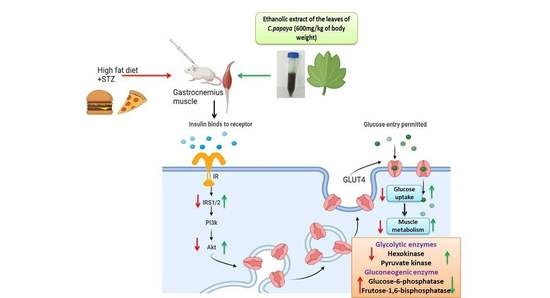
Effect of Carica papaya on IRS-1/Akt Signaling Mechanisms in High-Fat-Diet–Streptozotocin-Induced Type 2 Diabetic Experimental Rats: A Mechanistic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Collection of Plant Material
2.3. Animals
2.4. Induction of T2DM
2.5. Experimental Design
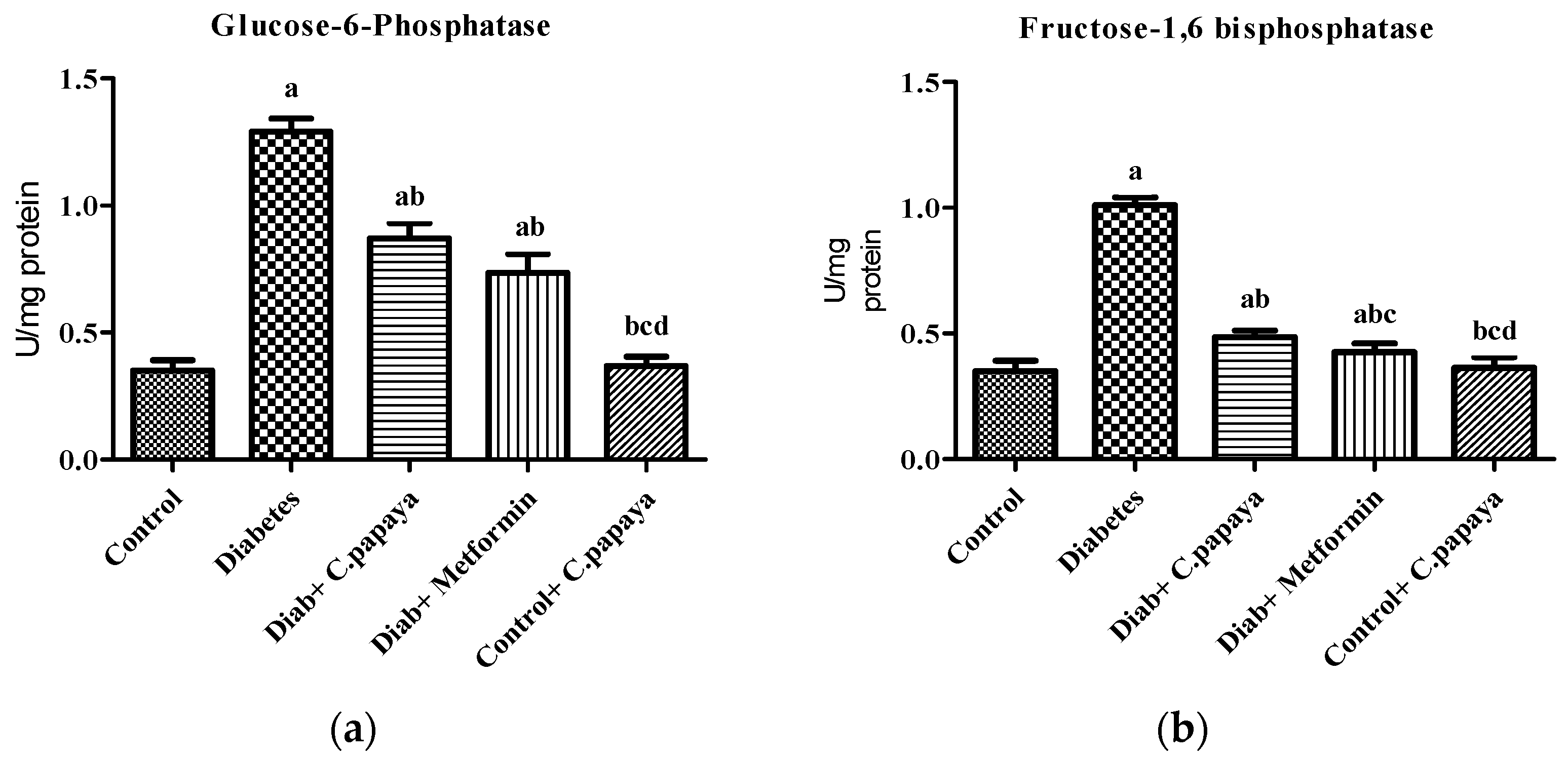
2.6. Determination of Gluconeogenic Enzymes
2.6.1. Glucose-6-Phosphatase Assay
2.6.2. Fructose-1,6 Bisphosphatase Assay
2.7. Determination of Glycolytic Enzymes
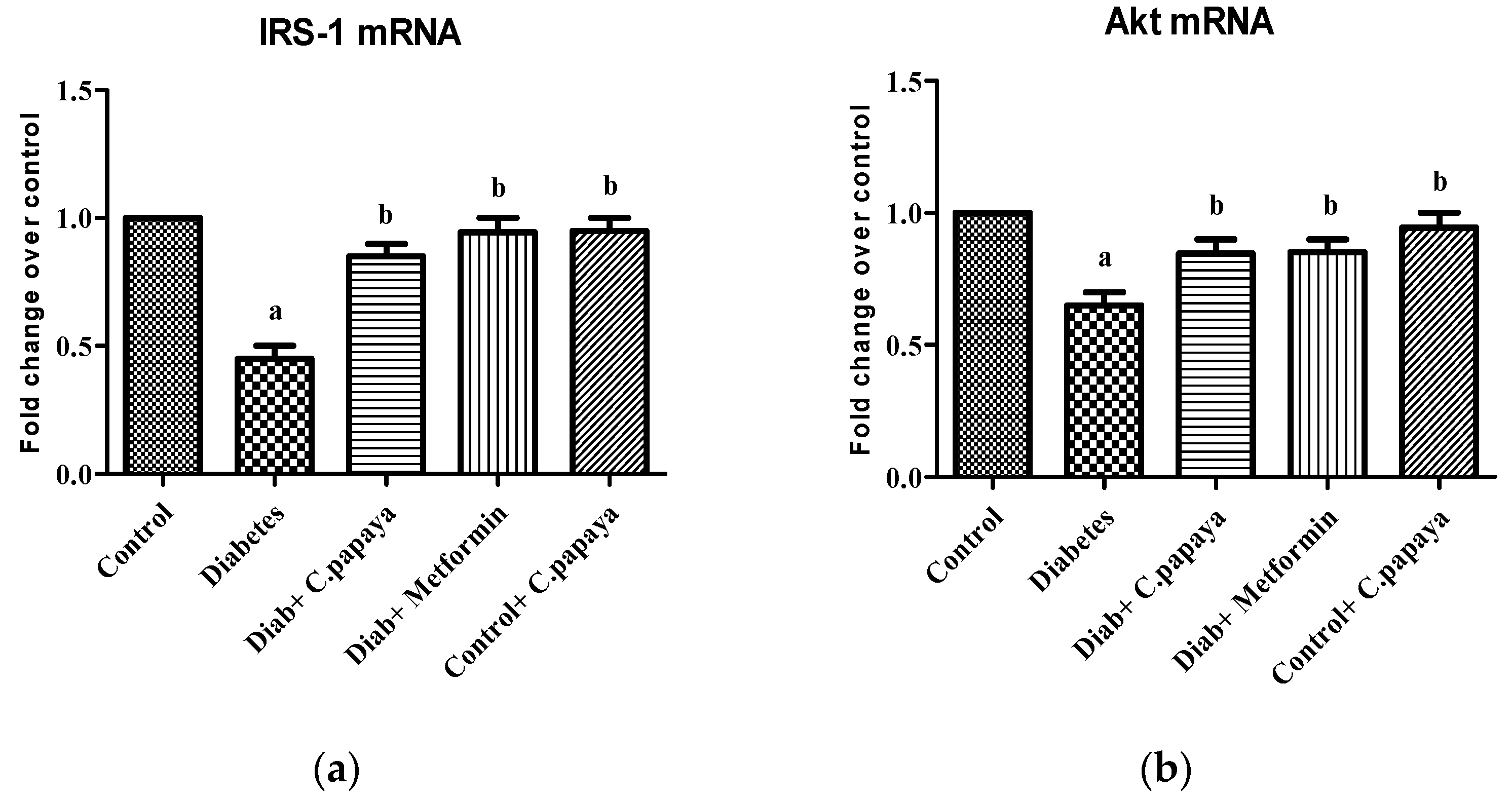
2.8. mRNA Expression Analysis
Total RNA Isolation, cDNA Conversion, and Real-Time PCR
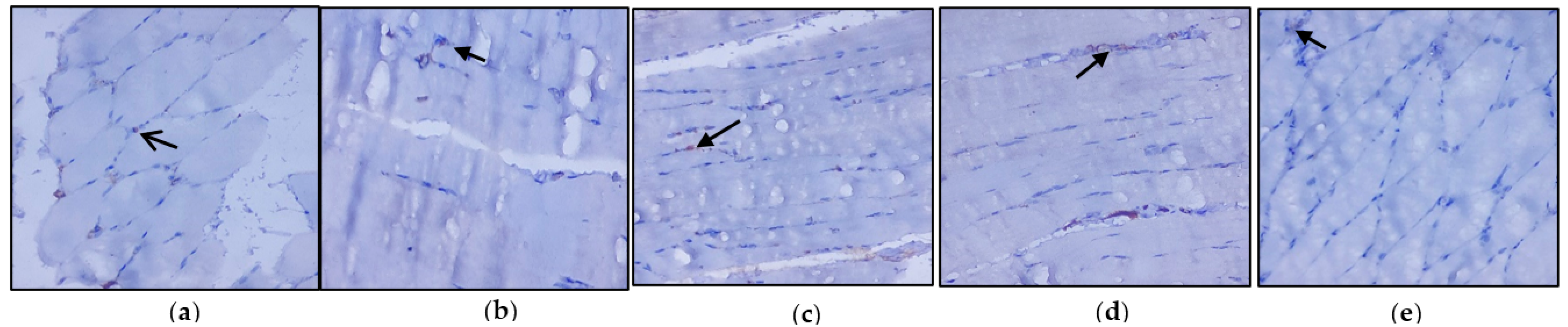
2.9. Immunohistochemical Analysis
2.10. Statistical Analysis
2.11. Molecular Docking
2.11.1. Compound/Ligand Preparation
2.11.2. Protein Preparation
2.11.3. Molecular Docking Procedure
2.12. Molecular Simulation and Dynamics
Molecular Simulation and Dynamics Study of Proposed Compounds and IRS-1 and Akt Complex
3. Results
3.1. Estimation of Gluconeogenic Enzymes and Glycolytic Enzymes
3.2. Effect of C. papaya on mRNA Expression of IRS-1 and Akt
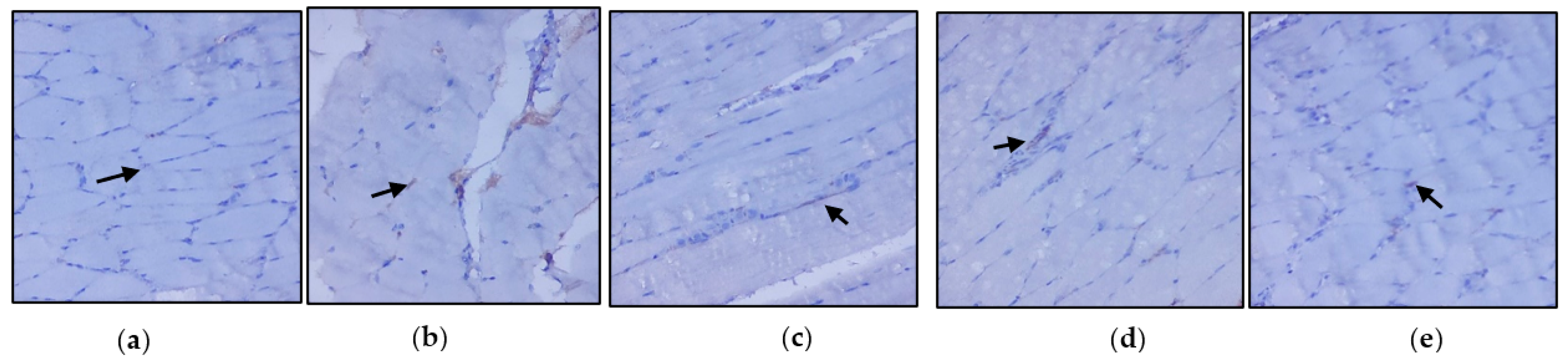
3.3. Evaluation of Immunohistochemical Changes in Skeletal Muscle
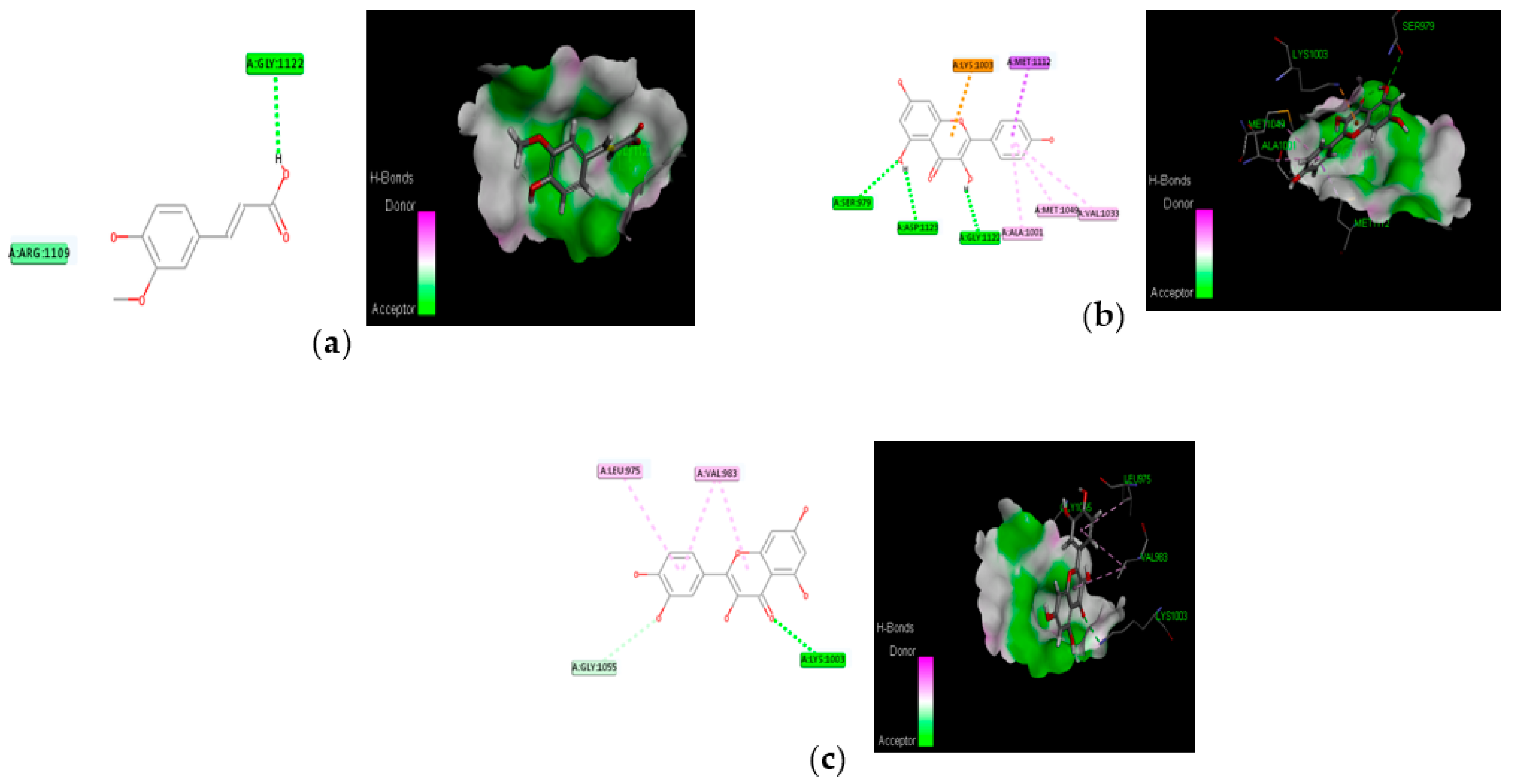
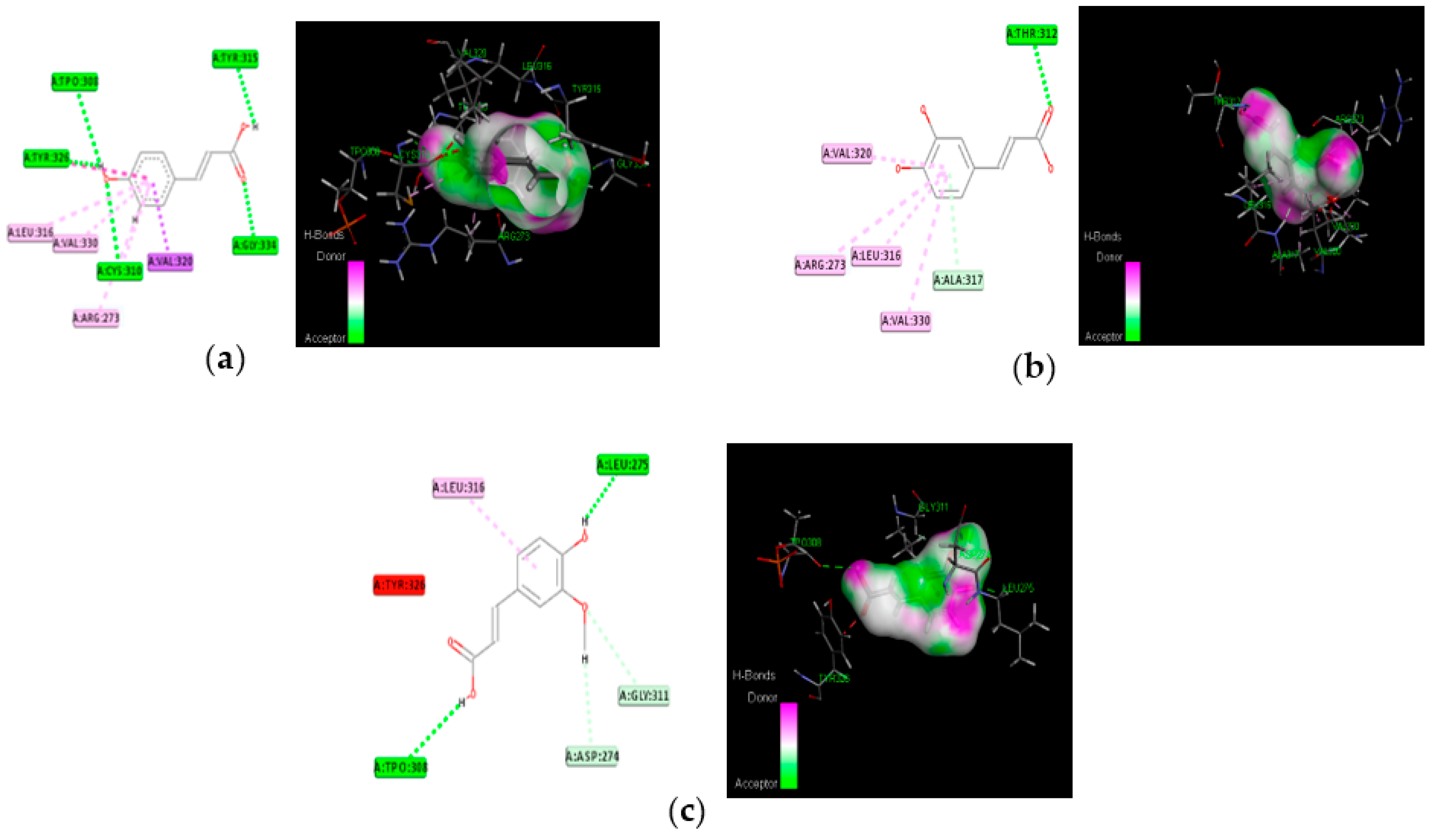
3.4. Molecular Docking
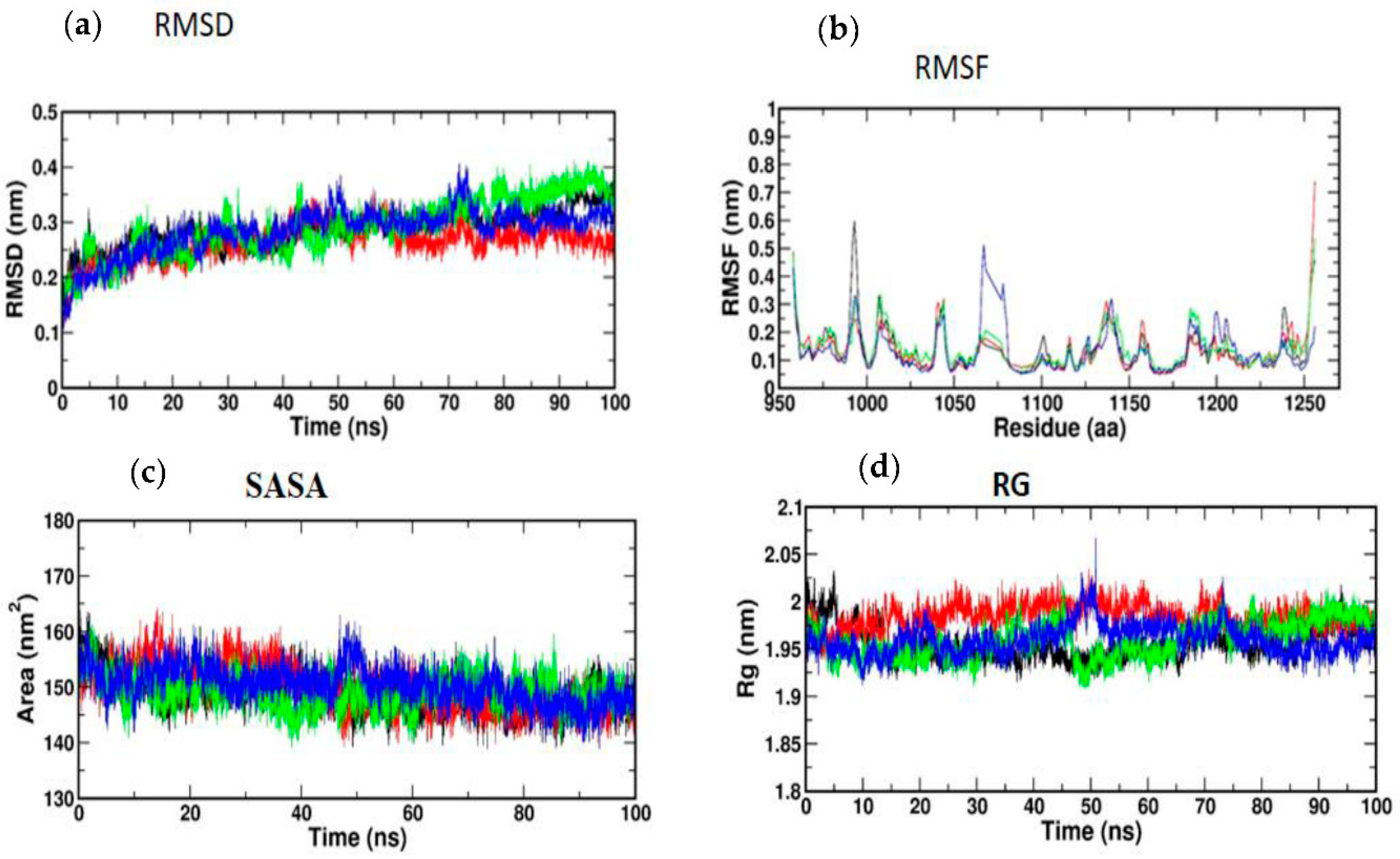
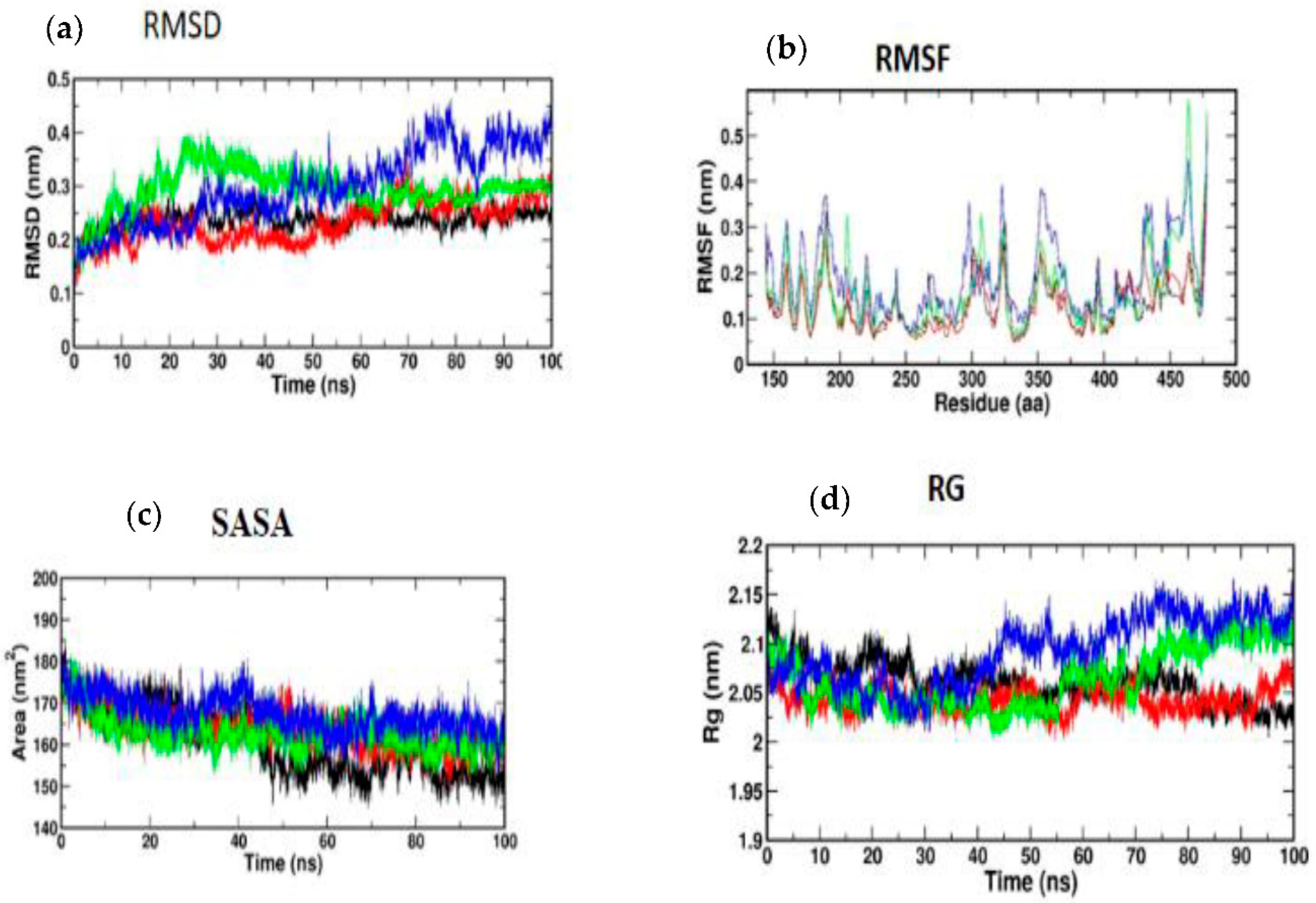
3.5. Molecular Simulation and Dynamics Study of Docked Complex
3.5.1. Molecular Dynamic Simulation of IRS-1
3.5.2. Molecular Dynamic Simulation of Akt
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Öhman, T.; Teppo, J.; Datta, N.; Mäkinen, S.; Varjosalo, M.; Koistinen, H.A. Skeletal muscle proteomes reveal downregulation of mitochondrial proteins in transition from prediabetes into type 2 diabetes. iScience 2021, 24, 102712. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; AlKaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Koistinen, H.A.; Zierath, J.R. Regulation of glucose transport in human skeletal muscle. Ann. Med. 2002, 34, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Hulett, N.A.; Scalzo, R.L.; Reusch, J.E.B. Glucose Uptake by Skeletal Muscle within the Contexts of Type 2 Diabetes and Exercise: An Integrated Approach. Nutrients 2022, 14, 647. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.I.; Rothman, D.L.; Jue, T.; Stein, P.; DeFronzo, R.A.; Shulman, R.G. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N. Engl. J. Med. 1990, 322, 223–228. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef] [Green Version]
- Petersen, K.F.; Shulman, G.I. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am. J. Cardiol. 2002, 90, 11G–18G. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Findings in redox biology: From H2O2 to oxidative stress. J. Biol. Chem. 2020, 295, 13458–13473. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Nawaratne, R.; Gray, A.; Jørgensen, C.H.; Downes, C.P.; Siddle, K.; Sethi, J.K. Regulation of insulin receptor substrate 1 pleckstrin homology domain by protein kinase C: Role of serine 24 phosphorylation. Mol. Endocrinol. 2006, 20, 1838–1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, R.M.; Moura, L.P.; Muñoz, V.R.; Silva, A.S.; Gaspar, R.S.; Ropelle, E.R.; Pauli, J.R. Molecular mechanisms of glucose uptake in skeletal muscle at rest and in response to exercise. Mot. Rev. Educ. Fis. 2017, 23, e101609. [Google Scholar] [CrossRef] [Green Version]
- Krook, A.; Björnholm, M.; Galuska, D.; Jiang, X.J.; Fahlman, R.; Myers, M.G., Jr.; Wallberg-Henriksson, H.; Zierath, J.R. Characterization of signal transduction and glucose transport in skeletal muscle from type 2 diabetic patients. Diabetes 2000, 49, 284–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meo, S.D.; Iossa, S.; Venditti, P. Skeletal muscle insulin resistance: Role of mitochondria and other ROS sources. J. Endocrinol. 2017, 233, R15–R42. [Google Scholar] [CrossRef] [Green Version]
- Phielix, E.; Mensink, M. Type 2 diabetes mellitus and skeletal muscle metabolic function. Physiol. Behav. 2008, 94, 252–258. [Google Scholar] [CrossRef]
- Kelley, D.E.; Mandarino, L.J. Fuel selection in human skeletal muscle in insulin resistance: A reexamination. Diabetes 2000, 49, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, V.; Uchida, T.; Yenush, L.; Davis, R.; White, M.F. The c-Jun NH (2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J. Biol. Chem. 2000, 275, 9047–9054. [Google Scholar] [CrossRef] [Green Version]
- Balbaa, M.; Abdulmalek, S.A.; Khalil, S. Oxidative stress and expression of insulin signaling proteins in the brain of diabetic rats: Role of Nigella sativa oil and antidiabetic drugs. PLoS ONE 2017, 12, e0172429. [Google Scholar] [CrossRef] [Green Version]
- Khalid, M.; Alkaabi, J.; Khan, M.A.B.; Adem, A. Insulin Signal Transduction Perturbations in Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 8590. [Google Scholar] [CrossRef]
- Sharma, M.; Aggarwal, S.; Nayar, U.; Vikram, N.K.; Misra, A.; Luthra, K. Differential expression of insulin receptor substrate-1(IRS-1) in visceral and subcutaneous adipose depots of morbidly obese subjects undergoing bariatric surgery in a tertiary care center in north India; SNP analysis and correlation with metabolic profile. Diabetes Metab. Syndr. 2021, 15, 981–986. [Google Scholar] [CrossRef]
- Eckstein, S.S.; Weigert, C.; Lehmann, R. Divergent Roles of IRS (Insulin Receptor Substrate) 1 and 2 in Liver and Skeletal Muscle. Curr. Med. Chem. 2017, 24, 1827–1852. [Google Scholar] [CrossRef] [PubMed]
- Gual, P.; Le Marchand-Brustel, Y.; Tanti, J.F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 2005, 87, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Suer, F.E.O.; Mergen, H.; Bolu, E.; Ozata, M. Molecular scanning for mutations in the insulin receptor substrate-1 (IRS-1) gene in Turkish with type 2 diabetes mellitus. Endocr. J. 2005, 52, 593–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandipati, K.C.; Subramanian, S.; Agrawal, D.K. Protein kinases: Mechanisms and downstream targets in inflammation-mediated obesity and insulin resistance. Mol. Cell Biochem. 2017, 426, 27–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Yu, X.H.; Yan, Y.G.; Wang, C.; Wang, W.J. PI3K/Akt signaling in osteosarcoma. Clin Chim Acta. 2015, 444, 182–192. [Google Scholar] [CrossRef]
- Liu, P.; Gan, W.; Chin, Y.R.; Ogura, K.; Guo, J.; Zhang, J.; Wang, B.; Blenis, J.; Cantley, L.C.; Toker, A.; et al. PtdIns(3,4,5)P3-Dependent Activation of the mTORC2 Kinase Complex. Cancer Discov. 2015, 5, 1194–1209. [Google Scholar] [CrossRef] [Green Version]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [Green Version]
- Risso, G.; Blaustein, M.; Pozzi, B.; Mammi, P.; Srebrow, A. Akt/PKB: One kinase, many modifications. Biochem. J. 2015, 468, 203–214. [Google Scholar] [CrossRef]
- Chao, P.C.; Li, Y.; Chang, C.H.; Shieh, J.P.; Cheng, J.T.; Cheng, K.C. Investigation of insulin resistance in the popularly used four rat models of type-2 diabetes. Biomed. Pharmacother. 2018, 101, 155–161. [Google Scholar] [CrossRef]
- Koide, H.; Oda, T. Pathological occurrence of glucose-6-phosphatase in serum in liver diseases. Clin. Chim. Acta 1959, 4, 554–561. [Google Scholar]
- Fiske, C.H.; Subbarow, J. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Gancedo, J.M.; Gancedo, C. Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non fermenting yeasts. Arch. Mikrobiol. 1971, 76, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Brandstrup, N.; Kirk, J.E.; Bruni, C. The hexokinase and phosphoglucoisomerase activities of aortic and pulmonary artery tissue in individuals of various ages. J. Gerontol. 1957, 12, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Valentine, W.N.; Tanaka, K.R. Pyruvate kinase: Clinical aspects. Methods Enzymol. 1966, 9, 468–473. [Google Scholar]
- Prasad, M.; Jayaraman, S.; Rajagopal, P.; Veeraraghavan, V.P.; Kumar, P.K.; Piramanayagam, S.; Pari, L. Diosgenin inhibits ER stress-induced inflammation in aorta via iRhom2/TACE mediated signaling in experimental diabetic rats: An in vivo and in silico approach. Chem. Biol. Interact. 2022, 358, 109885. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Alonso, A.; Fernandez, R.; Patterson, A.M. Regulation of insulin receptor substrate-1 in the liver, skeletal muscle and adipose tissue of rats throughout pregnancy. Gynecol. Endocrinol. 2003, 17, 187–197. [Google Scholar]
- Indumathi, D.; Jayashree, S.; Selvaraj, J.; Sathish, S.; Mayilvanan, C.; Akilavalli, N.; Balasubramanian, K. Effect of bisphenol-A on insulin signal transduction and glucose oxidation in skeletal muscle of adult male albino rat. Hum. Exp. Toxicol. 2013, 9, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Christy, J.; Shankari, S.; Majeed, I.; Anand, D.A. Deciphering the Synergistic Mechanism of Cortistatin towards Cancer Targets using Network Pharmacology Approach. Indian J. Pharm. Educ. Res. 2021, 55, 1017–1027. [Google Scholar] [CrossRef]
- Christy, J.; Harini; Vasudevan, S.; Lingesan, P.; Anand, D.A. Deciphering the molecular interplay between pelvic inflammatory disease (PID) and ovarian cancer (OC)—A network biology approach. Gene Rep. 2021, 25, 101405. [Google Scholar] [CrossRef]
- Schüttelkopf, A.W.; van Aalten, D.M. PRODRG: A tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 1355–1363. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.D.; Corkey, B.E.; Istfan, N.W.; Apovian, C.M. Hyperinsulinemia: An Early Indicator of Metabolic Dysfunction. J. Endocr. Soc. 2019, 3, 1727–1747. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Zhao, C.; Yang, C.; Wai, S.; Zhang, Y.; Portillo, M.P.; Paoli, P.; Wu, Y.; Cheang, W.S.; Liu, B.; Carpéné, C.; et al. Regulation of glucose metabolism by bioactive phytochemicals for the management of type 2 diabetes mellitus. Crit. Rev. Food Sci. Nutr. 2019, 59, 830–847. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.S.M.; Kamalakkannan, N. Rutin improves glucose homeostasis in streptozotocin diabetic tissues by altering glycolytic and gluconeogenic enzymes. J. Biochem. Mol. Toxicol. 2006, 20, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Latha, M.; Pari, L. Antihyperglycaemic effect of Cassia auriculata in experimental diabetes and its effects on key metabolic enzymes involved in carbohydrate metabolism. Clin. Exp. Pharmacol. Physiol. 2003, 30, 38–43. [Google Scholar] [CrossRef]
- Kanadi, M.A.; Alhassan, A.J.; Yaradua, A.I.; Nasir, A.; Wudil, A.M. Sub-fractions from Carica Papaya Seed Extracts Can Prevent Potassium Bromate-induced Changes in Activities of Renal Brush Border Membrane Enzymes and Some Enzymes of Carbohydrate Metabolism in the Kidney of Rats. Asian J. Biochem. Genet. Mol. 2021, 8, 1–9. [Google Scholar] [CrossRef]
- Oyenihi, A.B.; Langa, S.O.P.; Mukaratirwa, S.; Masola, B. Effects of Centella asiatica on skeletal muscle structure and key enzymes of glucose and glycogen metabolism in type 2 diabetic rats. Biomed. Pharmacother. 2019, 112, 108715. [Google Scholar] [CrossRef]
- Kalaivani, K.; Sankaranarayanan, C. Modulatory effect of isopulegol on hepatic key enzymes of glucose metabolism in high-fat diet/streptozotocin-induced diabetic rats. Arch. Physiol. Biochem. 2021, 127, 318–326. [Google Scholar] [CrossRef]
- Jayachandran, M.; Zhang, T.; Ganesan, K.; Xu, B.; Chung, S.S.M. Isoquercetin ameliorates hyperglycemia and regulates key enzymes of glucose metabolism via insulin signaling pathway in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2018, 82, 112–120. [Google Scholar] [CrossRef]
- Pari, L.; Rajarajeswari, N. Efficacy of coumarin on hepatic key enzymes of glucose metabolism in chemical induced type 2 diabetic rats. Chem.-Biol. Interact. 2009, 181, 292–296. [Google Scholar] [CrossRef]
- Gothandam, K.; Ganesan, V.S.; Ayyasamy, T.; Ramalingam, S. Antioxidant potential of theaflavin ameliorates the activities of key enzymes of glucose metabolism in high fat diet and streptozotocin–induced diabetic rats. Redox Rep. 2019, 24, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juárez-Rojop, I.E.; Tovilla-Zárate, C.A.; Aguilar-Domínguez, D.E.; Lobato-García, C.E.; Blé-Castillo, J.L.; López-Meraz, L.; Díaz-Zagoya, J.C.; Bermúdez-Ocaña, D.Y. Phytochemical screening and hypoglycemic activity of Carica papaya leaf in streptozotocin-induced diabetic rats. Rev. Bras. Farmacogn. 2014, 24, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Peng, S.; Chen, F.; Liu, L.; Li, Z.; Zeng, G.; Huang, Q. Protective effects of pioglitazone on vascular endothelial cell dysfunction induced by high glucose via inhibition of IKKα/β–NFκB signaling mediated by PPARγ in vitro. Can. J. Physiol. Pharmacol. 2017, 95, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Copps, K.D.; White, M.F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef] [Green Version]
- Chuang, W.T.; Yen, C.C.; Huang, C.S.; Chen, H.W.; Lii, C.K. Benzyl Isothiocyanate Ameliorates High-Fat Diet-Induced Hyperglycemia by Enhancing Nrf2-Dependent Antioxidant Defense-Mediated IRS-1/AKT/TBC1D1 Signaling and GLUT4 Expression in Skeletal Muscle. J. Agric. Food Chem. 2020, 68, 15228–15238. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of skeletal muscle in insulin resistance and glucose uptake. Compr. Physiol. 2011, 10, 785–809. [Google Scholar]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xu, W.; Huang, X.; Zhao, Y.; Ren, Q.; Hong, Z.; Huang, M.; Xing, X. Fucoxanthin ameliorates hyperglycemia, hyperlipidemia and insulin resistance in diabetic mice partially through IRS-1/PI3K/Akt and AMPK pathways. J. Funct. Foods 2018, 48, 515–524. [Google Scholar] [CrossRef]
- Cai, S.; Sun, W.; Fan, Y.; Guo, X.; Xu, G.; Xu, T.; Hou, Y.; Zhao, B.; Feng, X.; Liu, T. Effect of mulberry leaf (Folium Mori) on insulin resistance via IRS-1/PI3K/Glut-4 signalling pathway in type 2 diabetes mellitus rats. Pharm. Biol. 2016, 54, 2685–2691. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Sun, W.; Luo, G.; Wu, L.; Xu, G.; Hou, D.; Hou, Y.; Guo, X.; Mu, X.; Qin, L.; et al. Panax notoginseng saponins alleviate skeletal muscle insulin resistance by regulating the IRS 1–PI 3K–AKT signaling pathway and GLUT 4 expression. FEBS Open Bio 2019, 9, 1008–1019. [Google Scholar] [CrossRef] [Green Version]
- Jung, T.W.; Kim, H.C.; Kim, H.U.; Park, T.; Park, J.; Kim, U.; Kim, M.K.; Jeong, J.H. Asprosin attenuates insulin signaling pathway through PKCδ-activated ER stress and inflammation in skeletal muscle. J. Cell. Physiol. 2019, 234, 20888–20899. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Badrachalam, R.; Shanmugam, S.N.; Balraj, M.; Kasthuri, R.; Danavel, A.; Babu, S. Effect of β-Caryophyllene on insulin resistance in skeletal muscle of high fat diet and fructose-induced type-2 diabetic rats. Bioinformation 2021, 17, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nishina, P.M.; Naggert, J.K. Degradation of IRS1 leads to impaired glucose uptake in adipose tissue of the type 2 diabetes mouse model TALLYHO/Jng. J. Endocrinol. 2009, 203, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yu, L.; Zhao, C. Dioscin attenuates high fat diet induced insulin resistance of adipose tissue through the IRS 1/PI3K/Akt signaling pathway. Mol. Med. Rep. 2019, 19, 1230–1237. [Google Scholar] [CrossRef] [PubMed]

| S. No | Gene Name | Primer Sequence | Reference |
|---|---|---|---|
| 1 | Rat βactin | Sense primer: 5′-AAG TCC CTC ACC CTC CCA AAA G-3′ Antisense primer: 5′-AAG CAA TGC TGT CAC CTT CCC-3′ | [35] |
| 2 | IRS-1 | Sense primer: 5′-GCC AAT CTT CAT CCA GTT GCT-3′ Antisense primer: 5′-CAT CGT GAA GAA GGC ATA GGG-3 | [36] |
| 3 | Akt | Sense primer: 5′-GGA AGC CTT CAG TTT GGA TCC CAA-3′ Antisense primer: 5′-AGT GGA AAT CCA GTT CCG AGC TTG-3′ | [37] |
| S. No. | Compound Name |
|---|---|
| 1 | Caffeic_acid |
| 2 | Chlorogenic_acid |
| 3 | Kaempferol |
| 4 | Quercetin |
| 5 | Rutin |
| 6 | p-coumaric_acid |
| 7 | trans-ferulic_acid |
| S. No | Compound Name | Lig Score1_Drei Ding | Lig Score2_Drei Ding | PLP 1 | PLP 2 | JAIN | PMF | Docking Score |
|---|---|---|---|---|---|---|---|---|
| IK3A | ||||||||
| 1 | Trans-ferulic acid | 1.64 | 3.37 | 38.93 | 36.6 | −1.2 | 34.9 | 37.161 |
| 2 | Quercetin | 2.69 | 3.56 | 52.33 | 58.2 | −0.84 | 52.63 | 49.741 |
| 3 | Kaempferol | 0.32 | 1.75 | 52.03 | 65.41 | 0.75 | 67.22 | 49.413 |
| 4 | Rutin | 3.33 | 4.24 | 109.67 | 113.31 | 1.14 | 73.52 | 103.327 |
| 5 | p-coumaric acid | No interaction | ||||||
| 6 | Chlorogenic acid | 3.96 | 4.6 | 75.2 | 75.63 | −0.37 | 64.06 | 71.235 |
| 7 | Protocatechuic acid | No interaction | ||||||
| 8 | Caffeic acid | No interaction | ||||||
| 3QKM | ||||||||
| 1 | Trans-ferulic acid | 1.02 | 0.14 | 64.19 | 71.24 | 2.36 | −8.12 | 58.136 |
| 2 | Quercetin | −18.41 | −31.47 | 9.64 | 54.3 | 5.87 | −37.75 | 0.656 |
| 3 | Kaempferol | −16.08 | −28.66 | 12.82 | 52.39 | 6.57 | −27.04 | 4.939 |
| 4 | Rutin | No interaction | ||||||
| 5 | p-coumaric acid | 0.26 | −0.99 | 55.11 | 62.57 | 3.43 | 7.61 | 50.999 |
| 6 | Chlorogenic acid | No interaction | ||||||
| 7 | Protocatechuic acid | No interaction | ||||||
| 8 | Caffeic acid | −2.19 | −4.55 | 54.21 | 59.96 | 3.52 | −2.24 | 51.777 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, J.R.; Janaki, C.S.; Jayaraman, S.; Periyasamy, V.; Balaji, T.; Vijayamalathi, M.; Veeraraghavan, V.P. Effect of Carica papaya on IRS-1/Akt Signaling Mechanisms in High-Fat-Diet–Streptozotocin-Induced Type 2 Diabetic Experimental Rats: A Mechanistic Approach. Nutrients 2022, 14, 4181. https://doi.org/10.3390/nu14194181
Roy JR, Janaki CS, Jayaraman S, Periyasamy V, Balaji T, Vijayamalathi M, Veeraraghavan VP. Effect of Carica papaya on IRS-1/Akt Signaling Mechanisms in High-Fat-Diet–Streptozotocin-Induced Type 2 Diabetic Experimental Rats: A Mechanistic Approach. Nutrients. 2022; 14(19):4181. https://doi.org/10.3390/nu14194181
Chicago/Turabian StyleRoy, Jeane Rebecca, Coimbatore Sadagopan Janaki, Selvaraj Jayaraman, Vijayalakshmi Periyasamy, Thotakura Balaji, Madhavan Vijayamalathi, and Vishnu Priya Veeraraghavan. 2022. "Effect of Carica papaya on IRS-1/Akt Signaling Mechanisms in High-Fat-Diet–Streptozotocin-Induced Type 2 Diabetic Experimental Rats: A Mechanistic Approach" Nutrients 14, no. 19: 4181. https://doi.org/10.3390/nu14194181
APA StyleRoy, J. R., Janaki, C. S., Jayaraman, S., Periyasamy, V., Balaji, T., Vijayamalathi, M., & Veeraraghavan, V. P. (2022). Effect of Carica papaya on IRS-1/Akt Signaling Mechanisms in High-Fat-Diet–Streptozotocin-Induced Type 2 Diabetic Experimental Rats: A Mechanistic Approach. Nutrients, 14(19), 4181. https://doi.org/10.3390/nu14194181