Is Abdominal Obesity a Risk Factor for the Incidence of Vitamin D Insufficiency and Deficiency in Older Adults? Evidence from the ELSA Study
Abstract
1. Introduction
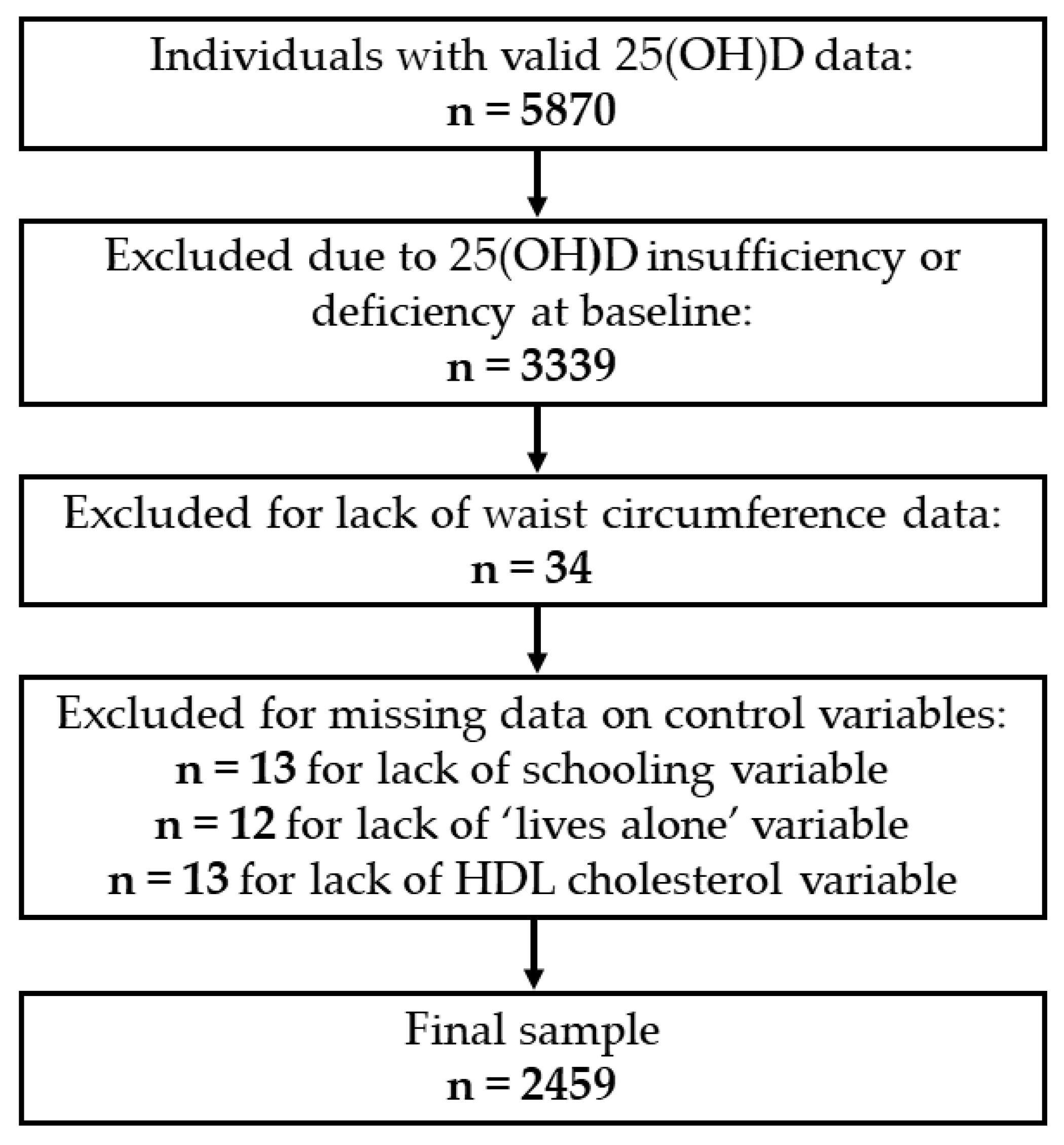
2. Materials and Methods
2.1. Study Population
2.2. Vitamin D Assessment
2.3. Abdominal Obesity
2.4. Covariates
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Schoor, N.M.; Lips, P. Worldwide vitamin D status. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.; O’Halloran, A.M.; Carey, D.; Healy, M.; O’Connor, D.; Moore, P.; Shannon, T.; Molloy, A.M.; Kenny, R.A. The Prevalence of Vitamin D Deficiency and the Determinants of 25(OH)D Concentration in Older Irish Adults: Data from The Irish Longitudinal Study on Ageing (TILDA). J. Gerontol. A Biol. Sci. Med. 2018, 73, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Aspell, N.; Laird, E.; Healy, M.; Shannon, T.; Lawlor, B.; O’Sullivan, M. The Prevalence and Determinants of Vitamin D Status in Community-Dwelling Older Adults: Results from the English Longitudinal Study of Ageing (ELSA). Nutrients 2019, 11, 1253. [Google Scholar] [CrossRef]
- Liu, X.; Baylin, A.; Levy, P.D. Vitamin D deficiency and insufficiency among US adults: Prevalence, predictors and clinical implications. Br. J. Nutr. 2018, 119, 928–936. [Google Scholar] [CrossRef]
- Benedik, E. Sources of vitamin D for humans. Int. J. Vitam. Nutr. Res. 2022, 92, 118–125. [Google Scholar] [CrossRef]
- Snijder, M.B.; van Dam, R.M.; Visser, M.; Deeg, D.J.; Dekker, J.M.; Bouter, L.M.; Seidell, J.C.; Lips, P. Adiposity in relation to vitamin D status and parathyroid hormone levels: A population-based study in older men and women. J. Clin. Endocrinol. Metab. 2005, 90, 4119–4123. [Google Scholar] [CrossRef]
- Ding, C.; Parameswaran, V.; Blizzard, L.; Burgess, J.; Jones, G. Not a simple fat-soluble vitamin: Changes in serum 25-(OH)D levels are predicted by adiposity and adipocytokines in older adults. J. Intern. Med. 2010, 268, 501–510. [Google Scholar] [CrossRef]
- Sousa-Santos, A.R.; Afonso, C.; Santos, A.; Borges, N.; Moreira, P.; Padrão, P.; Fonseca, I.; Amaral, T.F. The association between 25(OH)D levels, frailty status and obesity indices in older adults. PLoS ONE 2018, 13, e0198650. [Google Scholar] [CrossRef]
- Earthman, C.P.; Beckman, L.M.; Masodkar, K.; Sibley, S.D. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: Considerations and implications. Int. J. Obes. 2012, 36, 387–396. [Google Scholar] [CrossRef]
- Brock, K.; Huang, W.Y.; Fraser, D.R.; Ke, L.; Tseng, M.; Stolzenberg-Solomon, R.; Peters, U.; Ahn, J.; Purdue, M.; Mason, R.S.; et al. Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. J. Steroid Biochem. Mol. Biol. 2010, 121, 462–466. [Google Scholar] [CrossRef] [PubMed]
- McCarroll, K.; Beirne, A.; Casey, M.; McNulty, H.; Ward, M.; Hoey, L.; Molloy, A.; Laird, E.; Healy, M.; Strain, J.J.; et al. Determinants of 25-hydroxyvitamin D in older Irish adults. Age Ageing 2015, 44, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, P.; Zhu, Y.; Chang, H.; Wang, X.; Liu, W.; Zhang, Y.; Huang, G. Higher visceral fat area increases the risk of vitamin D insufficiency and deficiency in Chinese adults. Nutr. Metab. 2015, 12, 50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- González-Molero, I.; Rojo-Martínez, G.; Morcillo, S.; Gutierrez, C.; Rubio, E.; Pérez-Valero, V.; Esteva, I.; Ruiz de Adana, M.S.; Almaraz, M.C.; Colomo, N.; et al. Hypovitaminosis D and incidence of obesity: A prospective study. Eur. J. Clin. Nutr. 2013, 67, 680–682. [Google Scholar] [CrossRef]
- Bosello, O.; Vanzo, A. Obesity paradox and aging. Eat. Weight Disord. 2021, 26, 27–35. [Google Scholar] [CrossRef]
- Mindell, J.; Biddulph, J.P.; Hirani, V.; Stamatakis, E.; Craig, R.; Nunn, S.; Shelton, N. Cohort profile: The health survey for England. Int. J. Epidemiol. 2012, 41, 1585–1593. [Google Scholar] [CrossRef]
- National Heart, Lung, Blood Institute; National Institute of Diabetes, & Kidney Diseases (US). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report; No. 98; National Heart, Lung, and Blood Institute: Bethesda, MD, USA, 1998. [Google Scholar] [PubMed]
- Joint Health Surveys Unit, National Centre Social Research and University College London Research Department of Epidemiology and Public Health. The Health Survey for England Physical Activity Validation Study: Substantive Report; NHS Information Centre for Health and Social Care: Leeds, UK, 2008. [Google Scholar]
- Luiz, M.M.; Máximo, R.; Oliveira, D.C.; Ramírez, P.C.; de Souza, A.F.; Delinocente, M.L.B.; Steptoe, A.; de Oliveira, C.; Alexandre, T. Association of Serum 25-Hydroxyvitamin D Deficiency with Risk of Incidence of Disability in Basic Activities of Daily Living in Adults > 50 Years of Age. J. Nutr. 2020, 150, 2977–2984. [Google Scholar] [CrossRef]
- Steffick, D.E. Documentation of Affective Functioning Measures in the Health and Retirement Study; HRS Documentation Report DR-005; Survey Research Center at the Institute for Social Research: Ann Arbor, MI, USA, 2000. [Google Scholar]
- World Health Organization (Ed.) Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2000; ISBN 978-92-4-120894-9. [Google Scholar]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O.; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of Inflammation and Cardiovascular Disease. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Fedder, D.O.; Koro, C.E.; L’Italien, G.J. New National Cholesterol Education Program III Guidelines for Primary Prevention Lipid-Lowering Drug Therapy. Circulation 2002, 105, 152–156. [Google Scholar] [CrossRef]
- LoPinto-Khoury, C.; Brennan, L.; Mintzer, S. Impact of carbamazepine on vitamin D levels: A meta-analysis. Epilepsy Res. 2021, 178, 106829. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, G.; Greenland, S. Simulation Study of Confounder-Selection Strategies. Am. J. Epidemiol. 1993, 138, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Bani-issa, W.; Eldeirawi, K.; Harfil, S.; Fakhry, R. Vitamin D Deficiency and Its Determinants in Adults: A Sample from Community-Based Settings in the United Arab Emirates. Int. J. Endocrinol. 2017, 2017, 3906306. [Google Scholar] [CrossRef] [PubMed]
- Stokić, E.; Kupusinac, A.; Tomić-Naglić, D.; Zavišić, B.K.; Mitrović, M.; Smiljenić, D.; Soskić, S.; Isenović, E. Obesity and vitamin D deficiency: Trends to promote a more proatherogenic cardiometabolic risk profile. Angiology 2015, 66, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C.; Sainsbury, A.; Stock Conference 2008 Working Group. Sex differences in obesity and the regulation of energy homeostasis. Obes. Rev. 2009, 10, 154–167. [Google Scholar] [CrossRef]
- Price, G.M.; Uauy, R.; Breeze, E.; Bulpitt, C.J.; Fletcher, A.E. Weight, shape, and mortality risk in older persons: Elevated waist-hip ratio, not high body mass index, is associated with a greater risk of death. Am. J. Clin. Nutr. 2006, 84, 449–460. [Google Scholar] [CrossRef]
- de Carvalho, D.H.T.; Scholes, S.; Santos, J.L.F.; de Oliveira, C.; Alexandre, T.S. Does Abdominal Obesity Accelerate Muscle Strength Decline in Older Adults? Evidence from the English Longitudinal Study of Ageing. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1105–1111. [Google Scholar] [CrossRef]
- Swainson, M.G.; Batterham, A.M.; Tsakirides, C.; Rutherford, Z.H.; Hind, K. Prediction of whole-body fat percentage and visceral adipose tissue mass from five anthropometric variables. PLoS ONE 2017, 12, e0177175. [Google Scholar] [CrossRef]
- Targher, G.; Bertolini, L.; Scala, L.; Cigolini, M.; Zenari, L.; Falezza, G.; Arcaro, G. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 517–524. [Google Scholar] [CrossRef]
- Wamberg, L.; Pedersen, S.B.; Rejnmark, L.; Richelsen, B. Causes of vitamin D deficiency and effect of vitamin D supplementation on metabolic complications in obesity: A review. Curr. Obes. Rep. 2015, 4, 429–440. [Google Scholar] [CrossRef]
- Jamka, M.; Woźniewicz, M.; Walkowiak, J.; Bogdański, P.; Jeszka, J.; Stelmach-Mardas, M. The effect of vitamin D supplementation on selected inflammatory biomarkers in obese and overweight subjects: A systematic review with meta-analysis. Eur. J. Nutr. 2016, 55, 2163–2176. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Grundy, S.M.; Li, X.; Adams-Huet, B.; Vega, G.L. Comparison of visceral fat mass measurement by dual-X-ray absorptiometry and magnetic resonance imaging in a multiethnic cohort: The Dallas Heart Study. Nutr. Diabetes 2016, 6, e221. [Google Scholar] [CrossRef]
- Fang, H.; Berg, E.; Cheng, X.; Shen, W. How to best assess abdominal obesity. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Ponnalagu, S.D.; Bi, X.; Henry, C.J. Is waist circumference more strongly associated with metabolic risk factors than waist-to-height ratio in Asians? Nutrition 2019, 60, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Osayande, O.E.; Azekhumen, G.N.; Obuzor, E.O. A comparative study of different body fat measuring instruments. Niger. J. Physiol. Sci. 2018, 33, 125–128. [Google Scholar]
| Total (n = 2459) | Without Abdominal Obesity (n = 1386) | With Abdominal Obesity (n = 1073) | |
|---|---|---|---|
| Age, years | 66.6 ± 8.5 | 66.1 ± 8.6 | 67.3 ± 8.3 * |
| Age group, (%) | |||
| 50–59 | 22.1 | 24.2 | 19.4 * |
| 60–69 | 43.0 | 43.3 | 42.6 |
| 70–79 | 27.6 | 25.4 | 30.4 |
| ≥80 years | 7.3 | 7.1 | 7.6 |
| Gender, women (%) | 53.9 | 49.4 | 59.7 * |
| Race, white (%) | 98.9 | 99.1 | 98.6 |
| Living alone, (%) | 14.3 | 15.2 | 13.1 |
| Schooling years, (%) | |||
| >13 years | 34.2 | 40.3 | 26.3 * |
| 12 to 13 years | 28.9 | 28.0 | 30.0 |
| ≤11 years | 36.9 | 31.7 | 43.7 * |
| Total household wealth, (%) | |||
| Highest quintile | 27.1 | 31.3 | 21.6 * |
| 4th quintile | 24.1 | 23.8 | 24.6 |
| 3rd quintile | 21.9 | 21.1 | 22.9 |
| 2nd quintile | 15.4 | 14.4 | 16.8 |
| Lowest quintile | 9.9 | 7.9 | 12.4 * |
| Not applicable | 1.6 | 1.5 | 1.7 |
| Total (n = 2459) | Without Abdominal Obesity (n = 1386) | With Abdominal Obesity (n = 1073) | |
|---|---|---|---|
| Smoking status, (%) | |||
| Non-smoker | 39.4 | 40.3 | 38.2 |
| Ex-smoker | 52.8 | 50.9 | 55.4 |
| Smoker | 7.8 | 8.8 | 6.4 |
| Alcohol intake, (%) | |||
| Never/rarely | 15.1 | 12.8 | 18.0 * |
| Frequently | 40.9 | 39.8 | 42.5 |
| Daily | 38.5 | 42.1 | 33.9 * |
| Not applicable | 5.5 | 5.3 | 5.6 |
| Physical activity, inactive (%) | 3.3 | 2.8 | 4.0 |
| Clinical conditions, yes (%) | |||
| Systemic arterial hypertension | 35.3 | 27.0 | 46.0 * |
| Diabetes mellitus | 8.3 | 5.1 | 12.3 * |
| Cancer | 5.7 | 6.0 | 5.2 |
| Heart disease | 15.5 | 14.8 | 16.3 |
| Lung disease | 12.6 | 11.3 | 14.3 |
| Stroke | 2.7 | 2.0 | 3.5 |
| Osteoporosis | 10.3 | 10.0 | 10.5 |
| Osteoarthritis | 38.1 | 31.9 | 46.0 * |
| Depressive symptoms | 8.4 | 7.7 | 9.3 |
| Season, (%) | |||
| Summer | 31.8 | 30.2 | 33.9 |
| Spring | 4.8 | 5.1 | 4.5 |
| Autumn | 46.0 | 46.8 | 44.8 |
| Winter | 17.4 | 17.9 | 16.8 |
| BMI, (kg/m2) | 27.2 ± 4.5 | 24.6 ± 2.8 | 30.6 ± 4.0 |
| BMI, (%) | |||
| Normal weight (≥18.5 and <25 kg/m2) | 31.5 | 52.4 | 4.6 * |
| Underweight (<18.5 kg/m2) | 1.1 | 1.8 | 0.1 * |
| Overweight (≥25 and <30 kg/m2) | 44.3 | 43.9 | 44.9 |
| Obesity (≥30 kg/m2) | 23.1 | 1.9 | 50.4 * |
| Vitamin D Supplementation, yes (%) | 4.5 | 3.8 | 5.3 |
| Use of carbamazepine, yes (%) | 1.8 | 2.0 | 1.7 |
| Biochemical measures, (%) | |||
| High C-reactive protein | 23.1 | 15.7 | 32.8 * |
| High total cholesterol | 62.3 | 66.2 | 57.2 * |
| Low HDL cholesterol | 7.7 | 4.0 | 12.5 * |
| High LDL cholesterol | 71.7 | 75.5 | 66.7 * |
| High triglycerides | 23.1 | 15.4 | 32.9 * |
| Relative Risk Ratio and 95% CI 1 | ||
|---|---|---|
| 25(OH)D Insufficiency (>30 to ≤50 nmol/L) | 25(OH)D Deficiency (≤30 nmol/L) | |
| Without abdominal obesity 2 | 1.00 | 1.00 |
| With abdominal obesity 3 | 1.36 (1.01–1.83) | 1.64 (1.05–2.58) |
| Relative Risk Ratio and 95% CI 1 | ||
|---|---|---|
| 25(OH)D Insufficiency (>30 to ≤50 nmol/L) | 25(OH)D Deficiency (≤30 nmol/L) | |
| Without abdominal obesity 2 | 1.00 | 1.00 |
| With abdominal obesity 3 | 1.38 (1.02–1.88) | 1.62 (1.02–2.56) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, T.B.P.; Luiz, M.M.; Delinocente, M.L.B.; Steptoe, A.; de Oliveira, C.; Alexandre, T.d.S. Is Abdominal Obesity a Risk Factor for the Incidence of Vitamin D Insufficiency and Deficiency in Older Adults? Evidence from the ELSA Study. Nutrients 2022, 14, 4164. https://doi.org/10.3390/nu14194164
da Silva TBP, Luiz MM, Delinocente MLB, Steptoe A, de Oliveira C, Alexandre TdS. Is Abdominal Obesity a Risk Factor for the Incidence of Vitamin D Insufficiency and Deficiency in Older Adults? Evidence from the ELSA Study. Nutrients. 2022; 14(19):4164. https://doi.org/10.3390/nu14194164
Chicago/Turabian Styleda Silva, Thaís Barros Pereira, Mariane Marques Luiz, Maicon Luís Bicigo Delinocente, Andrew Steptoe, Cesar de Oliveira, and Tiago da Silva Alexandre. 2022. "Is Abdominal Obesity a Risk Factor for the Incidence of Vitamin D Insufficiency and Deficiency in Older Adults? Evidence from the ELSA Study" Nutrients 14, no. 19: 4164. https://doi.org/10.3390/nu14194164
APA Styleda Silva, T. B. P., Luiz, M. M., Delinocente, M. L. B., Steptoe, A., de Oliveira, C., & Alexandre, T. d. S. (2022). Is Abdominal Obesity a Risk Factor for the Incidence of Vitamin D Insufficiency and Deficiency in Older Adults? Evidence from the ELSA Study. Nutrients, 14(19), 4164. https://doi.org/10.3390/nu14194164







