The Effect of Circulating Zinc, Selenium, Copper and Vitamin K1 on COVID-19 Outcomes: A Mendelian Randomization Study
Abstract
:1. Introduction
2. Material & Methods
2.1. Selection of Genetic Instruments—Relevance MR Criterion
2.1.1. GWAS Studies
2.1.2. Zinc Genetic Instruments
2.1.3. Selenium Genetic Instruments
2.1.4. Copper Genetic Instruments
2.1.5. Vitamin K1 Genetic Instruments
2.1.6. Sensitivity Analyses Using Subsignificant Hits
2.1.7. Independence MR Criterion
2.1.8. Exclusion Restriction MR Criterion
2.1.9. Selection of Outcomes
2.1.10. Statistical Analysis
2.1.11. Ethics Statement
3. Results
3.1. Power Analysis
3.2. Mendelian Randomization
3.2.1. Zinc
3.2.2. Selenium
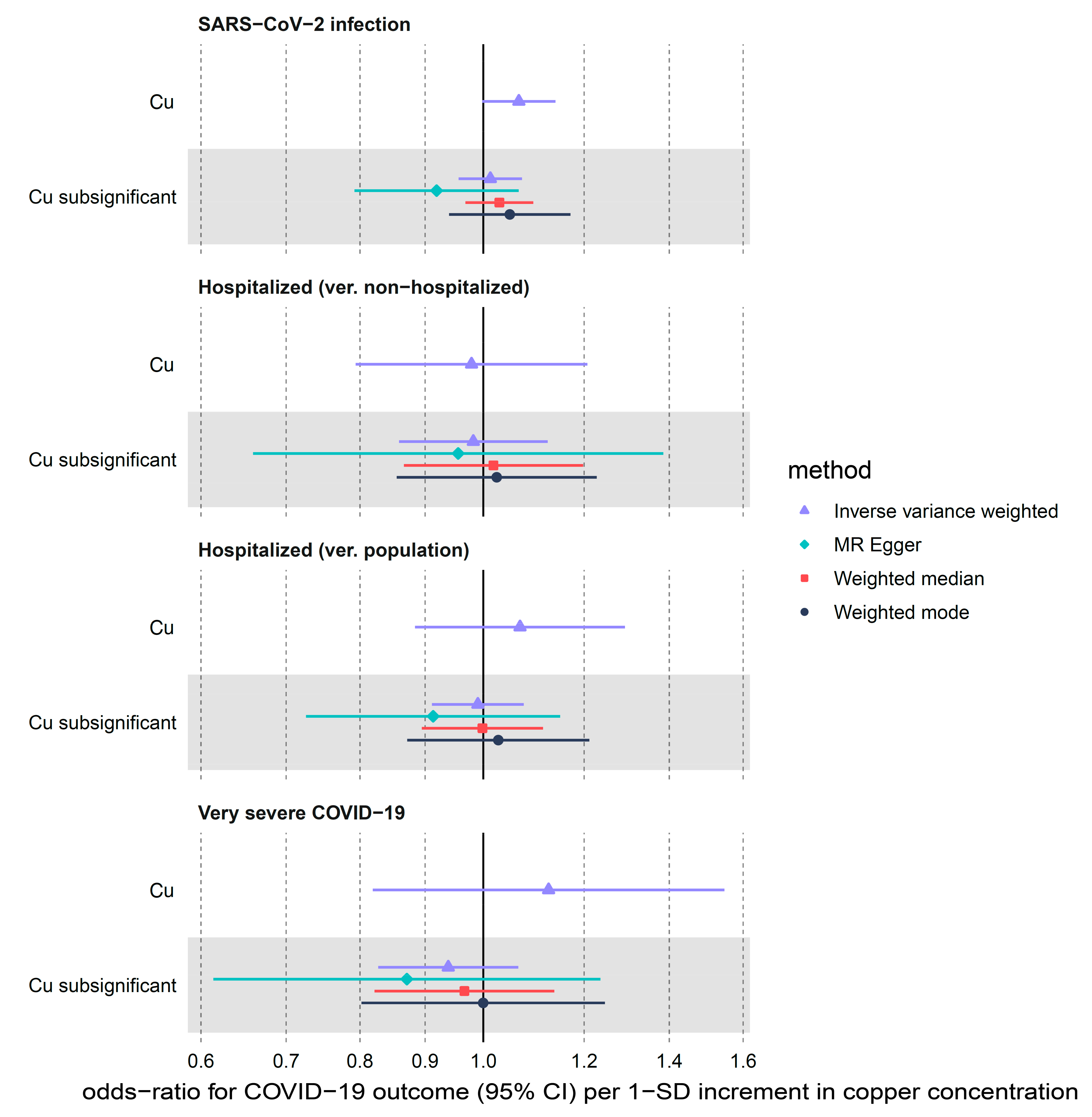
3.2.3. Copper
3.2.4. Vitamin K1
3.2.5. Pleiotropic Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Siemieniuk, R.A.C.; Bartoszko, J.J.; Ge, L.; Zeraatkar, D.; Izcovich, A.; Kum, E.; Pardo-Hernandez, H.; Qasim, A.; Martinez, J.P.D.; Rochwerg, B.; et al. Drug treatments for COVID-19: Living systematic review and network meta-analysis. BMJ 2020, 370, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bartoszko, J.J.; Siemieniuk, R.A.C.; Kum, E.; Qasim, A.; Zeraatkar, D.; Ge, L.; Han, M.A.; Sadeghirad, B.; Agarwal, A.; Agoritsas, T.; et al. Prophylaxis against COVID-19: Living systematic review and network meta-analysis. BMJ 2021, 373. [Google Scholar] [CrossRef] [PubMed]
- WHO Africa. Africa Faces 470 Million COVID-19 Vaccine Shortfall in 2021. Available online: https://www.afro.who.int/news/africa-faces-470-million-COVID-19-vaccine-shortfall-2021 (accessed on 16 September 2021).
- Akhtar, S.; Das, J.K.; Ismail, T.; Wahid, M.; Saeed, W.; Bhutta, Z.A. Nutritional perspectives for the prevention and mitigation of COVID-19. Nutr. Rev. 2020, 79, 289–300. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas 2021, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- White, C.M. Dietary Supplements Pose Real Dangers to Patients. Ann. Pharmacother. 2020, 54, 815–819. [Google Scholar] [CrossRef]
- Rayman, M.P. The argument for increasing selenium intake. Proc. Nutr. Soc. 2002, 61, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Fooladi, S.; Matin, S.; Mahmoodpoor, A. Copper as a potential adjunct therapy for critically ill COVID-19 patients. Clin. Nutr. ESPEN 2020, 40, 90–91. [Google Scholar] [CrossRef]
- Kudelko, M.; Yip, T.F.; Hei Law, G.C.; Lee, S.M.Y. Potential Beneficial Effects of Vitamin K in SARS-CoV-2 Induced Vascular Disease? Immuno 2021, 1, 17–29. [Google Scholar] [CrossRef]
- Keen, C.L.; Gershwin, M.E. Zinc deficiency and immune function. Annu. Rev. Nutr. 1990, 10, 415–431. [Google Scholar] [CrossRef]
- Dardenne, M. Zinc and immune function. Eur. J. Clin. Nutr. 2002, 56, S20–S23. [Google Scholar] [CrossRef]
- Joachimiak, M.P. Zinc against COVID-19? Symptom surveillance and deficiency risk groups. PLoS Negl. Trop. Dis. 2021, 15, e0008895. [Google Scholar] [CrossRef]
- Copaescu, A.; Smibert, O.; Gibson, A.; Phillips, E.J.; Trubiano, J.A. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J. Allergy Clin. Immunol. 2020, 146, 518–534.e1. [Google Scholar] [CrossRef]
- Mayor-Ibarguren, A.; Busca-Arenzana, C.; Robles-Marhuenda, Á. A Hypothesis for the Possible Role of Zinc in the Immunological Pathways Related to COVID-19 Infection. Front. Immunol. 2020, 11, 1736. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef]
- Chinni, V.; El-Khoury, J.; Perera, M.; Bellomo, R.; Jones, D.; Bolton, D.; Ischia, J.; Patel, O. Zinc supplementation as an adjunct therapy for COVID-19: Challenges and opportunities. Br. J. Clin. Pharmacol. 2021, 87, 3737–3746. [Google Scholar] [CrossRef]
- te Velthuis, A.J.W.; van den Worm, S.H.E.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn2+ Inhibits Coronavirus and Arterivirus RNA Polymerase Activity In Vitro and Zinc Ionophores Block the Replication of These Viruses in Cell Culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef]
- Panchariya, L.; Khan, W.A.; Kuila, S.; Sonkar, K.; Sahoo, S.; Ghoshal, A.; Kumar, A.; Verma, D.K.; Hasan, A.; Das, S.; et al. Zinc2+ ion inhibits SARS-CoV-2 main protease and viral replication in vitro. Chem. Commun. 2021. Epub ahead. [Google Scholar] [CrossRef]
- Singh, M.; Das, R.R. Zinc for the common cold. Cochrane Database Syst. Rev. 2013, CD001364. [Google Scholar] [CrossRef]
- Brooks, W.A.; Yunus, M.; Santosham, M.; Wahed, M.A.; Nahar, K.; Yeasmin, S.; Black, R.E. Zinc for severe pneumonia in very young children: Double-blind placebo-controlled trial. Lancet 2004, 363, 1683–1688. [Google Scholar] [CrossRef]
- Bermano, G.; Méplan, C.; Mercer, D.K.; Hesketh, J.E. Selenium and viral infection: Are there lessons for COVID-19? Br. J. Nutr. 2021, 125, 618–627. [Google Scholar] [CrossRef]
- Manzanares, W.; Moreira, E.; Hardy, G. Pharmaconutrition revisited for critically ill patients with coronavirus disease 2019 (COVID-19): Does selenium have a place? Nutrition 2021, 81, 110989. [Google Scholar] [CrossRef]
- Loscalzo, J. Keshan Disease, Selenium Deficiency, and the Selenoproteome. N. Engl. J. Med. 2014, 370, 1756–1760. [Google Scholar] [CrossRef]
- Broome, C.S.; McArdle, F.; Kyle, J.A.M.; Andrews, F.; Lowe, N.M.; Hart, C.A.; Arthur, J.R.; Jackson, M.J. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am. J. Clin. Nutr. 2004, 80, 154–162. [Google Scholar] [CrossRef]
- Chen, X.; Ren, F.; Hesketh, J.; Shi, X.; Li, J.; Gan, F.; Huang, K. Selenium blocks porcine circovirus type 2 replication promotion induced by oxidative stress by improving GPx1 expression. Free Radic. Biol. Med. 2012, 53, 395–405. [Google Scholar] [CrossRef]
- Amporndanai, K.; Meng, X.; Shang, W.; Jin, Z.; Rogers, M.; Zhao, Y.; Rao, Z.; Liu, Z.-J.; Yang, H.; Zhang, L.; et al. Inhibition mechanism of SARS-CoV-2 main protease by ebselen and its derivatives. Nat. Commun. 2021, 12, 3061. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Seale, L.A.; Torres, D.J.; Berry, M.J.; Pitts, M.W. A role for selenium-dependent GPX1 in SARS-CoV-2 virulence. Am. J. Clin. Nutr. 2020, 112, 447–448. [Google Scholar] [CrossRef]
- Tsuji, P.A.; Carlson, B.A.; Anderson, C.B.; Seifried, H.E.; Hatfield, D.L.; Howard, M.T. Dietary Selenium Levels Affect Selenoprotein Expression and Support the Interferon-γ and IL-6 Immune Response Pathways in Mice. Nutrients 2015, 7, 6529–6549. [Google Scholar] [CrossRef]
- Walston, J.; Xue, Q.; Semba, R.D.; Ferrucci, L.; Cappola, A.R.; Ricks, M.; Guralnik, J.; Fried, L.P. Serum Antioxidants, Inflammation, and Total Mortality in Older Women. Am. J. Epidemiol. 2005, 163, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Stadtman, T.C. Inhibition of NF-kappaB DNA binding and nitric oxide induction in human T cells and lung adenocarcinoma cells by selenite treatment. Proc. Natl. Acad. Sci. USA 1997, 94, 12904–12907. [Google Scholar] [CrossRef] [PubMed]
- Kiremidjian-Schumacher, L.; Roy, M.; Wishe, H.I.; Cohen, M.W.; Stotzky, G. Supplementation with selenium and human immune cell functions. Biol. Trace Elem. Res. 1994, 41, 115. [Google Scholar] [CrossRef] [PubMed]
- Ravaglia, G.; Forti, P.; Maioli, F.; Bastagli, L.; Facchini, A.; Mariani, E.; Savarino, L.; Sassi, S.; Cucinotta, D.; Lenaz, G. Effect of micronutrient status on natural killer cell immune function in healthy free-living subjects aged ≥90 y. Am. J. Clin. Nutr. 2000, 71, 590–598. [Google Scholar] [CrossRef]
- Hackler, J.; Heller, R.A.; Sun, Q.; Schwarzer, M.; Diegmann, J.; Bachmann, M.; Moghaddam, A.; Schomburg, L. Relation of Serum Copper Status to Survival in COVID-19. Nutrients 2021, 13, 1898. [Google Scholar] [CrossRef]
- Percival, S.S. Copper and immunity. Am. J. Clin. Nutr. 1998, 67, 1064S–1068S. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Imani, S.M.; Ladouceur, L.; Marshall, T.; Maclachlan, R.; Soleymani, L.; Didar, T.F. Antimicrobial Nanomaterials and Coatings: Current Mechanisms and Future Perspectives to Control the Spread of Viruses Including SARS-CoV-2. ACS Nano 2020, 14, 12341–12369. [Google Scholar] [CrossRef]
- Sagripanti, J.-L.; Lightfoote, M.M. Cupric and Ferric Ions Inactivate HIV. AIDS Res. Hum. Retrovir. 1996, 12, 333–336. [Google Scholar] [CrossRef]
- Horie, M.; Ogawa, H.; Yoshida, Y.; Yamada, K.; Hara, A.; Ozawa, K.; Matsuda, S.; Mizota, C.; Tani, M.; Yamamoto, Y.; et al. Inactivation and morphological changes of avian influenza virus by copper ions. Arch. Virol. 2008, 153, 1467–1472. [Google Scholar] [CrossRef]
- Rodriguez, K.; Saunier, F.; Rigaill, J.; Audoux, E.; Botelho-Nevers, E.; Prier, A.; Dickerscheit, Y.; Pillet, S.; Pozzetto, B.; Bourlet, T.; et al. Evaluation of in vitro activity of copper gluconate against SARS-CoV-2 using confocal microscopy-based high content screening. J. Trace Elem. Med. Biol. 2021, 68, 126818. [Google Scholar] [CrossRef]
- Tsang, T.; Posimo, J.M.; Gudiel, A.A.; Cicchini, M.; Feldser, D.M.; Brady, D.C. Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. Nat. Cell Biol. 2020, 22, 412–424. [Google Scholar] [CrossRef]
- Linneberg, A.; Kampmann, F.B.; Israelsen, S.B.; Andersen, L.R.; Jørgensen, H.L.; Sandholt, H.; Jørgensen, N.R.; Thysen, S.M.; Benfield, T. The Association of Low Vitamin K Status with Mortality in a Cohort of 138 Hospitalized Patients with COVID-19. Nutrients 2021, 13, 1985. [Google Scholar] [CrossRef]
- Dofferhoff, A.S.M.; Piscaer, I.; Schurgers, L.J.; Visser, M.P.J.; van den Ouweland, J.M.W.; de Jong, P.A.; Gosens, R.; Hackeng, T.M.; van Daal, H.; Lux, P.; et al. Reduced vitamin K status as a potentially modifiable risk factor of severe COVID-19. Clin. Infect. Dis. 2020, 73, e4039–e4046. [Google Scholar] [CrossRef]
- Janssen, R.; Visser, M.P.J.; Dofferhoff, A.S.M.; Vermeer, C.; Janssens, W.; Walk, J. Vitamin K metabolism as the potential missing link between lung damage and thromboembolism in Coronavirus disease 2019. Br. J. Nutr. 2021, 126, 191–198. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Haycock, P.C.; Burgess, S.; Wade, K.H.; Bowden, J.; Relton, C.; Davey Smith, G. Best (but oft-forgotten) practices: The design, analysis, and interpretation of Mendelian randomization studies. Am. J. Clin. Nutr. 2016, 103, 965–978. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Robberecht, H.; De Bruyne, T.; Davioud-Charvet, E.; Mackrill, J.; Hermans, N. Selenium Status in Elderly People: Longevity and Age-Related Diseases. Curr. Pharm. Des. 2019, 25, 1694–1706. [Google Scholar] [CrossRef]
- Mossink, J.P. Zinc as nutritional intervention and prevention measure for COVID-19 disease. BMJ Nutr. Prev. Health 2020, 3, 111–117. [Google Scholar] [CrossRef]
- Wessels, I.; Rolles, B.; Rink, L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front. Immunol. 2020, 11, 1712. [Google Scholar] [CrossRef]
- Larsson, S.C. Mendelian randomization as a tool for causal inference in human nutrition and metabolism. Curr. Opin. Lipidol. 2021, 32, 1–8. [Google Scholar] [CrossRef]
- Kodali, H.P.; Pavilonis, B.T.; Schooling, C.M. Effects of copper and zinc on ischemic heart disease and myocardial infarction: A Mendelian randomization study. Am. J. Clin. Nutr. 2018, 108, 237–242. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, C.; Sun, Y.; Francis, M.; Ryu, M.S.; Grider, A.; Ye, K. Genetically predicted circulating levels of copper and zinc are associated with osteoarthritis but not with rheumatoid arthritis. Osteoarthr. Cartil. 2021, 29, 1029–1035. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Papadimitriou, N.; Dimou, N.; Gill, D.; Lewis, S.J.; Martin, R.M.; Murphy, N.; Markozannes, G.; Zuber, V.; Cross, A.J.; et al. Genetically predicted circulating concentrations of micronutrients and risk of colorectal cancer among individuals of European descent: A Mendelian randomization study. Am. J. Clin. Nutr. 2021, 113, 1490–1502. [Google Scholar] [CrossRef]
- Yarmolinsky, J.; Bonilla, C.; Haycock, P.C.; Langdon, R.J.Q.; Lotta, L.A.; Langenberg, C.; Relton, C.L.; Lewis, S.J.; Evans, D.M.; Consortium, P.; et al. Circulating Selenium and Prostate Cancer Risk: A Mendelian Randomization Analysis. JNCI J. Natl. Cancer Inst. 2018, 110, 1035–1038. [Google Scholar] [CrossRef]
- Zwakenberg, S.R.; Remmelzwaal, S.; Beulens, J.W.J.; Booth, S.L.; Burgess, S.; Dashti, H.S.; Imamura, F.; Feskens, E.J.M.; van der Schouw, Y.T.; Sluijs, I. Circulating Phylloquinone Concentrations and Risk of Type 2 Diabetes: A Mendelian Randomization Study. Diabetes 2019, 68, 220–225. [Google Scholar] [CrossRef]
- Larsson, S.C.; Traylor, M.; Markus, H.S. Circulating Vitamin K1 Levels in Relation to Ischemic Stroke and Its Subtypes: A Mendelian Randomization Study. Nutrients 2018, 10, 1575. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yang, H. Ovarian cancer risk according to circulating zinc and copper concentrations: A meta-analysis and Mendelian randomization study. Clin. Nutr. 2021, 40, 2464–2468. [Google Scholar] [CrossRef]
- Elsworth, B.; Lyon, M.; Alexander, T.; Liu, Y.; Matthews, P.; Hallett, J.; Bates, P.; Palmer, T.; Haberland, V.; Smith, G.D.; et al. The MRC IEU OpenGWAS data infrastructure. bioRxiv 2020. [Google Scholar] [CrossRef]
- Milano, A.; McMahon, A.; Welter, D.; Bowler, E.; Hastings, E.; Cunningham, F.; MacArthur, J.; Morales, J.; Gil, L.; Cerezo, M.; et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Res. 2016, 45, D896–D901. [Google Scholar] [CrossRef]
- Evans, D.M.; Zhu, G.; Dy, V.; Heath, A.C.; Madden, P.A.F.; Kemp, J.P.; McMahon, G.; St Pourcain, B.; Timpson, N.J.; Golding, J.; et al. Genome-wide association study identifies loci affecting blood copper, selenium and zinc. Hum. Mol. Genet. 2013, 22, 3998–4006. [Google Scholar] [CrossRef] [PubMed]
- Vitoux, D.; Arnaud, J.; Chappuis, P. Are Copper, Zinc and Selenium in Erythrocytes Valuable Biological Indexes of Nutrition and Pathology ? J. Trace Elem. Med. Biol. 1999, 13, 113–128. [Google Scholar] [CrossRef]
- Combs, G.F., Jr. Biomarkers of Selenium Status. Nutrients 2015, 7, 2209–2236. [Google Scholar] [CrossRef]
- Cornelis, M.C.; Fornage, M.; Foy, M.; Xun, P.; Gladyshev, V.N.; Morris, S.; Chasman, D.I.; Hu, F.B.; Rimm, E.B.; Kraft, P.; et al. Genome-wide association study of selenium concentrations. Hum. Mol. Genet. 2014, 24, 1469–1477. [Google Scholar] [CrossRef]
- Dashti, H.S.; Shea, M.K.; Smith, C.E.; Tanaka, T.; Hruby, A.; Richardson, K.; Wang, T.J.; Nalls, M.A.; Guo, X.; Liu, Y.; et al. Meta-analysis of genome-wide association studies for circulating phylloquinone concentrations. Am. J. Clin. Nutr. 2014, 100, 1462–1469. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.A.M.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, s13742-015. [Google Scholar] [CrossRef]
- Auton, A.; Abecasis, G.R.; Altshuler, D.M.; Durbin, R.M.; Bentley, D.R.; Chakravarti, A.; Clark, A.G.; Donnelly, P.; Eichler, E.E.; Flicek, P.; et al. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Machiela, M.J.; Chanock, S.J. LDlink: A web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 2015, 31, 3555–3557. [Google Scholar] [CrossRef]
- Taylor, A.E.; Burgess, S.; Ware, J.J.; Gage, S.H.; Richards, J.B.; Davey Smith, G.; Munafò, M.R. Investigating causality in the association between 25(OH)D and schizophrenia. Sci. Rep. 2016, 6, 26496. [Google Scholar] [CrossRef]
- Kamat, M.A.; Blackshaw, J.A.; Young, R.; Surendran, P.; Burgess, S.; Danesh, J.; Butterworth, A.S.; Staley, J.R. PhenoScanner V2: An expanded tool for searching human genotype–phenotype associations. Bioinformatics 2019, 35, 4851–4853. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- COVID-19 Host Genetics Initiative. Mapping the human genetic architecture of COVID-19. Nature 2021, 600, 472–477. [Google Scholar] [CrossRef]
- Brion, M.-J.A.; Shakhbazov, K.; Visscher, P.M. Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol. 2012, 42, 1497–1501. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Yavorska, O.O.; Burgess, S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 2017, 46, 1734–1739. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Holmes, M.V.; Minelli, C.; Relton, C.L.; et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2019, 4, 186. [Google Scholar] [CrossRef]
- Marreiro, D.D.N.; Cruz, K.J.C.; Oliveira, A.D.; Morais, J.B.S.; Freitas, B.J.S.A.; Melo, S.R.D.S.; Santos, L.R.; Cardoso, B.E.P.; Dias, T.M.D.S. Antiviral and immunological activity of zinc and possible role in COVID-19. Br. J. Nutr. 2021, 1–8. [Google Scholar] [CrossRef]
- Thomas, S.; Patel, D.; Bittel, B.; Wolski, K.; Wang, Q.; Kumar, A.; Il’Giovine, Z.J.; Mehra, R.; McWilliams, C.; Nissen, S.E.; et al. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e210369. [Google Scholar] [CrossRef]
- Louca, P.; Murray, B.; Klaser, K.; Graham, M.S.; Mazidi, M.; Leeming, E.R.; Thompson, E.; Bowyer, R.; Drew, D.A.; Nguyen, L.H.; et al. Modest effects of dietary supplements during the COVID-19 pandemic: Insights from 445 850 users of the COVID-19 Symptom Study app. BMJ Nutr. Prev. Health 2021, 4, 149–157. [Google Scholar] [CrossRef]
- Yasui, Y.; Yasui, H.; Suzuki, K.; Saitou, T.; Yamamoto, Y.; Ishizaka, T.; Nishida, K.; Yoshihara, S.; Gohma, I.; Ogawa, Y. Analysis of the predictive factors for a critical illness of COVID-19 during treatment—relationship between serum zinc level and critical illness of COVID-19. Int. J. Infect. Dis. 2020, 100, 230–236. [Google Scholar] [CrossRef]
- Heller, R.A.; Sun, Q.; Hackler, J.; Seelig, J.; Seibert, L.; Cherkezov, A.; Minich, W.B.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2021, 38, 101764. [Google Scholar] [CrossRef]
- Jothimani, D.; Kailasam, E.; Danielraj, S.; Nallathambi, B.; Ramachandran, H.; Sekar, P.; Manoharan, S.; Ramani, V.; Narasimhan, G.; Kaliamoorthy, I.; et al. COVID-19: Poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. 2020, 100, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Vogel-González, M.; Talló-Parra, M.; Herrera-Fernández, V.; Pérez-Vilaró, G.; Chillón, M.; Nogués, X.; Gómez-Zorrilla, S.; López-Montesinos, I.; Arnau-Barrés, I.; Sorli-Redó, M.L.; et al. Low Zinc Levels at Admission Associates with Poor Clinical Outcomes in SARS-CoV-2 Infection. Nutrients 2021, 13, 562. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Gowda, S.; Mundkur, L. An exploratory study of selenium status in healthy individuals and in patients with COVID-19 in a south Indian population: The case for adequate selenium status. Nutrition 2021, 82, 111053. [Google Scholar] [CrossRef]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef]
- Skalny, A.V.; Timashev, P.S.; Aschner, M.; Aaseth, J.; Chernova, L.N.; Belyaev, V.E.; Grabeklis, A.R.; Notova, S.V.; Lobinski, R.; Tsatsakis, A.; et al. Serum Zinc, Copper, and Other Biometals Are Associated with COVID-19 Severity Markers. Metabolites 2021, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Zhang, A.-R.; Lu, Q.-B.; Zhang, X.-A.; Zhang, Z.-J.; Guan, X.-G.; Che, T.-L.; Yang, Y.; Li, H.; Liu, W.; et al. Association between fatality rate of COVID-19 and selenium deficiency in China. BMC Infect. Dis. 2021, 21, 452. [Google Scholar] [CrossRef]
- Stefanowicz, F.; Gashut, R.A.; Talwar, D.; Duncan, A.; Beulshausen, J.F.; McMillan, D.C.; Kinsella, J. Assessment of plasma and red cell trace element concentrations, disease severity, and outcome in patients with critical illness. J. Crit. Care 2014, 29, 214–218. [Google Scholar] [CrossRef]
- Nichol, C.; Herdman, J.; Sattar, N.; O’Dwyer, P.J.; O’Reilly, D.S.J.; Littlejohn, D.; Fell, G. Changes in the Concentrations of Plasma Selenium and Selenoproteins after Minor Elective Surgery: Further Evidence for a Negative Acute Phase Response? Clin. Chem. 1998, 44, 1764–1766. [Google Scholar] [CrossRef] [PubMed]
- Oestreicher, P.; Cousins, R.J. Copper and Zinc Absorption in the Rat: Mechanism of Mutual Antagonism. J. Nutr. 1985, 115, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Stafford, S.L.; Bokil, N.J.; Achard, M.E.S.; Kapetanovic, R.; Schembri, M.A.; McEwan, A.G.; Sweet, M.J. Metal ions in macrophage antimicrobial pathways: Emerging roles for zinc and copper. Biosci. Rep. 2013, 33, e00049. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Malarstig, A. Using Mendelian randomization to assess and develop clinical interventions: Limitations and benefits. J. Comp. Eff. Res. 2013, 2, 209–212. [Google Scholar] [CrossRef]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Hólm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef]
- C Reactive Protein Coronary Heart Disease Genetics Collaboration. Association between C reactive protein and coronary heart disease: Mendelian randomisation analysis based on individual participant data. BMJ 2011, 342, d548. [Google Scholar] [CrossRef]
- Griffith, G.J.; Morris, T.T.; Tudball, M.J.; Herbert, A.; Mancano, G.; Pike, L.; Sharp, G.C.; Sterne, J.; Palmer, T.M.; Davey Smith, G.; et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat. Commun. 2020, 11, 5749. [Google Scholar] [CrossRef]
- Hui, L.L.; Nelson, E.A.S.; Lin, S.L.; Zhao, J.V. The role of vitamin C in pneumonia and COVID-19 infection in adults with European ancestry: A Mendelian randomisation study. Eur. J. Clin. Nutr. 2021. [Google Scholar] [CrossRef]
- Butler-Laporte, G.; Nakanishi, T.; Mooser, V.; Morrison, D.R.; Abdullah, T.; Adeleye, O.; Mamlouk, N.; Kimchi, N.; Afrasiabi, Z.; Rezk, N.; et al. Vitamin D and COVID-19 susceptibility and severity in the COVID-19 Host Genetics Initiative: A Mendelian randomization study. PLoS Med. 2021, 18, 1–14. [Google Scholar] [CrossRef]
- Amin, H.A.; Drenos, F. No evidence that vitamin D is able to prevent or affect the severity of COVID-19 in individuals with European ancestry: A Mendelian randomisation study of open data. BMJ Nutr. Prev. Health 2021, 4, 42–48. [Google Scholar] [CrossRef]



| Exposure | Outcome | n SNPs | IVW Odds Ratio (95% CI) | IVW p-Value 1 | Cochrane’s Q | Cochrane’s Q p-Value 1 | MR-Egger Intercept | MR-Egger Intercept p-Value 1 |
|---|---|---|---|---|---|---|---|---|
| Zn | SARS-CoV-2 infection | 2 | 0.97 (0.87–1.08) | 0.548 | 0.73 | 0.394 | NA 2 | NA 2 |
| Zn | Hospitalized (ver. non-hospitalized) | 2 | 0.99 (0.69–1.44) | 0.971 | 0.02 | 0.889 | NA 2 | NA 2 |
| Zn | Hospitalized (ver. population) | 2 | 1.06 (0.81–1.39) | 0.663 | 1.62 | 0.203 | NA 2 | NA 2 |
| Zn | Very severe COVID-19 | 2 | 1.21 (0.79–1.86) | 0.386 | 2.09 | 0.148 | NA 2 | NA 2 |
| Zn subsignificant | SARS-CoV-2 infection | 12 | 1.01 (0.98–1.05) | 0.489 | 7.32 | 0.772 | 0.898 | 0.468 |
| Zn subsignificant | Hospitalized (ver. non-hospitalized) | 12 | 0.97 (0.85–1.11) | 0.688 | 14.09 | 0.228 | 0.717 | 0.496 |
| Zn subsignificant | Hospitalized (ver. population) | 12 | 0.98 (0.91–1.06) | 0.623 | 13.40 | 0.268 | 0.108 | 0.424 |
| Zn subsignificant | Very severe COVID-19 | 12 | 0.92 (0.81–1.04) | 0.161 | 13.37 | 0.270 | 0.845 | 0.340 |
| Se meta-analysis | SARS-CoV-2 infection | 2 | 1.03 (0.95–1.11) | 0.506 | 1.68 | 0.195 | NA 2 | NA 2 |
| Se meta-analysis | Hospitalized (ver. non-hospitalized) | 2 | 0.91 (0.75–1.11) | 0.347 | 0.58 | 0.445 | NA 2 | NA 2 |
| Se meta-analysis | Hospitalized (ver. population) | 2 | 0.98 (0.87–1.10) | 0.715 | 0.28 | 0.599 | NA 2 | NA 2 |
| Se meta-analysis | Very severe COVID-19 | 2 | 0.99 (0.83–1.17) | 0.864 | 0.22 | 0.638 | NA 2 | NA 2 |
| Se ALSPAC subsignificant | SARS-CoV-2 infection | 12 | 0.99 (0.95–1.03) | 0.704 | 9.66 | 0.561 | 0.104 | 0.457 |
| Se ALSPAC subsignificant | Hospitalized (ver. non-hospitalized) | 12 | 1.01 (0.88–1.16) | 0.844 | 12.15 | 0.353 | 0.675 | 0.439 |
| Se ALSPAC subsignificant | Hospitalized (ver. population) | 12 | 1.03 (0.95–1.12) | 0.453 | 4.62 | 0.948 | 0.262 | 0.522 |
| Se ALSPAC subsignificant | Very severe COVID-19 | 12 | 1.06 (0.94–1.19) | 0.369 | 6.77 | 0.817 | 0.278 | 0.642 |
| Se QIMR subsignificant | SARS-CoV-2 infection | 15 | 1.00 (0.97–1.03) | 0.974 | 9.35 | 0.808 | 0.973 | 0.392 |
| Se QIMR subsignificant | Hospitalized (ver. non-hospitalized) | 15 | 1.04 (0.94–1.16) | 0.412 | 17.82 | 0.215 | 0.050 | 0.352 |
| Se QIMR subsignificant | Hospitalized (ver. population) | 15 | 1.06 (1.00–1.12) | 0.033 | 13.47 | 0.490 | 0.212 | 0.363 |
| Se QIMR subsignificant | Very severe COVID-19 | 15 | 1.07 (0.99–1.16) | 0.069 | 11.77 | 0.624 | 0.679 | 0.371 |
| Cu | SARS-CoV-2 infection | 2 | 1.07 (1.00–1.14) | 0.057 | 0.66 | 0.415 | NA 2 | NA 2 |
| Cu | Hospitalized (ver. non-hospitalized) | 2 | 0.98 (0.79–1.21) | 0.842 | 0.00 | 0.984 | NA 2 | NA 2 |
| Cu | Hospitalized (ver. population) | 2 | 1.07 (0.88–1.29) | 0.493 | 2.24 | 0.135 | NA 2 | NA 2 |
| Cu | Very severe COVID-19 | 2 | 1.13 (0.82–1.55) | 0.467 | 2.84 | 0.092 | NA 2 | NA 2 |
| Cu subsignificant | SARS-CoV-2 infection | 7 | 1.01 (0.96–1.07) | 0.662 | 11.30 | 0.080 | 0.022 | 0.227 |
| Cu subsignificant | Hospitalized (ver. non-hospitalized) | 7 | 0.98 (0.86–1.12) | 0.792 | 1.39 | 0.967 | 0.006 | 0.882 |
| Cu subsignificant | Hospitalized (ver. population) | 7 | 0.99 (0.91–1.08) | 0.816 | 5.09 | 0.532 | 0.018 | 0.493 |
| Cu subsignificant | Very severe COVID-19 | 7 | 0.94 (0.83–1.07) | 0.326 | 3.87 | 0.694 | 0.017 | 0.672 |
| vit. K1 subsignificant | SARS-CoV-2 infection | 3 | 0.99 (0.93–1.05) | 0.677 | 0.95 | 0.621 | 0.507 | 0.000 |
| vit. K1 subsignificant | Hospitalized (ver. non-hospitalized) | 3 | 1.06 (0.88–1.28) | 0.565 | 0.50 | 0.779 | 0.697 | 0.000 |
| vit. K1 subsignificant | Hospitalized (ver. population) | 3 | 0.98 (0.87–1.09) | 0.662 | 0.62 | 0.732 | 0.593 | 0.000 |
| vit. K1 subsignificant | Very severe COVID-19 | 3 | 0.93 (0.72–1.19) | 0.546 | 4.42 | 0.110 | 0.349 | 0.084 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobczyk, M.K.; Gaunt, T.R. The Effect of Circulating Zinc, Selenium, Copper and Vitamin K1 on COVID-19 Outcomes: A Mendelian Randomization Study. Nutrients 2022, 14, 233. https://doi.org/10.3390/nu14020233
Sobczyk MK, Gaunt TR. The Effect of Circulating Zinc, Selenium, Copper and Vitamin K1 on COVID-19 Outcomes: A Mendelian Randomization Study. Nutrients. 2022; 14(2):233. https://doi.org/10.3390/nu14020233
Chicago/Turabian StyleSobczyk, Maria K., and Tom R. Gaunt. 2022. "The Effect of Circulating Zinc, Selenium, Copper and Vitamin K1 on COVID-19 Outcomes: A Mendelian Randomization Study" Nutrients 14, no. 2: 233. https://doi.org/10.3390/nu14020233
APA StyleSobczyk, M. K., & Gaunt, T. R. (2022). The Effect of Circulating Zinc, Selenium, Copper and Vitamin K1 on COVID-19 Outcomes: A Mendelian Randomization Study. Nutrients, 14(2), 233. https://doi.org/10.3390/nu14020233





