. [Google Scholar] [CrossRef]
- Louiselle, A.E.; Niemiec, S.M.; Zgheib, C.; Liechty, K.W. Macrophage polarization and diabetic wound healing. Transl. Res. 2021, 236, 109–116. [Google Scholar] [CrossRef]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef]
- Nolte, M.; Margadant, C. Controlling Immunity and Inflammation through Integrin-Dependent Regulation of TGF-β. Trends Cell Biol. 2020, 30, 49–59. [Google Scholar] [CrossRef]
- Cheng, Z.; Kishore, R. Potential role of hydrogen sulfide in diabetes-impaired angiogenesis and ischemic tissue repair. Redox Biol. 2020, 37, 101704. [Google Scholar] [CrossRef]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic Krill (Euphausia superba) Oil: A Comprehensive Review of Chemical Composition, Extraction Technologies, Health Benefits, and Current Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 514–534. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Y.; Sun, X.; Meng, H.; Hao, W.; Yin, J.; Ma, F.; Guo, X.; Du, L.; Sun, L.; et al. Krill Oil Turns Off TGF-β1 Profibrotic Signaling in the Prevention of Diabetic Nephropathy. J. Agric. Food Chem. 2022, 70, 9865–9876. [Google Scholar] [CrossRef]
- Sun, X.; Sun, X.; Meng, H.; Wu, J.; Guo, X.; Du, L.; Wu, H. Krill Oil Inhibits NLRP3 Inflammasome Activation in the Prevention of the Pathological Injuries of Diabetic Cardiomyopathy. Nutrients 2022, 14, 368. [Google Scholar] [CrossRef]
- Liu, F.; Smith, A.D.; Solano-Aguilar, G.; Wang, T.T.Y.; Pham, Q.; Beshah, E.; Tang, Q.; Urban, J.F., Jr.; Xue, C.; Li, R.W. Mechanistic insights into the attenuation of intestinal inflammation and modulation of the gut microbiome by krill oil using in vitro and in vivo models. Microbiome 2020, 8, 83. [Google Scholar] [CrossRef]
- Ru, Q.; Tian, X.; Xiong, Q.; Xu, C.; Chen, L.; Wu, Y. Krill Oil Alleviated Methamphetamine-Induced Memory Impairment via the MAPK Signaling Pathway and Dopaminergic Synapse Pathway. Front. Pharmacol. 2021, 12, 756822. [Google Scholar] [CrossRef]
- Seth, N.; Chopra, D.; Lev-Tov, H. Fish Skin Grafts with Omega-3 for Treatment of Chronic Wounds: Exploring the Role of Omega-3 Fatty Acids in Wound Healing and A Review of Clinical Healing Outcomes. Surg. Technol. Int. 2022, 40, 38–46. [Google Scholar]
- Karoud, W.; Ghlissi, Z.; Krichen, F.; Kallel, R.; Bougatef, H.; Zarai, Z.; Boudawara, T.; Sahnoun, Z.; Sila, A.; Bougatef, A. Oil from hake (Merluccius merluccius): Characterization, antioxidant activity, wound healing and anti-inflammatory effects. J. Tissue Viability 2020, 29, 138–147. [Google Scholar] [CrossRef]
- Zhang, K.; Jiao, X.; Zhou, L.; Wang, J.; Wang, C.; Qin, Y.; Wen, Y. Nanofibrous composite aerogel with multi-bioactive and fluid gating characteristics for promoting diabetic wound healing. Biomaterials 2021, 276, 121040. [Google Scholar] [CrossRef]
- Sharifiaghdam, M.; Shaabani, E.; Faridi-Majidi, R.; De Smedt, S.C.; Braeckmans, K.; Fraire, J.C. Macrophages as a therapeutic target to promote diabetic wound healing. Mol. Ther. 2022, 30, 2891–2908. [Google Scholar] [CrossRef]
- Borges, P.A.; Waclawiak, I.; Georgii, J.L.; Fraga-Junior, V.D.S.; Barros, J.F.; Lemos, F.S.; Russo-Abrahão, T.; Saraiva, E.M.; Takiya, C.M.; Coutinho-Silva, R.; et al. Adenosine Diphosphate Improves Wound Healing in Diabetic Mice Through P2Y (12) Receptor Activation. Front. Immunol. 2021, 12, 651740. [Google Scholar] [CrossRef]
- Su, C.H.; Li, W.P.; Tsao, L.C.; Wang, L.C.; Hsu, Y.P.; Wang, W.J.; Liao, M.C.; Lee, C.L.; Yeh, C.S. Enhancing Microcirculation on Multitriggering Manner Facilitates Angiogenesis and Collagen Deposition on Wound Healing by Photoreleased NO from Hemin-Derivatized Colloids. ACS Nano 2019, 13, 4290–4301. [Google Scholar] [CrossRef]
- Elbialy, Z.I.; Assar, D.H.; Abdelnaby, A.; Asa, S.A.; Abdelhiee, E.Y.; Ibrahim, S.S.; Abdel-Daim, M.M.; Almeer, R.; Atiba, A. Healing potential of Spirulina platensis for skin wounds by modulating bFGF, VEGF, TGF-ß1 and α-SMA genes expression targeting angiogenesis and scar tissue formation in the rat model. Biomed. Pharmacother. 2021, 137, 111349. [Google Scholar] [CrossRef]
- Talbott, H.E.; Mascharak, S.; Griffin, M.; Wan, D.C.; Longaker, M.T. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell 2022, 29, 1161–1180. [Google Scholar] [CrossRef]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Niu, H.; Liu, Z.; Dang, Y.; Shen, J.; Zayed, M.; Ma, L.; Guan, J. Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci. Adv. 2021, 7, eabj0153. [Google Scholar] [CrossRef]
- Boniakowski, A.E.; Kimball, A.S.; Jacobs, B.N.; Kunkel, S.L.; Gallagher, K.A. Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing. J. Immunol. 2017, 199, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, Y.; He, W.; Mu, X.; Wu, X.; Deng, J.; Nie, X. Fibroblasts: Immunomodulatory factors in refractory diabetic wound healing. Front. Immunol. 2022, 13, 918223. [Google Scholar] [CrossRef]
- Hwang, S.M.; Kim, Y.U.; Kim, J.K.; Chun, Y.S.; Kwon, Y.S.; Ku, S.K.; Song, C.H. Preventive and Therapeutic Effects of Krill Oil on Obesity and Obesity-Induced Metabolic Syndromes in High-Fat Diet-Fed Mice. Mar. Drugs 2022, 20, 483. [Google Scholar] [CrossRef]
- Braunwald, E. Diabetes, heart failure, and renal dysfunction: The vicious circles. Prog. Cardiovasc. Dis. 2019, 62, 298–302. [Google Scholar] [CrossRef]
- Chen, T.Y.; Wen, T.K.; Dai, N.T.; Hsu, S.H. Cryogel/hydrogel biomaterials and acupuncture combined to promote diabetic skin wound healing through immunomodulation. Biomaterials 2021, 269, 120608. [Google Scholar] [CrossRef]
- Ha, D.H.; Kim, H.K.; Lee, J.; Kwon, H.H.; Park, G.H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef]
- Peta, K.T.; Ambele, M.A.; Pepper, M.S. Similarities between Tumour Immune Response and Chronic Wound Microenvironment: Influence of Mesenchymal Stromal/Stem Cells. J. Immunol. Res. 2021, 2021, 6649314. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef] [PubMed]
- Guillamat-Prats, R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells 2021, 10, 1729. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Pham, T.L.; Kakazu, A.; Bazan, H.E.P. Recovery of Corneal Sensitivity and Increase in Nerve Density and Wound Healing in Diabetic Mice After PEDF Plus DHA Treatment. Diabetes 2017, 66, 2511–2520. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Cameron-Smith, D.; Garg, M.; Sinclair, A.J. Docosapentaenoic acid (22:5n-3): A review of its biological effects. Prog. Lipid Res. 2011, 50, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Chae, S.Y.; Park, R.; Hong, S.W. Surface-mediated high antioxidant and anti-inflammatory effects of astaxanthin-loaded ultrathin graphene oxide film that inhibits the overproduction of intracellular reactive oxygen species. Biomater. Res. 2022, 26, 30. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, W.; Meng, H.; Li, H.; Zheng, Y.; Song, C.; Jiang, Z.; Bai, X.; Zhang, Z.; Du, L.; Liu, P.; et al. Local Application of Krill Oil Accelerates the Healing of Artificially Created Wounds in Diabetic Mice. Nutrients 2022, 14, 4139. https://doi.org/10.3390/nu14194139
Hao W, Meng H, Li H, Zheng Y, Song C, Jiang Z, Bai X, Zhang Z, Du L, Liu P, et al. Local Application of Krill Oil Accelerates the Healing of Artificially Created Wounds in Diabetic Mice. Nutrients. 2022; 14(19):4139. https://doi.org/10.3390/nu14194139
Chicago/Turabian StyleHao, Wenhao, Huali Meng, Hui Li, Yan Zheng, Chunhong Song, Ziping Jiang, Xue Bai, Zhiyue Zhang, Lei Du, Pei Liu, and et al. 2022. "Local Application of Krill Oil Accelerates the Healing of Artificially Created Wounds in Diabetic Mice" Nutrients 14, no. 19: 4139. https://doi.org/10.3390/nu14194139




