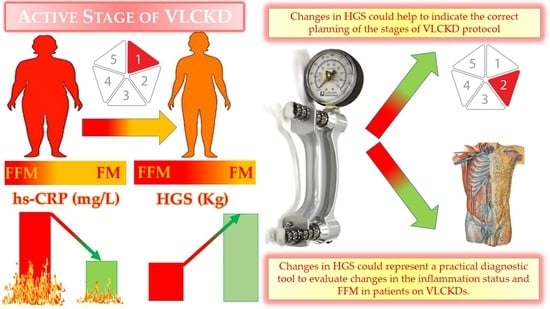
Impact of a Very Low-Calorie Ketogenic Diet (VLCKD) on Changes in Handgrip Strength in Women with Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Population Study
- Underweight, normal-weight, and overweight subjects;
- Male gender;
- Menopausal women, defined with at least 12 months of amenorrhea or LH levels > 30 IU/L;
- Pregnancy or breastfeeding;
- Type 1 and T2DM;
- Smokers;
- Women taking anti-inflammatory or anti-obesity drugs one month before enrollment in the study or women occasionally or currently taking medications that could influence musculoskeletal system and HGS, including anticonvulsants, steroidal and non-steroidal anti-inflammatory drugs, anti-thyroid agents, dopaminergic drugs, Parkinson’s disease drugs, or statins;
- Previous treatment with bariatric surgery;
- Clinical conditions such as immune, inflammatory, endocrine, or musculoskeletal diseases; liver or kidney failure; or neoplasms that could affect fluid balance, body composition, or HGS (ascertained after complete medical examination and appropriate laboratory tests);
- Subjects with implanted pacemakers or defibrillators (because of the potentially negative influence on the bioelectrical impedance analysis (BIA) device);
- Skin injury at the sites of electrode placement;
- Individuals unable to perform the HGS test due to conditions including osteoarticular or musculoskeletal diseases, pain, stroke, or hand injury.
- In addition, participants were evaluated at baseline, in the early follicular phase.
2.3. Handgrip Strength Test
2.4. Anthropometric Measurements
- Overweight (25.0–29.9 kg/m2);
- Obesity grade I (30.0–34.9 kg/m2);
- Obesity grade II (35.0–39.9 kg/m2);
- Obesity grade III (≥40.0 kg/m2).
2.5. VLCKD Intervention
2.6. Bioelectrical Impedance Analysis
2.7. Compliance to VLCKD
2.8. Assay Methods
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VLCKDs | very low-calorie ketogenic diets |
| EASO | European Association for the Study of Obesity |
| SIE | Italian Society of Endocrinology |
| T2DM | type 2 diabetes mellitus |
| NAFLD | non-alcoholic fatty liver disease |
| NYHA | New York Heart Association |
| PCOS | polycystic ovary syndrome |
| SGLT | sodium-dependent glucose cotransporters |
| KDs | ketogenic diets |
| HGS | handgrip strength |
| hs-CRP | high-sensitivity C-reactive protein |
| BMI | body mass index |
| BIA | bioelectrical impedance analysis |
| WC | waist circumference |
| ESPEN | European Society of Parenteral and Enteral Nutrition |
| R | resistance |
| Xc | reactance |
| CV | coefficient of variation |
| PhA | phase angle |
| SD | standard deviation |
| FM | fat mass |
| FFM | free fat mass |
References
- Barrea, L.; Muscogiuri, G.; Aprano, S.; Vetrani, C.; de Alteriis, G.; Varcamonti, L.; Verde, L.; Colao, A.; Savastano, S. Phase angle as an easy diagnostic tool for the nutritionist in the evaluation of inflammatory changes during the active stage of a very low-calorie ketogenic diet. Int. J. Obes. 2022, 46, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Caprio, M.; Watanabe, M.; Cammarata, G.; Feraco, A.; Muscogiuri, G.; Verde, L.; Colao, A.; Savastano, S.; on behalf of Obesity Programs of nutrition, Education, Research and Assessment (OPERA) Group. Could very low-calorie ketogenic diets turn off low grade inflammation in obesity? Emerging evidence. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Casanueva, F.F.; Castellana, M.; Bellido, D.; Trimboli, P.; Castro, A.I.; Sajoux, I.; Rodriguez-Carnero, G.; Gomez-Arbelaez, D.; Crujeiras, A.B.; Martinez-Olmos, M.A. Ketogenic diets as treatment of obesity and type 2 diabetes mellitus. Rev. Endocr. Metab. Disord. 2020, 21, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Castellana, M.; Conte, E.; Cignarelli, A.; Perrini, S.; Giustina, A.; Giovanella, L.; Giorgino, F.; Trimboli, P. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 5–16. [Google Scholar] [CrossRef]
- Muscogiuri, G.; El Ghoch, M.; Colao, A.; Hassapidou, M.; Yumuk, V.; Busetto, L. European Guidelines for Obesity Management in Adults with a Very Low-Calorie Ketogenic Diet: A Systematic Review and Meta-Analysis. Obes. Facts 2021, 14, 222–245. [Google Scholar] [CrossRef]
- Caprio, M.; Infante, M.; Moriconi, E.; Armani, A.; Fabbri, A.; Mantovani, G.; Mariani, S.; Lubrano, C.; Poggiogalle, E.; Migliaccio, S.; et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: Systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2019, 42, 1365–1386. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Salzano, C.; Savastano, S.; Colao, A. The management of very low-calorie ketogenic diet in obesity outpatient clinic: A practical guide. J. Transl. Med. 2019, 17, 356. [Google Scholar] [CrossRef] [Green Version]
- Barrea, L.; Verde, L.; Vetrani, C.; Marino, F.; Aprano, S.; Savastano, S.; Colao, A.; Muscogiuri, G. VLCKD: A real time safety study in obesity. J. Transl. Med. 2022, 20, 23. [Google Scholar] [CrossRef]
- Haensch, R. Bowenoid papulosis of the genito-anal region. Z. Hautkrankh. 1986, 61, 569–571. [Google Scholar]
- Hernandez, A.; Truckenbrod, L.; Federico, Q.; Campos, K.; Moon, B.; Ferekides, N.; Hoppe, M.; D’Agostino, D.; Burke, S. Metabolic switching is impaired by aging and facilitated by ketosis independent of glycogen. Aging 2020, 12, 7963. [Google Scholar] [CrossRef]
- Monda, V.; Polito, R.; Lovino, A.; Finaldi, A.; Valenzano, A.; Nigro, E.; Corso, G.; Sessa, F.; Asmundo, A.; Di Nunno, N.; et al. Short-term physiological effects of a very low-calorie ketogenic diet: Effects on adiponectin levels and inflammatory states. Int. J. Mol. Sci. 2020, 21, 3558. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Cancellara, P.; Pompei, P.; Moro, T. Ketogenic diet and skeletal muscle hypertrophy: A Frenemy relationship? J. Hum. Kinet. 2019, 68, 233–247. [Google Scholar] [CrossRef] [Green Version]
- Rondanelli, M.; Gasparri, C.; Peroni, G.; Faliva, M.A.; Naso, M.; Perna, S.; Bazire, P.; Sajuox, I.; Maugeri, R.; Rigon, C. The Potential Roles of Very Low Calorie, Very Low Calorie Ketogenic Diets and Very Low Carbohydrate Diets on the Gut Microbiota Composition. Front. Endocrinol. 2021, 12, 662591. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Faliva, M.A.; Gasparri, C.; Peroni, G.; Spadaccini, D.; Maugeri, R.; Nichetti, M.; Infantino, V.; Perna, S. Current opinion on dietary advice in order to preserve fat-free mass during a low-calorie diet. Nutrition 2020, 72, 110667. [Google Scholar] [CrossRef] [PubMed]
- Merra, G.; Gratteri, S.; De Lorenzo, A.; Barrucco, S.; Perrone, M.A.; Avolio, E.; Bernardini, S.; Marchetti, M.; Di Renzo, L. Effects of very-low-calorie diet on body composition, metabolic state, and genes expression: A randomized double-blind placebo-controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 329–345. [Google Scholar]
- Chen, L.; Nelson, D.R.; Zhao, Y.; Cui, Z.; Johnston, J.A. Relationship between muscle mass and muscle strength, and the impact of comorbidities: A population-based, Cross-sectional study of older adults in the United States. BMC Geriatr. 2013, 13, 74. [Google Scholar] [CrossRef] [Green Version]
- Reed, R.L.; Pearlmutter, L.; Yochum, K.; Meredith, K.E.; Mooradian, A.D. The Relationship between Muscle Mass and Muscle Strength in the Elderly. J. Am. Geriatr. Soc. 1991, 39, 555–561. [Google Scholar] [CrossRef]
- Hayashida, I.; Tanimoto, Y.; Takahashi, Y.; Kusabiraki, T.; Tamaki, J. Correlation between muscle strength and muscle mass, and their association with walking speed, in community-dwelling elderly Japanese individuals. PLoS ONE 2014, 9, e111810. [Google Scholar] [CrossRef]
- Porto, J.M.; Nakaishi, A.P.M.; Cangussu-Oliveira, L.M.; Freire Júnior, R.C.; Spilla, S.B.; de Abreu, D.C.C. Relationship between grip strength and global muscle strength in community-dwelling older people. Arch. Gerontol. Geriatr. 2019, 82, 273–278. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Tuttle, C.S.L.; Thang, L.A.N.; Maier, A.B. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res. Rev. 2020, 64, 101185. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Di Somma, C.; Annunziata, G.; Megna, M.; Falco, A.; Balato, A.; Colao, A.; Savastano, S. Coffee consumption, metabolic syndrome and clinical severity of psoriasis: Good or bad stuff? Arch. Toxicol. 2018, 92, 1831–1845. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Tarantino, G.; Somma, C.D.; Muscogiuri, G.; Macchia, P.E.; Falco, A.; Falco, A.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet and Circulating Levels of Sirtuin 4 in Obese Patients: A Novel Association. Oxidative Med. Cell. Longev. 2017, 2017, 6101254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrea, L.; Muscogiuri, G.; Di Somma, C.; Tramontano, G.; De Luca, V.; Illario, M.; Colao, A.; Savastano, S. Association between Mediterranean diet and hand grip strength in older adult women. Clin. Nutr. 2018, 38, 721–729. [Google Scholar] [CrossRef]
- Fess, E.E. Clinical Assessment Recommendations, Grip Strength, 3rd ed.; Clinical Assessment Recommendations; American Society of Hand Therapists: Chicago, IL, USA, 1992. [Google Scholar]
- Fernandes, S.G.G.; De Andrade, L.E.L.; Dos Santos Aguiar Gonçalves, R.S.; Da Câmara1, S.M.A.; Guerra, R.O.; MacIel, A.C.C. Cut-off points to screening for sarcopenia in community-dwelling older people residents in brazil. PeerJ 2021, 9, e12038. [Google Scholar] [CrossRef]
- Barrea, L.; Di Somma, C.; Macchia, P.E.; Falco, A.; Savanelli, M.C.; Orio, F.; Colao, A.; Savastano, S. Influence of nutrition on somatotropic axis: Milk consumption in adult individuals with moderate-severe obesity. Clin. Nutr. 2015, 36, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Muscogiuri, G.; Barrea, L.; Aprano, S.; Framondi, L.; Di Matteo, R.; Laudisio, D.; Pugliese, G.; Savastano, S.; Colao, A.; on behalf of the OPERA PREVENTION Project. Sleep Quality in Obesity: Does Adherence to the Mediterranean Diet Matter? Nutrients 2020, 12, 1364. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Aprano, S.; Framondi, L.; Di Matteo, R.; Laudisio, D.; Pugliese, G.; Savastano, S.; Colao, A. Chronotype and adherence to the mediterranean diet in obesity: Results from the opera prevention project. Nutrients 2020, 12, 1354. [Google Scholar] [CrossRef]
- WHO|World Health Organization. Body Mass Index—BMI. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 15 March 2021).
- National Center for Health Statistics. No Title. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/Anthropometry_Procedures_Manual.pdf (accessed on 21 May 2020).
- Yanovski, S.Z.; Hubbard, V.S.; Heymsfield, S.B.; Lukaski, H.C. Bioelectrical impedance analysis in body composition measurement: National Institutes of Health Technology Assessment Conference statement. Am. J. Clin. Nutr. 1996, 64, 524S–532S. [Google Scholar]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Laudisio, D.; Di Somma, C.; Salzano, C.; Pugliese, G.; de Alteriis, G.; Colao, A.; Savastano, S. Phase angle: A possible biomarker to quantify inflammation in subjects with obesity and 25(OH)D deficiency. Nutrients 2019, 11, 1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Di Somma, C.; Pugliese, G.; Salzano, C.; Colao, A.; Savastano, S. Somatotropic axis and obesity: Is there any role for the Mediterranean diet? Nutrients 2019, 11, 2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrea, L.; Muscogiuri, G.; Pugliese, G.; Laudisio, D.; de Alteriis, G.; Graziadio, C.; Colao, A.; Savastano, S. Phase Angle as an Easy Diagnostic Tool of Meta-Inflammation for the Nutritionist. Nutrients 2021, 13, 1446. [Google Scholar] [CrossRef] [PubMed]
- Bera, T.K. Bioelectrical impedance methods for noninvasive health monitoring: A review. J. Med. Eng. 2014, 2014, 381251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paoli, A.; Mancin, L.; Giacona, M.C.; Bianco, A.; Caprio, M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J. Transl. Med. 2020, 18, 104. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, C.E.; Phinney, S.D.; Fernandez, M.L.; Quann, E.E.; Wood, R.J.; Bibus, D.M.; Kraemer, W.J.; Feinman, R.D.; Volek, J.S. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids 2008, 43, 65–77. [Google Scholar] [CrossRef]
- Ilyas, Z.; Perna, S.; Alalwan, T.A.; Zahid, M.N.; Spadaccini, D.; Gasparri, C.; Peroni, G.; Faragli, A.; Alogna, A.; La Porta, E.; et al. The Ketogenic Diet: Is It an Answer for Sarcopenic Obesity? Nutrients 2022, 14, 620. [Google Scholar] [CrossRef]
- Watanabe, M.; Tozzi, R.; Risi, R.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Spera, G.; Lubrano, C.; Gnessi, L. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes. Rev. 2020, 21, e13024. [Google Scholar] [CrossRef] [Green Version]
- Barrea, L.; Caprio, M.; Camajani, E.; Verde, L.; Elce, A.; Frias-Toral, E.; Ceriani, F.; Cucalón, G.; Garcia-Velasquez, E.; El Ghoch, M.; et al. Clinical and nutritional management of very-low-calorie ketogenic diet (VLCKD) in patients with psoriasis and obesity: A practical guide for the nutritionist. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef]
- Camajani, E.; Feraco, A.; Basciani, S.; Gnessi, L.; Barrea, L.; Armani, A.; Caprio, M. VLCKD in Combination with Physical Exercise Preserves Skeletal Muscle Mass in Sarcopenic Obesity after Severe COVID-19 Disease: A Case Report. Healthcare 2022, 10, 573. [Google Scholar] [CrossRef]
- Kunz, H.E.; Michie, K.L.; Gries, K.J.; Zhang, X.; Ryan, Z.C.; Lanza, I.R. A Randomized Trial of the Effects of Dietary n3-PUFAs on Skeletal Muscle Function and Acute Exercise Response in Healthy Older Adults. Nutrients 2022, 14, 3537. [Google Scholar] [CrossRef] [PubMed]
- Salucci, S.; Bartoletti-Stella, A.; Bavelloni, A.; Aramini, B.; Blalock, W.L.; Fabbri, F.; Vannini, I.; Sambri, V.; Stella, F.; Faenza, I. Extra Virgin Olive Oil (EVOO), a Mediterranean Diet Component, in the Management of Muscle Mass and Function Preservation. Nutrients 2022, 14, 3567. [Google Scholar] [CrossRef] [PubMed]
- Merra, G.; Miranda, R.; Barrucco, S.; Gualtieri, P.; Mazza, M.; Moriconi, E.; Marchetti, M.; Chang, T.F.M.; De Lorenzo, A.; Di Renzo, L. Very-low-calorie ketogenic diet with aminoacid supplement versus very low restricted-calorie diet for preserving muscle mass during weight loss: A pilot double-blind study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2613–2621. [Google Scholar] [PubMed]
- Nunes, E.A.; Colenso-Semple, L.; McKellar, S.R.; Yau, T.; Ali, M.U.; Fitzpatrick-Lewis, D.; Sherifali, D.; Gaudichon, C.; Tomé, D.; Atherton, P.J.; et al. Systematic review and meta-analysis of protein intake to support muscle mass and function in healthy adults. J. Cachexia Sarcopenia Muscle 2022, 13, 795–810. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Khandelwal, D.; Kalra, S.; Gupta, P.; Dutta, D.; Aggarwal, S. Ketogenic diet in endocrine disorders: Current perspectives. J. Postgrad. Med. 2017, 63, 242. [Google Scholar] [PubMed]
- Zhu, H.; Bi, D.; Zhang, Y.; Kong, C.; Du, J.; Wu, X.; Wei, Q.; Qin, H. Ketogenic diet for human diseases: The underlying mechanisms and potential for clinical implementations. Signal Transduct. Target. Ther. 2022, 7, 11. [Google Scholar] [CrossRef]
- Deru, L.; Warren, C.; Wright, B.; Coleman, J.; Duersch, A.; Chan, H.; Hales, K.; Bailey, B.; Bikman, B. Understanding the endocrine response to macronutrients in the context of a ketogenic diet. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Devesa, J. The Complex World of Regulation of Pituitary Growth Hormone Secretion: The Role of Ghrelin, Klotho, and Nesfatins in It. Front. Endocrinol. 2021, 12, 636403. [Google Scholar] [CrossRef]
- Moreno, B.; Bellido, D.; Sajoux, I.; Goday, A.; Saavedra, D.; Crujeiras, A.B.; Casanueva, F.F. Comparison of a very low-calorie-ketogenic diet with a standard low-calorie diet in the treatment of obesity. Endocrine 2014, 47, 793–805. [Google Scholar] [CrossRef]
- Nimptsch, K.; Konigorski, S.; Pischon, T. Diagnosis of obesity and use of obesity biomarkers in science and clinical medicine. Metab. Clin. Exp. 2019, 92, 61–70. [Google Scholar] [CrossRef]



| VERY LOW-CALORIE KETOGENIC DIETS (VLCKDS) | |
|---|---|
| STRONG RECOMMENDATIONS | CONTRAINDICATIONS |
| Severe obesity | Pregnancy and breastfeeding kidney failure |
| Severe obesity before bariatric surgery | Moderate-to-severe chronic kidney disease |
| Sarcopenic obesity | Liver failure |
Obesity associated with:
| Rare disorders: porphyria, carnitine deficiency, carnitine palmitoyltransferase deficiency, carnitine-acylcarnitine translocase deficiency, mitochondrial fatty acid β-oxidation disorders, and pyruvate carboxylase deficiency |
| Pediatric obesity associated with epilepsy and/or with a high level of insulin resistance and/or comorbidities, not responsive to standardized diet | Respiratory failure unstable angina, hearth failure (NYHA III–IV), recent stroke or myocardial infarction (<12 months), and cardiac arrhythmias |
WEAK RECOMMENDATIONS
| Eating disorders and other severe mental illnesses, alcohol, and substance abuse |
| Type 1 diabetes mellitus, latent autoimmune diabetes in adults, β-cell failure in T2DM, use of SGLT2 inhibitors | |
| Active/severe infections and frail elderly patients, | |
| 48 h prior to elective surgery or invasive procedures and perioperative period | |
| Parameters | Participant Baseline Mean ± SD or Number (%) n = 247 | Participant 45th Day of VLCKD Mean ± SD or Number (%) n = 247 | ∆% | * p-Value |
|---|---|---|---|---|
| Weight (kg) | 99.4 ± 15.2 | 91.9 ± 14.3 | −7.5 ± 3.1 | <0.001 |
| BMI (kg/m2) | 37.3 ± 4.5 | 34.5 ± 4.3 | <0.001 | |
| Overweight (n, %) | - | 46, 18.6% | 18.6% | χ2 = 48.54, p < 0.001 |
| Grade I obesity (n, %) | 88, 35.6% | 94, 38.1% | 2.5% | χ2 = 0.22, p = 0.641 |
| Grade II obesity (n, %) | 94, 38.1% | 77, 31.2% | −6.9% | χ2 = 2.29, p = 0.130 |
| Grade III obesity (n, %) | 65, 26.3% | 30, 12.1% | −14.2% | χ2 = 15.07, p < 0.001 |
| WC (cm) | 108.0 ± 14.5 | 101.1 ± 13.8 | −6.3 ± 5.0 | <0.001 |
| WC < 88 cm | 18, 7.3% | 45, 18.2% | −10.9% | χ2 = 12.30, p < 0.001 |
| WC ≥ 88 cm | 229, 92.7% | 202, 81.8% | ||
| HGS (kg) | ||||
| <16 kg | 36, 14.6% | 9, 3.6% | +11.0% | χ2 = 16.53, p < 0.001 |
| ≥16 kg | 211, 85.4% | 238, 96.4% | ||
| Physical Activity | ||||
| Yes | 78, 31.6% | 78, 31.6% | 0% | χ2 = 0.00, p = 0.922 |
| No | 169, 68.4% | 169, 68.4% | ||
| BIA parameters | ||||
| R (Ω) | 468.8 ± 70.5 | 472.8 ± 64.4 | +1.4 ± 9.5 | 0.129 |
| Xc (Ω) | 45.7 ± 9.5 | 49.8 ± 9.8 | 10.3 ± 16.2 | <0.001 |
| FM (kg) | 44.5 ± 12.5 | 37.6 ± 11.5 | −15.6 ± 9.0 | <0.001 |
| FFM (kg) | 54.9 ± 5.7 | 54.4 ± 5.7 | −0.9 ± 4.5 | 0.001 |
| hs-CRP levels (mg/L) | 3.8 ± 4.3 | 1.9 ± 2.6 | −39.9 ± 44.6 | <0.001 |
| Participant Baseline n = 247 | |||
|---|---|---|---|
| Parameters | HGS <16 kg Mean ± SD n = 36 | HGS ≥16 kg Mean ± SD n = 211 | * p-Value |
| Age (years) | 36.7 ± 9.1 | 35.2 ± 10.7 | 0.424 |
| Weight (kg) | 111.0 ± 17.9 | 97.4 ± 13.7 | <0.001 |
| BMI (kg/m2) | 43.1 ± 3.7 | 36.3 ± 3.8 | <0.001 |
| WC (cm) | 119.1 ± 12.4 | 106.1 ± 14.0 | <0.001 |
| BIA parameters | |||
| R (Ω) | 494.2 ± 85.9 | 464.4 ± 66.8 | 0.019 |
| Xc (Ω) | 43.4 ± 8.9 | 46.1 ± 9.5 | 0.111 |
| FM (kg) | 57.9 ± 14.8 | 42.2 ± 10.5 | 0.018 |
| FFM (kg) | 53.1 ± 4.6 | 55.2 ± 5.8 | <0.001 |
| hs-CRP levels (mg/L) | 6.4 ± 6.8 | 3.3 ± 3.2 | 0.017 |
| Parameters | R | * p-Value |
|---|---|---|
| Age | −0.068 | 0.290 |
| ∆BMI (%) | −0.356 | <0.001 |
| ∆WC (%) | −0.176 | 0.006 |
| ∆R (%) | −0.334 | <0.001 |
| ∆Xc (%) | 0.051 | 0.425 |
| ∆FM (%) | −0.432 | <0.001 |
| ∆FFM (%) | 0.320 | <0.001 |
| ∆hs-CRP levels (%) | −0.171 | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrea, L.; de Alteriis, G.; Muscogiuri, G.; Vetrani, C.; Verde, L.; Camajani, E.; Aprano, S.; Colao, A.; Savastano, S. Impact of a Very Low-Calorie Ketogenic Diet (VLCKD) on Changes in Handgrip Strength in Women with Obesity. Nutrients 2022, 14, 4213. https://doi.org/10.3390/nu14194213
Barrea L, de Alteriis G, Muscogiuri G, Vetrani C, Verde L, Camajani E, Aprano S, Colao A, Savastano S. Impact of a Very Low-Calorie Ketogenic Diet (VLCKD) on Changes in Handgrip Strength in Women with Obesity. Nutrients. 2022; 14(19):4213. https://doi.org/10.3390/nu14194213
Chicago/Turabian StyleBarrea, Luigi, Giulia de Alteriis, Giovanna Muscogiuri, Claudia Vetrani, Ludovica Verde, Elisabetta Camajani, Sara Aprano, Annamaria Colao, and Silvia Savastano. 2022. "Impact of a Very Low-Calorie Ketogenic Diet (VLCKD) on Changes in Handgrip Strength in Women with Obesity" Nutrients 14, no. 19: 4213. https://doi.org/10.3390/nu14194213
APA StyleBarrea, L., de Alteriis, G., Muscogiuri, G., Vetrani, C., Verde, L., Camajani, E., Aprano, S., Colao, A., & Savastano, S. (2022). Impact of a Very Low-Calorie Ketogenic Diet (VLCKD) on Changes in Handgrip Strength in Women with Obesity. Nutrients, 14(19), 4213. https://doi.org/10.3390/nu14194213