Methionine Restriction Prevents Lipopolysaccharide-Induced Acute Lung Injury via Modulating CSE/H2S Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. LPS-Induced ALI Model and Drugs Administration
2.3. IL-1β, IL-6 and TNF-α Measurement in Bronchoalveolar Lavage Fluids
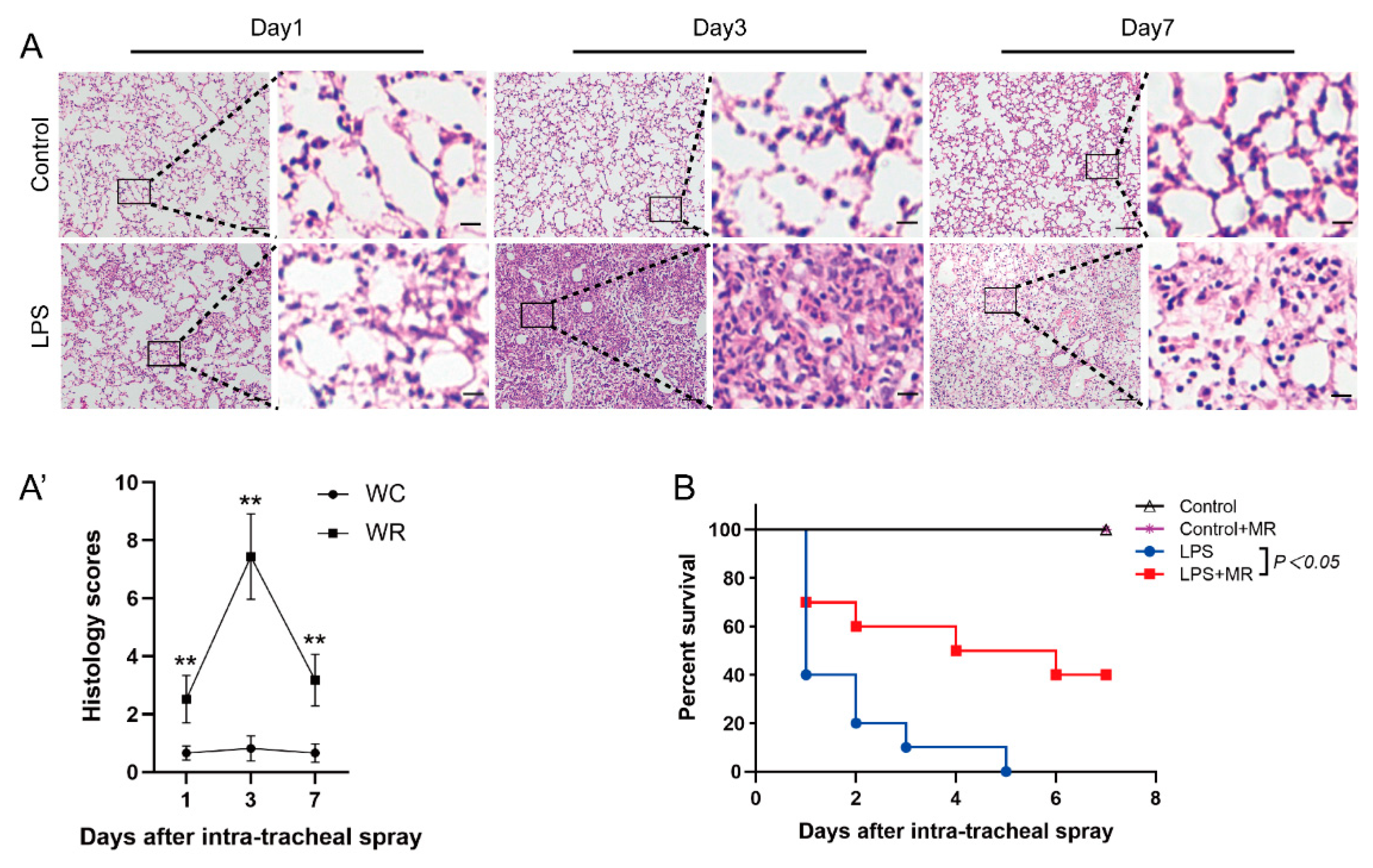
2.4. Histopathology
2.5. Immunochemistry
2.6. H2S Measurement in Plasma and Lung Tissues
2.7. Real-Time Quantitative PCR
2.8. Statistical Analysis
3. Results
3.1. LPS-Induced Lung Injury in Wild Mice
3.2. MR Attenuated LPS-Induced Lung Injury
3.3. MR Ameliorated LPS-Induced Alveolar Epithelial Cell Injuries
3.4. MR Attenuated LPS-Induced Inflammatory Response through TLR4/NF-κB/NLRP3 Signaling
3.5. MR Increased H2S Production by Upregulating CSE but Not CBS/MST in LPS-Induced ALI Mice
3.6. Inhibition of CSE Eliminated the Protective Effects of MR on LPS-Induced ALI and Reversed H2S Levels Increase upon MR
3.7. Exogenous H2S Administration Mimicked the Protective Effects of MR in Cse−/− Mice after LPS Administration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.P.; Bernard, G.R. Acute lung injury and the acute respiratory distress syndrome: A clinical review. Lancet 2007, 369, 1553–1564. [Google Scholar] [CrossRef]
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef]
- Kellner, M.; Noonepalle, S.; Lu, Q.; Srivastava, A.; Zemskov, E.; Black, S.M. ROS Signaling in the Pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS). Adv. Exp. Med. Biol. 2017, 967, 105–137. [Google Scholar] [CrossRef]
- Aujla, S.J.; Chan, Y.R.; Zheng, M.; Fei, M.; Askew, D.J.; Pociask, D.A.; Reinhart, T.A.; McAllister, F.; Edeal, J.; Gaus, K.; et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008, 14, 275–281. [Google Scholar] [CrossRef]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. J. Am. Med. Assoc. 2018, 319, 698–710. [Google Scholar] [CrossRef]
- Hine, C.; Harputlugil, E.; Zhang, Y.; Ruckenstuhl, C.; Lee, B.C.; Brace, L.; Longchamp, A.; Trevino-Villarreal, J.H.; Mejia, P.; Ozaki, C.K.; et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 2015, 160, 132–144. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- Anderson, R.M.; Shanmuganayagam, D.; Weindruch, R. Caloric restriction and aging: Studies in mice and monkeys. Toxicol. Pathol. 2009, 37, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Klein, S. Aging, adiposity, and calorie restriction. J. Am. Med. Assoc. 2007, 297, 986–994. [Google Scholar] [CrossRef]
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W.; et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009, 325, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Longchamp, A.; Mirabella, T.; Arduini, A.; MacArthur, M.R.; Das, A.; Trevino-Villarreal, J.H.; Hine, C.; Ben-Sahra, I.; Knudsen, N.H.; Brace, L.E.; et al. Amino Acid Restriction Triggers Angiogenesis via GCN2/ATF4 Regulation of VEGF and H2S Production. Cell 2018, 173, 117–129.e14. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Ogura, Y.; Monno, I.; Xu, J.; Koya, D. Effect of Methionine Restriction on Aging: Its Relationship to Oxidative Stress. Biomedicines 2021, 9, 130. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huang, Y.; Zhang, R.; Chen, Q.; Chen, J.; Zong, Y.; Liu, J.; Feng, S.; Liu, A.D.; Holmberg, L.; et al. Hydrogen sulfide upregulates KATP channel expression in vascular smooth muscle cells of spontaneously hypertensive rats. J. Mol. Med. 2015, 93, 439–455. [Google Scholar] [CrossRef]
- Guo, C.; Liang, F.; Shah Masood, W.; Yan, X. Hydrogen sulfide protected gastric epithelial cell from ischemia/reperfusion injury by Keap1 s-sulfhydration, MAPK dependent anti-apoptosis and NF-kappaB dependent anti-inflammation pathway. Eur. J. Pharmacol. 2014, 725, 70–78. [Google Scholar] [CrossRef]
- Du, J.; Huang, Y.; Yan, H.; Zhang, Q.; Zhao, M.; Zhu, M.; Liu, J.; Chen, S.X.; Bu, D.; Tang, C.; et al. Hydrogen sulfide suppresses oxidized low-density lipoprotein (ox-LDL)-stimulated monocyte chemoattractant protein 1 generation from macrophages via the nuclear factor kappaB (NF-kappaB) pathway. J. Biol. Chem. 2014, 289, 9741–9753. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhao, T.; Yuan, Y.; Hu, N.; Tang, X. Hydrogen sulfide (H2S) attenuates uranium-induced acute nephrotoxicity through oxidative stress and inflammatory response via Nrf2-NF-kappaB pathways. Chem. Biol. Interact. 2015, 242, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Fu, M.; Chen, X.; Guo, J.; Chen, B.; Tao, X. Dietary methionine restriction attenuates renal ischaemia/reperfusion-induced myocardial injury by activating the CSE/H2S/ERS pathway in diabetic mice. J. Cell. Mol. Med. 2020, 24, 9890–9897. [Google Scholar] [CrossRef]
- Cooke, D.; Ouattara, A.; Ables, G.P. Dietary methionine restriction modulates renal response and attenuates kidney injury in mice. FASEB J. 2018, 32, 693–702. [Google Scholar] [CrossRef]
- Liu, G.; Yu, L.; Fang, J.; Hu, C.A.; Yin, J.; Ni, H.; Ren, W.; Duraipandiyan, V.; Chen, S.; Al-Dhabi, N.A.; et al. Methionine restriction on oxidative stress and immune response in dss-induced colitis mice. Oncotarget 2017, 8, 44511–44520. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, G.; Zhou, Z.; Tang, Z.; Zhang, N.; Zhu, X.; Ni, X. Endogenous Hydrogen Sulfide Is an Important Factor in Maintaining Arterial Oxygen Saturation. Front. Pharmacol. 2021, 12, 677110. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ahmad, S.; Cai, M.; Rennie, J.; Fujisawa, T.; Crispi, F.; Baily, J.; Miller, M.R.; Cudmore, M.; Hadoke, P.W.; et al. Dysregulation of hydrogen sulfide producing enzyme cystathionine gamma-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation 2013, 127, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, S.; Zeng, M.; Lin, D.; Wang, Y.; Wen, X.; Xu, C.; Yang, L.; Fan, X.; Gong, Y.; et al. Apelin-13 Administration Protects Against LPS-Induced Acute Lung Injury by Inhibiting NF-κB Pathway and NLRP3 Inflammasome Activation. Cell. Physiol. Biochem. 2018, 49, 1918–1932. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yang, Z.; Huang, J.; Zhu, Y.; Zhao, H.; Unwith, S.; Gao, X.; Lu, K.; Ning, J. Inhibition of tyrosine kinases protects against lipopolysaccharide-induced acute lung injury by preventing nuclear export of Nrf2. J. Cell. Biochem. 2019, 120, 12331–12339. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Yang, C.; Qin, W.; Gu, J.; Hu, C.; Chen, A.; Ning, J.; Yi, B.; Lu, K. A self-organized actomyosin drives multiple intercellular junction disruption and directly promotes neutrophil recruitment in lipopolysaccharide-induced acute lung injury. FASEB J. 2018, 32, 6197–6211. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Tang, Z.; Li, Z.; Yang, X. Repeatability and Reproducibility of Quantitative Corneal Shape Analysis after Orthokeratology Treatment Using Image-Pro Plus Software. J. Ophthalmol. 2016, 2016, 1732476. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Wang, Z.J.; Ho, P.; Loke, Y.Y.; Zhu, Y.C.; Huang, S.H.; Tan, C.S.; Whiteman, M.; Lu, J.; Moore, P.K. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J. Appl. Physiol. 2007, 102, 261–268. [Google Scholar] [CrossRef]
- Mok, Y.Y.; Atan, M.S.; Yoke Ping, C.; Zhong Jing, W.; Bhatia, M.; Moochhala, S.; Moore, P.K. Role of hydrogen sulphide in haemorrhagic shock in the rat: Protective effect of inhibitors of hydrogen sulphide biosynthesis. Br. J. Pharmacol. 2004, 143, 881–889. [Google Scholar] [CrossRef]
- Borok, Z.; Lubman, R.L.; Danto, S.I.; Zhang, X.L.; Zabski, S.M.; King, L.S.; Lee, D.M.; Agre, P.; Crandall, E.D. Keratinocyte growth factor modulates alveolar epithelial cell phenotype in vitro: Expression of aquaporin. Am. J. Respir. Cell Mol. Biol. 1998, 18, 554–561. [Google Scholar] [CrossRef]
- Yudhawati, R.; Amin, M.; Rantam, F.A.; Prasetya, R.R.; Dewantari, J.R.; Nastri, A.M.; Poetranto, E.D.; Wulandari, L.; Lusida, M.I.; Koesnowidagdo, S.; et al. Bone marrow-derived mesenchymal stem cells attenuate pulmonary inflammation and lung damage caused by highly pathogenic avian influenza A/H5N1 virus in BALB/c mice. BMC Infect. Dis. 2020, 20, 823. [Google Scholar] [CrossRef]
- Desai, T.J.; Brownfield, D.G.; Krasnow, M.A. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 2014, 507, 190–194. [Google Scholar] [CrossRef]
- Moriwaki, K.; Chan, F.K. RIP3: A molecular switch for necrosis and inflammation. Genes Dev. 2013, 27, 1640–1649. [Google Scholar] [CrossRef]
- Giogha, C.; Lawlor, K.E. Sugar Fix Keeps RIPK3 at Bay. Immunity 2019, 50, 539–541. [Google Scholar] [CrossRef]
- Hoque, R.; Farooq, A.; Ghani, A.; Gorelick, F.; Mehal, W.Z. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 2014, 146, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Lu, T.; Zhan, L.; Zhou, C.; Zhang, N.; Lei, S.; Wang, Y.; Yang, J.; Yan, M.; Lv, G.; et al. Physicochemical characterization of polysaccharide from the leaf of Dendrobium officinale and effect on LPS induced damage in GES-1 cell. Int. J. Biol. Macromol. 2020, 149, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Orgeron, M.L.; Stone, K.P.; Wanders, D.; Cortez, C.C.; Van, N.T.; Gettys, T.W. The impact of dietary methionine restriction on biomarkers of metabolic health. Prog. Mol. Biol. Transl. Sci. 2014, 121, 351–376. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H.; Ueki, I. Dealing with methionine/homocysteine sulfur: Cysteine metabolism to taurine and inorganic sulfur. J. Inherit. Metab. Dis. 2011, 34, 17–32. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Tan, Z.; Shi, Y.; Yan, Y.; Liu, W.; Li, G.; Li, R. Impact of endogenous hydrogen sulfide on toll-like receptor pathway in renal ischemia/reperfusion injury in rats. Ren. Fail. 2015, 37, 727–733. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Ma, L.; Cao, X.; Xiao, J.; Chen, J.; Jiao, S.; Gao, Y.; Liu, C.; Duan, Z.; et al. Activation of vascular endothelial growth factor receptor-3 in macrophages restrains TLR4-NF-kappaB signaling and protects against endotoxin shock. Immunity 2014, 40, 501–514. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, S.; Teng, X.; Hu, Z.; Zhang, Z.; Qiu, X.; Tian, D.; Wu, Y. Hydrogen Sulfide Attenuates LPS-Induced Acute Kidney Injury by Inhibiting Inflammation and Oxidative Stress. Oxid. Med. Cell. Longev. 2018, 2018, 6717212. [Google Scholar] [CrossRef]
- Wang, H.; Sun, X.; Lu, Q.; Zemskov, E.A.; Yegambaram, M.; Wu, X.; Wang, T.; Tang, H.; Black, S.M. The mitochondrial redistribution of eNOS is involved in lipopolysaccharide induced inflammasome activation during acute lung injury. Redox Biol. 2021, 41, 101878. [Google Scholar] [CrossRef]
- Abderrazak, A.; Syrovets, T.; Couchie, D.; El Hadri, K.; Friguet, B.; Simmet, T.; Rouis, M. NLRP3 inflammasome: From a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015, 4, 296–307. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Offringa, A.K.; van Eijk, L.E.; Abdulle, A.E.; Hillebrands, J.L.; van der Voort, P.H.J.; van Goor, H.; van Hezik, E.J. N-Acetylcysteine and Hydrogen Sulfide in Coronavirus Disease 2019. Antioxid. Redox Signal 2021, 35. [Google Scholar] [CrossRef]
- Huang, Z.; Zhuang, X.; Xie, C.; Hu, X.; Dong, X.; Guo, Y.; Li, S.; Liao, X. Exogenous Hydrogen Sulfide Attenuates High Glucose-Induced Cardiotoxicity by Inhibiting NLRP3 Inflammasome Activation by Suppressing TLR4/NF-kappaB Pathway in H9c2 Cells. Cell. Physiol. Biochem. 2016, 40, 1578–1590. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, B.S.; Kim, J.I.; Kim, S.E.; Lee, J.; Oh, S.C.; Enkhbayar, P.; Matsushima, N.; Lee, H.; Yoo, O.J.; et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 2007, 130, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.H.; Park, H.H. Crystal structure of TIR domain of TLR6 reveals novel dimeric interface of TIR-TIR interaction for toll-like receptor signaling pathway. J. Mol. Biol. 2014, 426, 3305–3313. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, I.; Medzhitov, R. Two Modes of Ligand Recognition by TLRs. Cell 2007, 130, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Sen, N.; Paul, B.D.; Gadalla, M.M.; Mustafa, A.K.; Sen, T.; Xu, R.; Kim, S.; Snyder, S.H. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol. Cell. 2012, 45, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Faller, S.; Hausler, F.; Goeft, A.; von Itter, M.-N.A.; Gyllenram, V.; Hoetzel, A.; Spassov, S.G. Hydrogen sulfide limits neutrophil transmigration, inflammation, and oxidative burst in lipopolysaccharide-induced acute lung injury. Sci. Rep. 2018, 8, 14676. [Google Scholar] [CrossRef]
- Xu, X.; Li, H.; Gong, Y.; Zheng, H.; Zhao, D. Hydrogen sulfide ameliorated lipopolysaccharide-induced acute lung injury by inhibiting autophagy through PI3K/Akt/mTOR pathway in mice. Biochem. Biophys. Res. Commun. 2018, 507, 514–518. [Google Scholar] [CrossRef]
- Zimmermann, K.K.; Spassov, S.G.; Strosing, K.M.; Ihle, P.M.; Engelstaedter, H.; Hoetzel, A.; Faller, S. Hydrogen Sulfide Exerts Anti-oxidative and Anti-inflammatory Effects in Acute Lung Injury. Inflammation 2018, 41, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Aslami, H.; Heinen, A.; Roelofs, J.J.; Zuurbier, C.J.; Schultz, M.J.; Juffermans, N.P. Suspended animation inducer hydrogen sulfide is protective in an in vivo model of ventilator-induced lung injury. Intensive Care Med. 2010, 36, 1946–1952. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.W.; Wang, H.Y.; Guan, L.; Zhao, B. Regulatory effects of hydrogen sulfide on alveolar epithelial cell endoplasmic reticulum stress in rats with acute lung injury. World J. Emerg. Med. 2015, 6, 67–73. [Google Scholar] [CrossRef]
- Zhang, P.; Li, F.; Wiegman, C.H.; Zhang, M.; Hong, Y.; Gong, J.; Chang, Y.; Zhang, J.J.; Adcock, I.; Chung, K.F.; et al. Inhibitory effect of hydrogen sulfide on ozone-induced airway inflammation, oxidative stress, and bronchial hyperresponsiveness. Am. J. Respir. Cell. Mol. Biol. 2015, 52, 129–137. [Google Scholar] [CrossRef]
- Wagner, F.; Scheuerle, A.; Weber, S.; Stahl, B.; McCook, O.; Knoferl, M.W.; Huber-Lang, M.; Seitz, D.H.; Thomas, J.; Asfar, P.; et al. Cardiopulmonary, histologic, and inflammatory effects of intravenous Na2S after blunt chest trauma-induced lung contusion in mice. J. Trauma. 2011, 71, 1659–1667. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, J.; Xiang, L.; Yang, Z.; Chen, L.; Gu, J.; Lu, K.; Ma, D.; Zhao, H.; Yi, B.; Zhao, H.; et al. Methionine Restriction Prevents Lipopolysaccharide-Induced Acute Lung Injury via Modulating CSE/H2S Pathway. Nutrients 2022, 14, 322. https://doi.org/10.3390/nu14020322
Duan J, Xiang L, Yang Z, Chen L, Gu J, Lu K, Ma D, Zhao H, Yi B, Zhao H, et al. Methionine Restriction Prevents Lipopolysaccharide-Induced Acute Lung Injury via Modulating CSE/H2S Pathway. Nutrients. 2022; 14(2):322. https://doi.org/10.3390/nu14020322
Chicago/Turabian StyleDuan, Jiaxiang, Lunli Xiang, Zhen Yang, Li Chen, Jianteng Gu, Kaizhi Lu, Daqing Ma, Hailin Zhao, Bin Yi, Hongwen Zhao, and et al. 2022. "Methionine Restriction Prevents Lipopolysaccharide-Induced Acute Lung Injury via Modulating CSE/H2S Pathway" Nutrients 14, no. 2: 322. https://doi.org/10.3390/nu14020322






