The Short-Term Effect of Prunes in Improving Bone in Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Overview
2.2. Participant Inclusion Criteria
2.3. Participant Exclusion Criteria
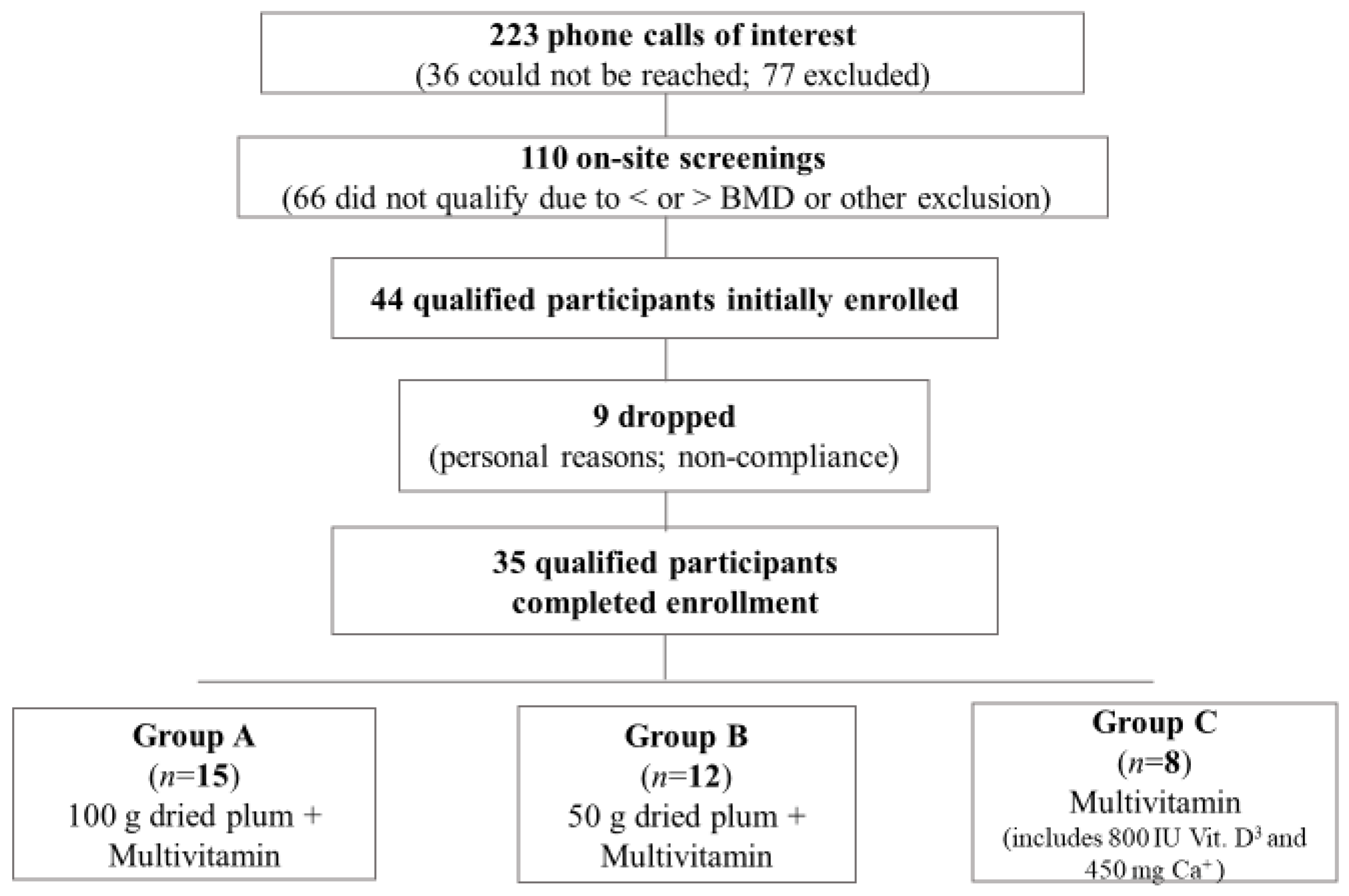
2.4. Prescreening
2.5. Screening
2.6. Intervention
2.7. Compliance
2.8. Questionnaires
2.8.1. Health and Medical History
2.8.2. Dietary Assessment
2.8.3. Physical Activity
2.9. Anthropometrics
2.10. Blood Collection and Processing
2.11. Urine Collection
2.12. Bone Density
2.13. TBS
2.14. Markers of Inflammation
2.15. Markers of Antioxidant Activity
2.16. Markers of Bone Formation
2.17. Markers of Bone Turnover
2.18. Markers of Bone Health and Inflammation
2.19. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Anthropometrics and Vitals
3.3. Physical Activity
3.4. Dietary Intake
3.5. Serum Biomarkers of Inflammation and Bone Health
3.6. Trabecular Bone Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.H.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [Green Version]
- McGowan, J.; Sharrock, W.; Khosla, S.; Lukert, B.; Orwoll, E. Handout on Health: Osteoporosis. In NIH Osteoporosis and Related Bone Diseases National Resource Center; National Institute of Arthritis and Musculoskeletal and Skin Diseases: Bethesda, MD, USA, 2011. [Google Scholar]
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res. 2014, 29, 2520–2526. [Google Scholar] [CrossRef] [Green Version]
- Caruso, C.; Lio, D.; Cavallone, L.; Franceschi, C. Aging, longevity, inflammation, and cancer. Ann. N. Y. Acad. Sci. 2004, 1028, 1–13. [Google Scholar] [CrossRef]
- Boyd, J.H.; Divangahi, M.; Yahiaoui, L.; Gvozdic, D.; Qureshi, S.; Petrof, B.J. Toll-like receptors differentially regulate CC and CXC chemokines in skeletal muscle via NF-kappaB and calcineurin. Infect. Immun. 2006, 74, 6829–6838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, G.R.; Michell, B.J.; van Denderen, B.J.; Watt, M.J.; Carey, A.L.; Fam, B.C.; Andrikopoulos, S.; Proietto, J.; Görgün, C.Z.; Carling, D.; et al. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006, 4, 465–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzetti, E.; Leeuwenburgh, C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp. Gerontol. 2006, 41, 1234–1238. [Google Scholar] [CrossRef]
- Faulkner, J.A.; Larkin, L.M.; Claflin, D.R.; Brooks, S.V. Age-related changes in the structure and function of skeletal muscles. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1091–1096. [Google Scholar] [CrossRef]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Semba, R.D.; Lauretani, F.; Ferrucci, L. Carotenoids as protection against sarcopenia in older adults. Arch. Biochem. Biophys. 2007, 458, 141–145. [Google Scholar] [CrossRef] [Green Version]
- National Osteoporosis Foundation. Available online: https://www.nof.org/ (accessed on 10 October 2019).
- Schreurs, A.S.; Shirazi-Fard, Y.; Shahnazari, M.; Alwood, J.S.; Truong, T.A.; Tahimic, C.G.; Limoli, C.L.; Turner, N.D.; Halloran, B.; Globus, R.K. Prune diet protects from bone loss caused by ionizing radiation. Sci. Rep. 2016, 6, 21343. [Google Scholar] [CrossRef] [Green Version]
- Franklin, M.; Bu, S.Y.; Lerner, M.R.; Lancaster, E.A.; Bellmer, D.; Marlow, D.; Lightfoot, S.A.; Arjmandi, B.H.; Brackett, D.J.; Lucas, E.A.; et al. Dried plum prevents bone loss in a male osteoporosis model via IGF-I and the RANK pathway. Bone 2006, 39, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.Y.; Lucas, E.A.; Franklin, M.; Marlow, D.; Brackett, D.J.; Boldrin, E.A.; Devareddy, L.; Arjmandi, B.H.; Smith, B.J. Comparison of dried plum supplementation and intermittent PTH in restoring bone in osteopenic orchidectomized rats. Osteoporos. Int. 2007, 18, 931–942. [Google Scholar] [CrossRef]
- Deyhim, F.; Stoecker, B.J.; Brusewitz, G.H.; Devareddy, L.; Arjmandi, B.H. Dried plum reverses bone loss in an osteopenic rat model of osteoporosis. Menopause 2005, 12, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.D.; Lucas, E.A.; Hooshmand, S.; Campbell, S.; Akhter, M.P.; Arjmandi, B.H. Addition of fructooligosaccharides and dried plums to soy-based diets reverses bone loss in the ovariectomized rat. Evid. Based Complement. Alternat. Med. 2011, 2011, 836267. [Google Scholar] [CrossRef] [Green Version]
- Arjmandi, B.H.; Johnson, C.D.; Campbell, S.C.; Hooshmand, S.; Chai, S.C.; Akhter, M.P. Combining fructooligosaccharide and prune has the greatest effect on restoring bone mineral density among select functional foods and bioactive compounds. J. Med. Food 2010, 13, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Chai, S.C.; Saadat, R.L.; Payton, M.E.; Brummel-Smith, K.; Arjmandi, B.H. Comparative effects of prune and dried apple on bone in postmenopausal women. Br. J. Nutr. 2011, 106, 923–930. [Google Scholar] [CrossRef]
- Hooshmand, S.; Brisco, J.R.; Arjmandi, B.H. The effect of dried plums on serum levels of receptor activator of NF-κB ligand, osteoprotegerin and sclerostin in osteopenic postmenopausal women: A randomised controlled trial. Br. J. Nutr. 2014, 112, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Weaver, C.M.; Alekel, D.L.; Ward, W.E.; Ronis, M.J. Flavonoid intake and bone health. J. Nutr. Gerontol. Geriatr. 2012, 31, 239–253. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Arjmandi, B.H. Viewpoint: Dried plums, an emerging functional food that may effectively improve bone health. Ageing Res. Rev. 2009, 8, 122–127. [Google Scholar] [CrossRef]
- González-Cebrino, F.; García-Parra, J.; Contador, R.; Tabla, R.; Ramírez, R. Effect of high-pressure processing and thermal treatment on quality attributes and nutritional compounds of “Songold” plum purée. J. Food Sci. 2012, 77, C866–C873. [Google Scholar] [CrossRef]
- Sallis, J.F.; Haskell, W.L.; Wood, P.D.; Fortmann, S.P.; Rogers, T.; Blair, S.N.; Paffenbarger, R.S. Physical activity assessment methodology in the Five-City Project. Am. J. Epidemiol. 1985, 121, 91–106. [Google Scholar] [CrossRef]
- McCloskey, E.V.; Odén, A.; Harvey, N.C.; Leslie, W.D.; Hans, D.; Johansson, H.; Barkmann, R.; Boutroy, S.; Brown, J.; Chapurlat, R.; et al. A Meta-Analysis of Trabecular Bone Score in Fracture Risk Prediction and Its Relationship to FRAX. J. Bone Miner. Res. 2016, 31, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Cauley, J.A.; Greendale, G.A.; Crandall, C.J.; Danielson, M.E.; Ouchi, Y.; Karlamangla, A.S. C-reactive protein, bone strength, and nine-year fracture risk: Data from the Study of Women’s Health Across the Nation (SWAN). J. Bone Miner. Res. 2013, 28, 1688–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mlakar, S.J.; Osredkar, J.; Prezelj, J.; Marc, J. The antioxidant enzyme GPX1 gene polymorphisms are associated with low BMD and increased bone turnover markers. Dis. Markers 2010, 29, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Feresin, R.G.; Navaei, N.; Figueroa, A.; Elam, M.L.; Akhavan, N.S.; Hooshmand, S.; Pourafshar, S.; Payton, M.E.; Arjmandi, B.H. Effects of daily blueberry consumption on circulating biomarkers of oxidative stress, inflammation, and antioxidant defense in postmenopausal women with pre- and stage 1-hypertension: A randomized controlled trial. Food Funct. 2017, 8, 372–380. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Johnson, S.A.; Pourafshar, S.; Navaei, N.; George, K.S.; Hooshmand, S.; Chai, S.C.; Akhavan, N.S. Bone-Protective Effects of Dried Plums in Postmenopausal Women: Efficacy and Possible Mechanisms. Nutrients 2017, 9, 496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaffen, D.; Tunstall, A.; Fajardo, J.; Ramachandran, P.; Kern, M.; Hooshmand, S. Effects of Dried Plum on Bone Biomarkers in Men. Curr. Dev. Nutr. 2019, 3, 57. [Google Scholar] [CrossRef] [Green Version]
- Harvey, N.C.; Glüer, C.C.; Binkley, N.; McCloskey, E.V.; Brandi, M.L.; Cooper, C.; Kendler, D.; Lamy, O.; Laslop, A.; Camargos, B.M.; et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 2015, 78, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Simonelli, C.; Leib, E.; Mossman, N.; Winzenrieth, R.; Hans, D.; McClung, M. Creation of an age-adjusted, dual-energy x-ray absorptiometry-derived trabecular bone score curve for the lumbar spine in non-Hispanic US White women. J. Clin. Densitom. 2014, 17, 314–319. [Google Scholar] [CrossRef]
- Spinasanta, S. FRAX® and Clinical Risk Factors. Available online: https://www.endocrineweb.com/professional/meetings/frax-clinical-risk-factors (accessed on 10 October 2019).
- Ahmed, T.; Sadia, H.; Batool, S.; Janjua, A.; Shuja, F. Use of prunes as a control of hypertension. J. Ayub Med. Coll. Abbottabad 2010, 22, 28–31. [Google Scholar]
- Arjmandi, B.H.; Khalil, D.A.; Lucas, E.A.; Georgis, A.; Stoecker, B.J.; Hardin, C.; Payton, M.E.; Wild, R.A. Dried Plums improve indices of bone formation in postmenopausal women. J. Womens Health Gend. Based Med. 2002, 11, 61–68. [Google Scholar] [CrossRef]
- Findlay, D.M.; Atkins, G.J. Relationship between serum RANKL and RANKL in bone. Osteoporos. Int. 2011, 22, 2597–2602. [Google Scholar] [CrossRef]
- Drake, M.T.; Srinivasan, B.; Mödder, U.I.; Peterson, J.M.; McCready, L.K.; Riggs, B.L.; Dwyer, D.; Stolina, M.; Kostenuik, P.; Khosla, S. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J. Clin. Endocrinol. Metab. 2010, 95, 5056–5062. [Google Scholar] [CrossRef]
- Findlay, D.; Chehade, M.; Tsangari, H.; Neale, S.; Hay, S.; Hopwood, B.; Pannach, S.; O’Loughlin, P.; Fazzalari, N. Circulating RANKL is inversely related to RANKL mRNA levels in bone in osteoarthritic males. Arthritis Res. Ther. 2008, 10, R2. [Google Scholar] [CrossRef] [Green Version]
- Reyes-García, R.; Muñoz-Torres, M.; García, D.F.; Mezquita-Raya, P.; García Salcedo, J.A.; de Dios Luna, J. Effects of alendronate treatment on serum levels of osteoprotegerin and total receptor activator of nuclear factor kappaB in women with postmenopausal osteoporosis. Menopause 2010, 17, 140–144. [Google Scholar] [CrossRef]
- Hooshmand, S.; Kern, M.; Metti, D.; Shamloufard, P.; Chai, S.C.; Johnson, S.A.; Payton, M.E.; Arjmandi, B.H. The effect of two doses of dried plums on bone density and bone biomarkers in osteopenic postmenopausal women: A randomized, controlled trial. Osteoporos. Int. 2016, 27, 2271–2279. [Google Scholar] [CrossRef]
- Stern, A.; Laughlin, G.A.; Bergstrom, J.; Barrett-Connor, E. The sex-specific association of serum osteoprotegerin and receptor activator of nuclear factor kappaB legend with bone mineral density in older adults: The Rancho Bernardo study. Eur. J. Endocrinol. 2007, 156, 555–562. [Google Scholar] [CrossRef]
- Naumnik, B.; Klejna, K.; Koc-Żórawska, E.; Myśliwiec, M. Age and gender predict OPG level and OPG/sRANKL ratio in maintenance hemodialysis patients. Adv. Med. Sci. 2013, 58, 382–387. [Google Scholar] [CrossRef] [Green Version]
- Kostenuik, P.J. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr. Opin. Pharmacol. 2005, 5, 618–625. [Google Scholar] [CrossRef]
- Jabbar, S.; Drury, J.; Fordham, J.N.; Datta, H.K.; Francis, R.M.; Tuck, S.P. Osteoprotegerin, RANKL and bone turnover in postmenopausal osteoporosis. J. Clin. Pathol. 2011, 64, 354–357. [Google Scholar] [CrossRef]

| Measures | 100 g DP | 50 g DP | Control | p Value |
|---|---|---|---|---|
| Age (years) | 65.9 ± 7.3 | 69.1 ± 5.9 | 64.9 ± 5.9 | 0.316 |
| Height (cm) | 173.8 ± 4.4 | 175.1 ± 9.0 | 174.6 ± 10.3 | 0.914 |
| Weight (kg) | 74.7 ± 13.7 | 82.1 ± 13.7 | 87.6 ± 24.2 | 0.206 |
| BMI (kg/m2) | 24.6 ± 3.7 | 24.3 ± 8.7 | 28.6 ± 6.9 | 0.312 |
| WC (cm) | 89.8 ± 13.4 | 95.7 ± 12.6 | 99.4 ± 15.3 | 0.296 |
| HC (cm) | 97.1 ± 7.3 | 106.7 ± 9.7 | 103.7 ± 12.3 | 0.063 |
| BMD T-score (L1–L4) | −1.01 ± 1.21 | −0.60 ± 0.93 | −0.78 ± 0.67 | 0.678 |
| 100 g DP (mean ±SD) | 50 g DP (mean ±SD) | Control (mean ±SD) | Trt*Time | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measures | Pre | Post | p Value | Pre | Post | p Value | Pre | Post | p Value | p Value |
| Weight (kg) | 75.9 ± 13.4 | 75.5 ± 13.6 | 0.146 | 82.1 ± 13.7 | 81.8 ± 13.0 | 0.540 | 86.5 ± 25.9 | 86.4 ± 25.8 | 0.863 | 0.438 |
| BMI (kg/m2) | 25.0 ± 3.5 | 24.9 ± 3.5 | 0.132 | 26.5 ± 4.3 | 26.4 ± 4.0 | 0.538 | 28.6± 2.5 | 28.7± 2.4 | 0.540 | 0.415 |
| WHR | 0.93 ± 0.08 | 0.92 ± 0.09 | 0.473 | 0.90 ± 0.08 | 0.91 ± 0.05 | 0.344 | 0.96 ± 0.06 | 0.95± 0.07 | 0.648 | 0.420 |
| SBP (mmHg) | 133.2 ± 23.2 | 130.8 ± 18.0 | 0.432 | 138.3± 16.3 | 129.8 ± 12.2 | 0.042 * | 149.7 ± 18.3 | 139.4 ± 13.5 | 0.003* | 0.171 |
| DBP (mmHg) | 76.9 ± 12.8 | 76.3 ± 11.8 | 0.834 | 77.6 ± 13.4 | 75.7 ± 9.3 | 0.448 | 81.3 ± 11.2 | 80.1 ±12.7 | 0.565 | 0.920 |
| Pulse (bpm) | 62.1 ± 7.3 | 62.0 ± 6.6 | 0.982 | 67.6 ± 16.1 | 64.3 ± 13.9 | 0.186 | 66.7± 9.6 | 68.9 ± 7.4 | 0.204 | 0.222 |
| 100 g DP | 50 g DP | Control | Trt*Time | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measures | Pre | Post | p Value | Pre | Post | p Value | Pre | Post | p Value | p Value |
| Total PA (hrs/week) | 13.0 ± 14.3 | 13.1 ± 13.1 | 0.995 | 9 ± 7.0 | 15.2 ± 11.9 | 0.058 | 4.6 ± 4.9 | 3.8 ± 5.6 | 0.526 | 0.274 |
| 100 g DP (mean ± SD) | 50 g DP (mean ± SD) | Control (mean ± SD) | Trt*Time | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measures | Pre | Post | p Value | Pre | Post | p Value | Pre | Post | p Value | p Value |
| Total kcal | 1920 ± 400 | 1927 ± 682 | 0.964 | 1590 ± 459 | 1617 ± 450 | 0.853 | 1647 ± 157 | 1637 ± 403 | 0.953 | 0.989 |
| Protein (g) | 87.1 ± 26.8 | 92.7 ± 33.5 | 0.461 | 77.8 ± 23.6 | 92.3 ± 61.6 | 0.523 | 69.9 ± 13.4 | 62.7 ± 9.5 | 0.253 | 0.745 |
| CHO (g) | 214.2 ± 62.1 | 256.1 ± 85.8 | 0.062 | 188.2 ± 86.4 | 173.9 ± 80.0 | 0.603 | 222.0 ± 47.4 | 231.5 ± 66.6 | 0.807 | 0.265 |
| Total Fiber (g) | 26.8 ± 13.1 | 30.4 ± 13.1 | 0.230 | 17.6 ± 7.7 | 19.7 ± 6.9 | 0.099 | 22.8 ± 9.9 | 20.5 ± 11.6 | 0.408 | 0.543 |
| Sugar (g) | 82.7 ± 24.1 | 91.4 ± 41.6 | 0.450 | 70.8 ± 43.5 | 78.9 ± 33.5 | 0.410 | 68.0 ± 17.4 | 89.9 ± 53.1 | 0.358 | 0.759 |
| Total Fat (g) | 73.9 ± 20.2 | 67.1 ± 29.3 | 0.409 | 56.2 ± 20.2 | 65.4 ± 31.7 | 0.362 | 53.2 ± 7.2 | 51.8 ± 13.3 | 0.801 | 0.401 |
| Saturated Fat (g) | 22.2 ± 8.2 | 18.7 ± 9.4 | 0.070 | 18.3 ± 7.5 | 25.6 ± 27.5 | 0.398 | 16.8 ± 3.0 | 15.2 ± 2.6 | 0.516 | 0.407 |
| MUFA (g) | 16.9 ± 7.1 | 11.8 ± 7.0 | 0.001 * | 11.9 ± 8.1 | 13.5 ± 7.0 | 0.579 | 8.5 ± 2.0 | 8.5 ± 1.4 | 0.987 | 0.091 |
| PUFA (g) | 10.5 ± 3.7 | 7.2 ± 3.4 | 0.032 * | 6.6 ± 3.6 | 10.4 ± 7.9 | 0.119 | 8.3 ± 2.8 | 7.6 ± 3.4 | 0.675 | 0.029* |
| Trans Fat (g) | 0.18 ± 0.34 | 0.22 ± 0.23 | 0.651 | 0.20 ± 0.22 | 3.0 ± 8.5 | 0.323 | 0.13 ± 0.16 | 0.51 ± 0.63 | 0.201 | 0.548 |
| Cholesterol (mg) | 284.3 ± 160.6 | 254.0 ± 151.6 | 0.442 | 317.3 ± 178.6 | 261.6 ± 150.4 | 0.322 | 207.6 ± 101.4 | 181.4 ± 88.2 | 0.688 | 0.903 |
| Potassium (mg) | 1791 ± 489 | 2215 ± 772 | 0.043 * | 1800 ± 634 | 1724 ± 601 | 0.730 | 1626 ± 354 | 1439 ± 639 | 0.693 | 0.181 |
| Sodium (mg) | 2328 ± 732 | 2536 ± 1327 | 0.553 | 2494 ± 1117 | 2294 ± 1574 | 0.636 | 1802 ± 408 | 2108 ± 467 | 0.226 | 0.632 |
| 100 g DP (mean ± SD) | 50 g DP (mean ± SD) | Control (mean ± SD) | Treatment*Time | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Pre | Post | p Value | Pre | Post | p Value | Pre | Post | p Value | p Value |
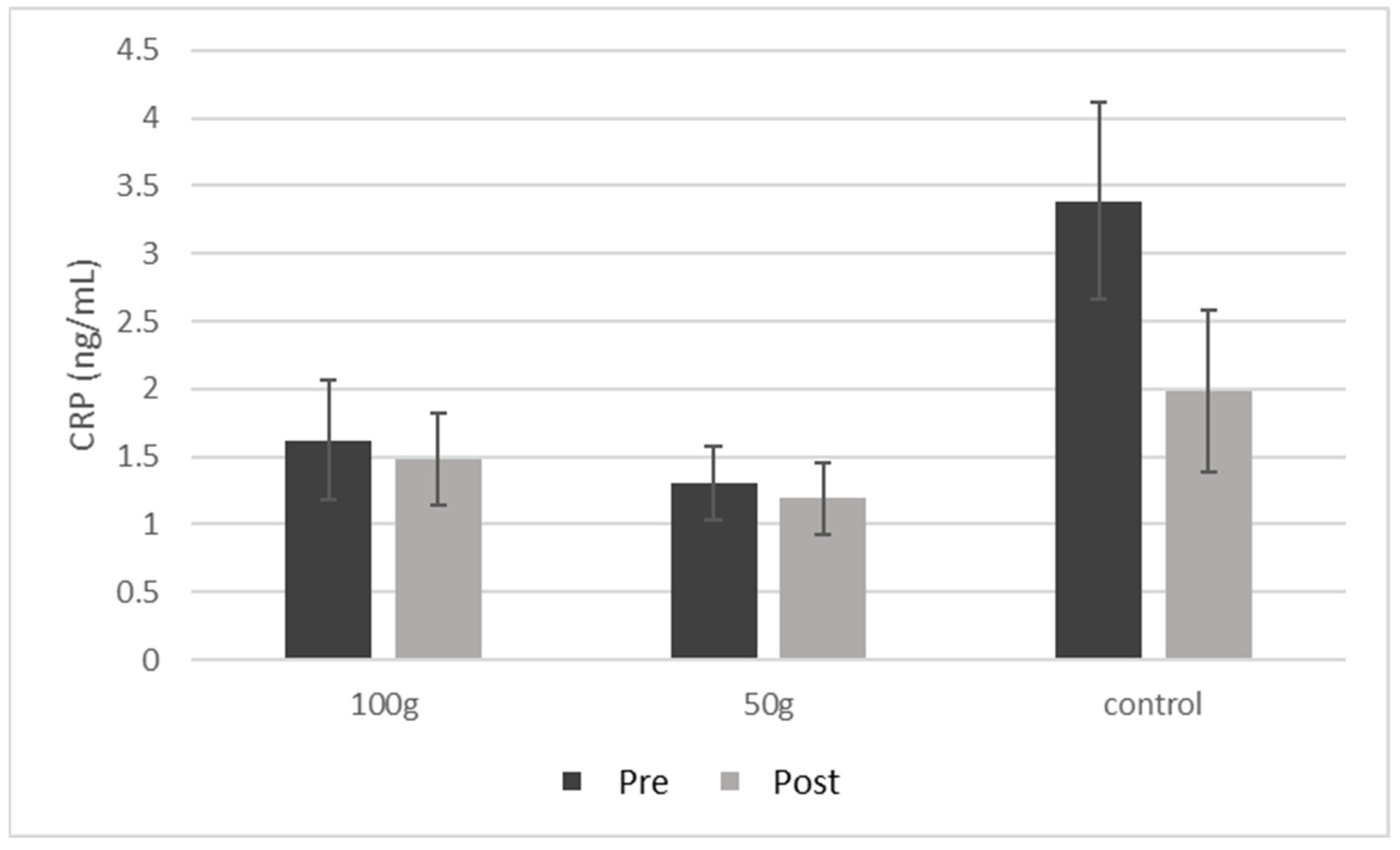
| CRP (ng/mL) | 1.62 ± 1.6 | 1.48 ± 1.2 | 0.188 | 1.30 ± 0.8 | 1.19 ± 0.8 | 0.252 | 3.39 ± 1.6 | 1.98 ± 1.3 | 0.216 | 0.171 |
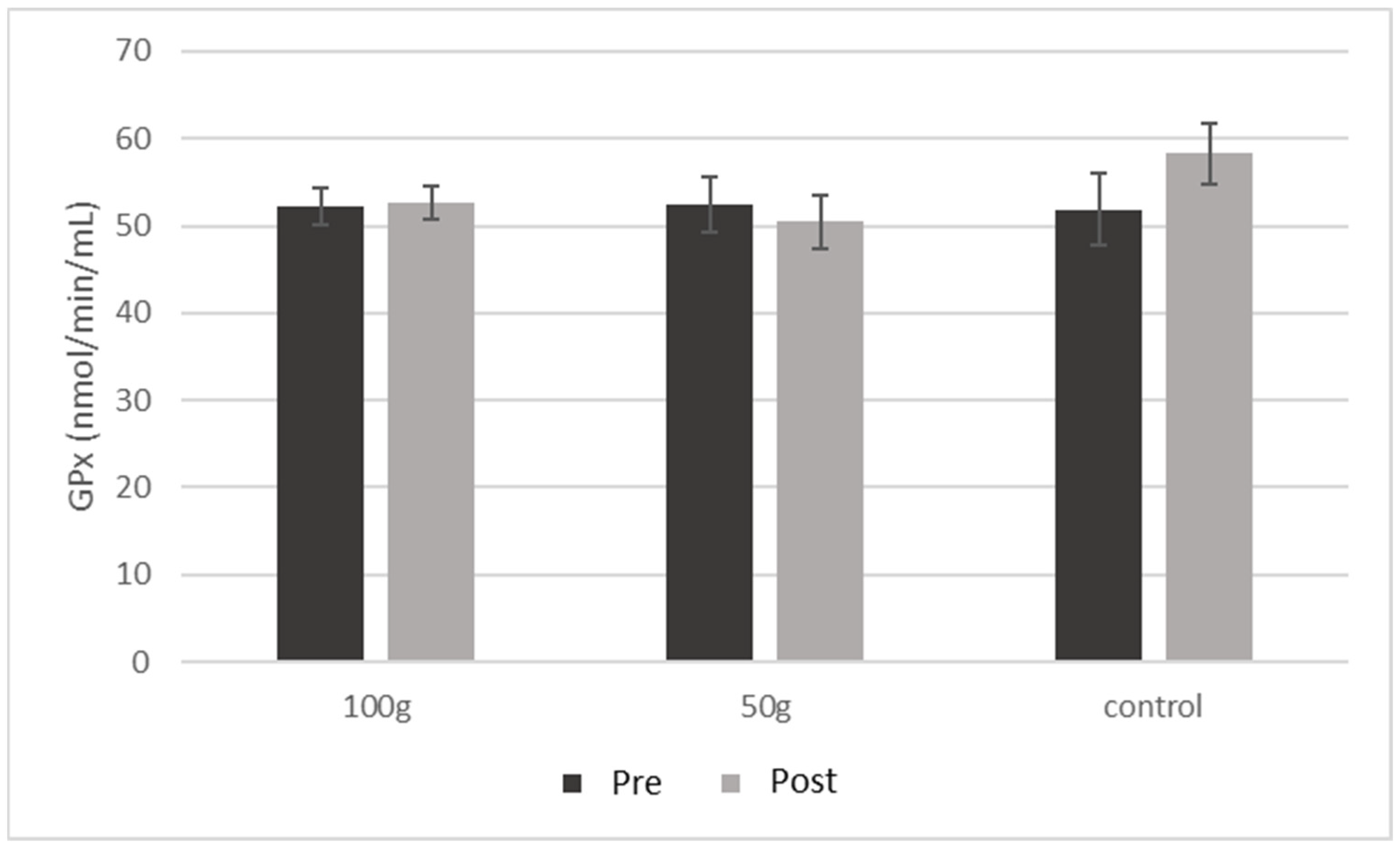
| GPx (nmol/min/mL) | 52.31 ± 8.2 | 52.66 ± 7.7 | 0.919 | 52.44 ± 10.5 | 50.43 ± 10.0 | 0.699 | 51.81 ± 10.9 | 58.27 ± 9.2 | 0.419 | 0.545 |
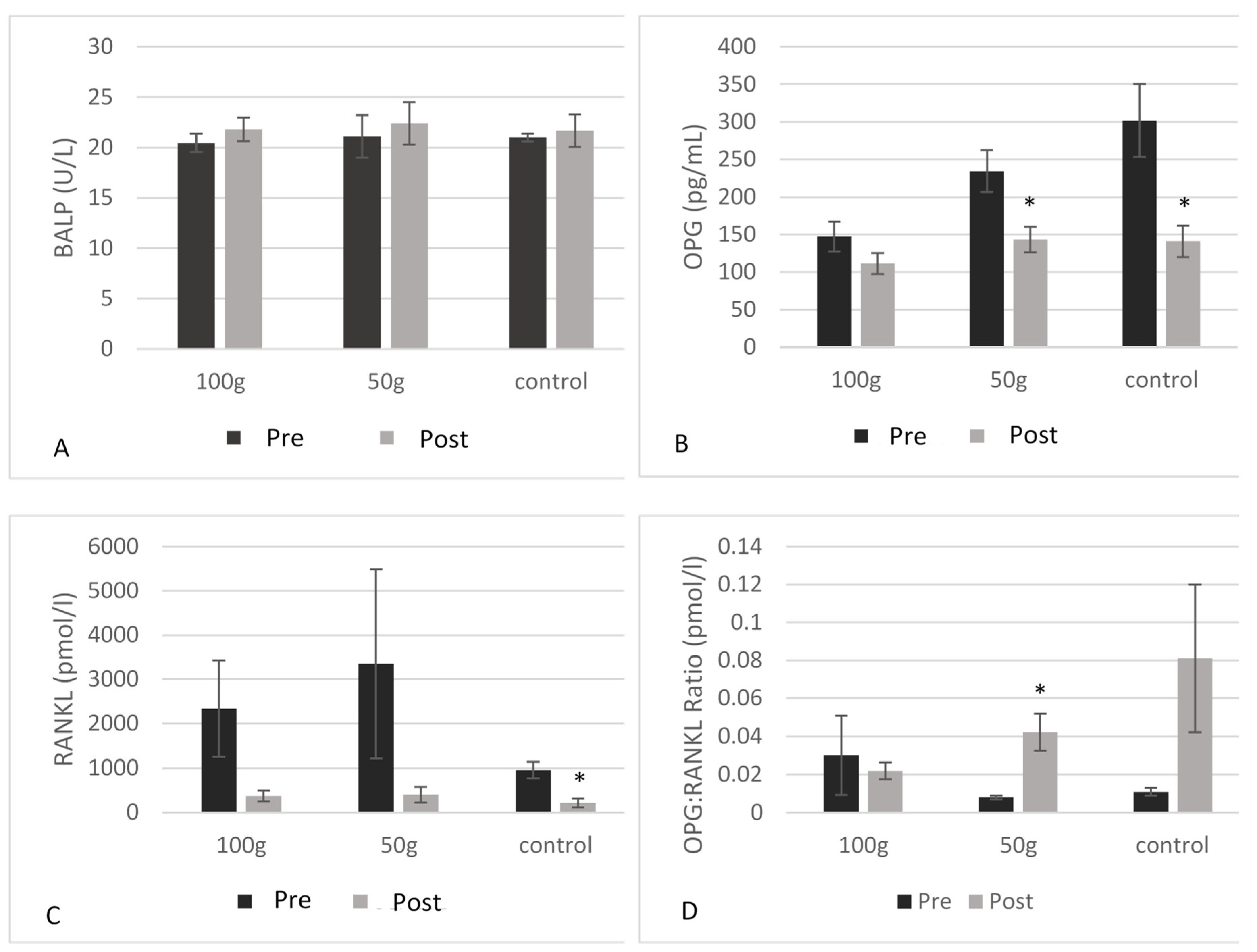
| OPG (pg/mL) | 147.2 ± 80.99 | 111.4 ± 57.87 | 0.155 | 234.5 ± 92.84 | 143.2 ± 56.60 | 0.003 * | 301.6 ± 128.4 | 140.9 ± 55.20 | 0.004 * | 0.019 * |
| RANKL (pmol/L) | 2337 ± 2885 | 373.6 ± 330.7 | 0.129 | 3347 ± 4776 | 403.0 ± 398.9 | 0.208 | 955.2 ± 420.1 | 210.4 ± 222.6 | 0.010 * | 0.535 |
| OPG: RANKL ratio (pmol/l) | 0.030 ± 0.051 | 0.022 ± 0.011 | 0.685 | 0.008 ± 0.002 | 0.042 ± 0.020 | 0.041 * | 0.011 ± 0.004 | 0.081 ± 0.069 | 0.217 | 0.029 * |
| OC (ng/mL) | 10.67 ± 2.39 | 7.13 ± 1.69 | <0.001 * | 10.54 ± 2.25 | 8.98 ± 2.58 | 0.040 * | 12.01 ± 2.55 | 8.86 ± 2.45 | 0.049 * | 0.105 |
| Sclerostin (pg/mL) | 143.8 ± 58.5 | 110.0 ± 62.7 | 0.088 | 146.7 ± 80.9 | 121.5 ± 44.8 | 0.460 | 131.7 ± 63.1 | 136.1 ± 58.3 | 0.839 | 0.543 |
| BALP (U/L) | 20.47 ± 3.4 | 21.80 ± 4.3 | 0.141 | 21.10 ± 5.5 | 22.4 ± 5.5 | 0.300 | 20.99 ± 3.7 | 21.67 ± 4.3 | 0.516 | 0.289 |
| TRAP-5b (U/L) | 2.89 ± 1.12 | 3.00 ± 1.39 | 0.651 | 2.78 ± 1.28 | 3.18 ± 1.71 | 0.362 | 2.76 ± 0.91 | 3.45 ± 1.41 | 0.284 | 0.135 |
| Risk Factor | Correlation (r) | Significance (p Value) |
|---|---|---|
| Age | −0.233 | 0.013 * |
| Ethnicity a | 0.109 | N/A |
| Physical activity levels | −0.031 | 0.866 |
| Body weight | 0.252 | 0.007 * |
| Family history a | 0.279 | N/A |
| Smoking status a | 0.424 | N/A |
| Alcohol consumption | −0.063 | 0.694 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
George, K.S.; Munoz, J.; Ormsbee, L.T.; Akhavan, N.S.; Foley, E.M.; Siebert, S.C.; Kim, J.-S.; Hickner, R.C.; Arjmandi, B.H. The Short-Term Effect of Prunes in Improving Bone in Men. Nutrients 2022, 14, 276. https://doi.org/10.3390/nu14020276
George KS, Munoz J, Ormsbee LT, Akhavan NS, Foley EM, Siebert SC, Kim J-S, Hickner RC, Arjmandi BH. The Short-Term Effect of Prunes in Improving Bone in Men. Nutrients. 2022; 14(2):276. https://doi.org/10.3390/nu14020276
Chicago/Turabian StyleGeorge, Kelli S., Joseph Munoz, Lauren T. Ormsbee, Neda S. Akhavan, Elizabeth M. Foley, Shalom C. Siebert, Jeong-Su Kim, Robert C. Hickner, and Bahram H. Arjmandi. 2022. "The Short-Term Effect of Prunes in Improving Bone in Men" Nutrients 14, no. 2: 276. https://doi.org/10.3390/nu14020276
APA StyleGeorge, K. S., Munoz, J., Ormsbee, L. T., Akhavan, N. S., Foley, E. M., Siebert, S. C., Kim, J.-S., Hickner, R. C., & Arjmandi, B. H. (2022). The Short-Term Effect of Prunes in Improving Bone in Men. Nutrients, 14(2), 276. https://doi.org/10.3390/nu14020276







