Application of a Machine Learning Technology in the Definition of Metabolically Healthy and Unhealthy Status: A Retrospective Study of 2567 Subjects Suffering from Obesity with or without Metabolic Syndrome
Abstract
:1. Introduction
- (1)
- Describe the cohort of patients at the time of their first access to our obesity specialisation centre with a rigorous collection of anthropometric, clinical and metabolic data.
- (2)
- Apply AI with a logic ML approach in the obese subgroup of patients to identify new parameters possibly involved mechanistically in the pathogenesis of the metabolic syndrome (either clinical, biochemical or instrumental), which could help distinguish MUO from MHO patients and define the best model capable of predicting the development of MUO, with a special focus on IGF-1 zSDS.
2. Materials and Methods
2.1. Study Design
- −
- Inclusion criteria: age ≥18 years old and body mass index ≥30 kg/m2.
- −
- Exclusion criteria: (1) pregnancy or breastfeeding; (2) patients with type 1 diabetes mellitus and severe chronic liver or kidney dysfunction; (3) tobacco habit and alcohol abuse; (4) current medication with drugs that could lead to weight gain.
2.2. Subjects and Measurements
2.2.1. Anthropometric Measurements
2.2.2. Routine Laboratory Assessments
2.2.3. Hormonal Assessments
2.2.4. Dual-Energy X-ray Absorptiometry
2.3. Characteristics of the Logic Machine Learning (LML)
3. Results
3.1. Population
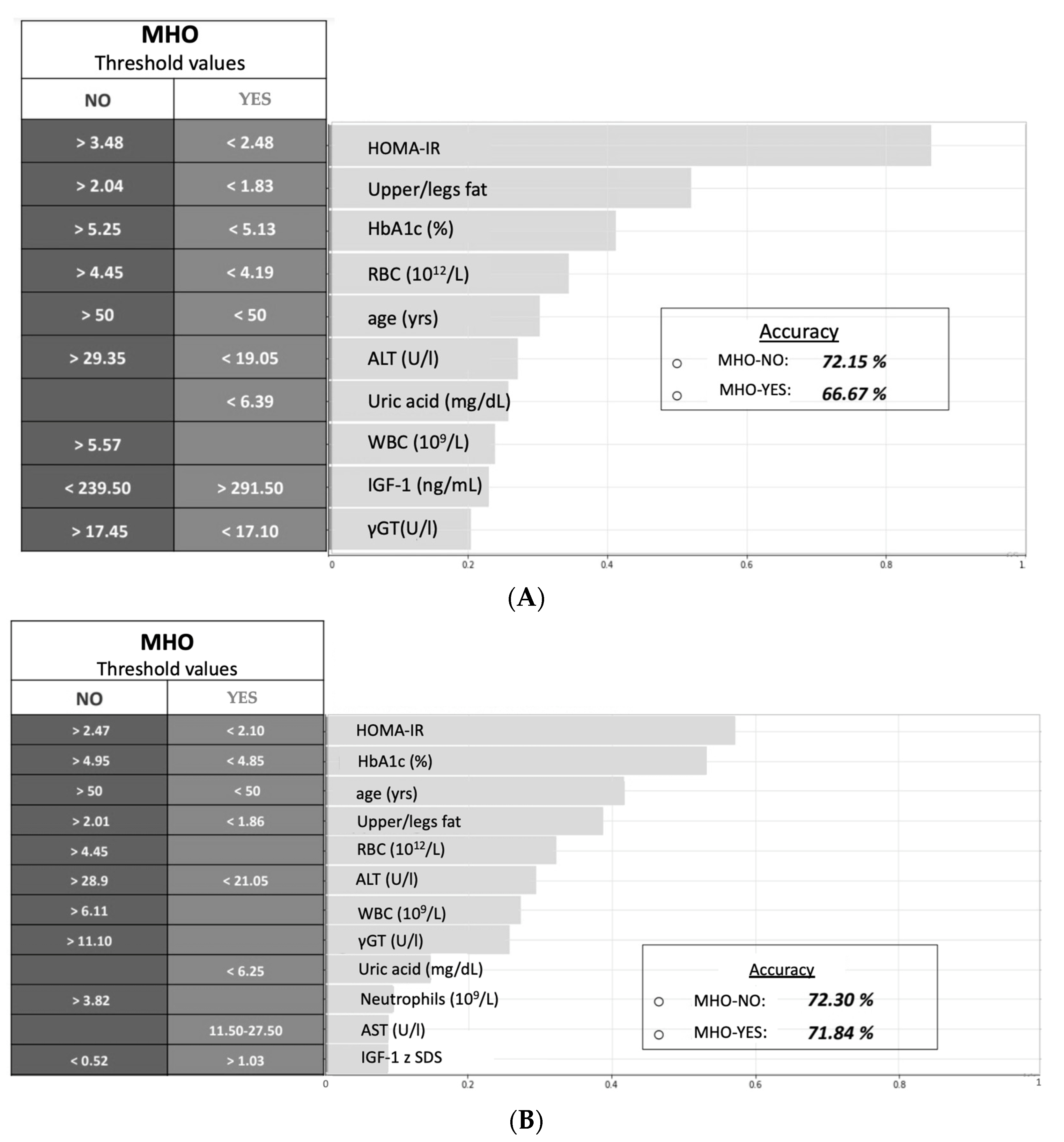
3.2. Logic Machine Learning
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gómez González, E.; Gómez Gutiérrez, E. Artificial Intelligence in Medicine and Healthcare: Applications, Availability and Societal Impact; Publications Office of the European Union: Luxembourg, 2020; ISBN 9789276184546. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC120214 (accessed on 13 January 2022).
- Giorda, C.B.; Pisani, F.; De Micheli, A.; Ponzani, P.; Russo, G.; Guaita, G.; Zilich, R.; Musacchio, N. Determinants of good metabolic control without weight gain in type 2 diabetes management: A machine learning analysis. BMJ Open Diabetes Res. Care 2020, 8, e001362. [Google Scholar] [CrossRef]
- Chen, C. Ascent of machine learning in medicine. Nat. Mater. 2019, 18, 407. [Google Scholar] [CrossRef]
- Dugan, T.M.; Mukhopadhyay, S.; Carroll, A.; Downs, S. Machine Learning Techniques for Prediction of Early Childhood Obesity. Appl. Clin. Inform. 2015, 6, 506–520. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Tawfik, H. Machine Learning Approach for the Early Prediction of the Risk of Overweight and Obesity in Young People. In Proceedings of the Computational Science—ICCS 2020; Krzhizhanovskaya, V.V., Závodszky, G., Lees, M.H., Dongarra, J.J., Sloot, P.M.A., Brissos, S., Teixeira, J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 523–535. [Google Scholar]
- Lin, Z.; Feng, W.; Liu, Y.; Ma, C.; Arefan, D.; Zhou, D.; Cheng, X.; Yu, J.; Gao, L.; Du, L.; et al. Machine Learning to Identify Metabolic Subtypes of Obesity: A Multi-Center Study. Front. Endocrinol. (Lausanne) 2021, 12, 843. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization. Health Topics. Obesity. 2019. Available online: https://www.who.int/topics/obesity/en/ (accessed on 20 November 2021).
- Watanabe, M.; Risi, R.; De Giorgi, F.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Lubrano, C.; Lenzi, A.; Gnessi, L. Obesity treatment within the Italian national healthcare system tertiary care centers: What can we learn ? Eat. Weight Disord.—Stud. Anorexia, Bulim. Obes. 2020, 26, 771–778. [Google Scholar] [CrossRef]
- Després, J.P. Body Fat Distribution and Risk of Cardiovascular Disease: An Update. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef] [Green Version]
- Tsatsoulis, A.; Paschou, S.A. Metabolically Healthy Obesity: Criteria, Epidemiology, Controversies, and Consequences. Curr. Obes. Rep. 2020, 9, 109–120. [Google Scholar] [CrossRef]
- Donini, L.M.; Merola, G.; Poggiogalle, E.; Lubrano, C.; Gnessi, L.; Mariani, S.; Migliaccio, S.; Lenzi, A. Disability, Physical Inactivity, and Impaired Health-Related Quality of Life Are Not Different in Metabolically Healthy vs. Unhealthy Obese Subjects. Nutrients 2016, 8, 759. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhu, X.; Chen, Z.; Yang, P.; Liu, L.; Liu, X.; Wu, L.; He, Q.; Li, Y. Natural histories of metabolite BMI phenotypes and their impacts on cardiovascular disease risk over a decade-long follow-up. Obes. Res. Clin. Pract. 2021, 15, 579–586. [Google Scholar] [CrossRef]
- Samocha-Bonet, D.; Dixit, V.D.; Kahn, C.R.; Leibel, R.L.; Lin, X.; Nieuwdorp, M.; Pietiläinen, K.H.; Rabasa-Lhoret, R.; Roden, M.; Scherer, P.E.; et al. Metabolically healthy and unhealthy obese—The 2013 Stock Conference report. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2014, 15, 697–708. [Google Scholar] [CrossRef]
- Samocha-Bonet, D.; Chisholm, D.J.; Tonks, K.; Campbell, L.V.; Greenfield, J.R. Insulin-sensitive obesity in humans—A “favorable fat” phenotype? Trends Endocrinol. Metab. 2012, 23, 116–124. [Google Scholar] [CrossRef]
- Michalsen, V.L.; Wild, S.H.; Kvaløy, K.; Svartberg, J.; Melhus, M.; Broderstad, A.R. Obesity measures, metabolic health and their association with 15-year all-cause and cardiovascular mortality in the SAMINOR 1 Survey: A population-based cohort study. BMC Cardiovasc. Disord. 2021, 21, 510. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Lubrano, C.; Gnessi, L.; Mariani, S.; Di Martino, M.; Catalano, C.; Lenzi, A.; Donini, L.M. The decline in muscle strength and muscle quality in relation to metabolic derangements in adult women with obesity. Clin. Nutr. 2019, 38, 2430–2435. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.U.; Schulze, M.B. Metabolically healthy obesity: The low-hanging fruit in obesity treatment? Lancet Diabetes Endocrinol. 2018, 6, 249–258. [Google Scholar] [CrossRef]
- Rosén, T.; Bengtsson, B.Å. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet 1990, 336, 285–288. [Google Scholar] [CrossRef]
- Vahl, N.; Klausen, I.; Christiansen, J.S.; Jørgensen, J.O.L. Growth hormone (GH) status is an independent determinant of serum levels of cholesterol and triglycerides in healthy adults. Clin. Endocrinol. 1999, 51, 309–316. [Google Scholar] [CrossRef]
- Laughlin, G.A.; Barrett-Connor, E.; Criqui, M.H.; Kritz-Silverstein, D. The Prospective Association of Serum Insulin-Like Growth Factor I (IGF-I) and IGF-Binding Protein-1 Levels with All Cause and Cardiovascular Disease Mortality in Older Adults: The Rancho Bernardo Study. J. Clin. Endocrinol. Metab. 2004, 89, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Colao, A.; Spiezia, S.; Di Somma, C.; Pivonello, R.; Marzullo, P.; Rota, F.; Musella, T.; Auriemma, R.S.; De Martino, M.C.; Lombardi, G. Circulating insulin-like growth factor-I levels are correlated with the atherosclerotic profile in healthy subjects independently of age. J. Endocrinol. Investig. 2005, 28, 440–448. [Google Scholar] [CrossRef]
- Miller, K.K.; Biller, B.M.K.; Lipman, J.G.; Bradwin, G.; Rifai, N.; Klibanski, A. Truncal adiposity, relative growth hormone deficiency, and cardiovascular risk. J. Clin. Endocrinol. Metab. 2005, 90, 768–774. [Google Scholar] [CrossRef] [Green Version]
- Bancu, I.; Navarro Díaz, M.; Serra, A.; Granada, M.; Lopez, D.; Romero, R.; Bonet, J. Low insulin-like growth factor-1 level in obesity nephropathy: A new risk factor? PLoS ONE 2016, 11, e0154451. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Masieri, S.; Costantini, D.; Tozzi, R.; De Giorgi, F.; Gangitano, E.; Tuccinardi, D.; Poggiogalle, E.; Mariani, S.; Basciani, S.; et al. Overweight and obese patients with nickel allergy have a worse metabolic profile compared to weight matched non-allergic individuals. PLoS ONE 2018, 13, e0202683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risi, R.; Masieri, S.; Poggiogalle, E.; Watanabe, M.; Caputi, A.; Tozzi, R.; Gangitano, E.; Masi, D.; Mariani, S.; Gnessi, L.; et al. Nickel Sensitivity Is Associated with GH-IGF1 Axis Impairment and Pituitary Abnormalities on MRI in Overweight and Obese Subjects. Int. J. Mol. Sci. 2020, 21, 9733. [Google Scholar] [CrossRef]
- Le Roith, D. Insulin-Like Growth Factors. N. Engl. J. Med. 1997, 336, 633–640. [Google Scholar] [CrossRef]
- Colao, A.; Di Somma, C.; Cascella, T.; Pivonello, R.; Vitale, G.; Grasso, L.F.S.; Lombardi, G.; Savastano, S. Relationships between serum IGF1 levels, blood pressure, and glucose tolerance: An observational, exploratory study in 404 subjects. Eur. J. Endocrinol. 2008, 159, 389–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasquali, R.; Casanueva, F.; Haluzik, M.; van Hulsteijn, L.; Ledoux, S.; Monteiro, M.P.; Salvador, J.; Santini, F.; Toplak, H.; Dekkers, O.M. European Society of Endocrinology Clinical Practice Guideline: Endocrine work-up in obesity. Eur. J. Endocrinol. 2020, 182, G1–G32. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, I.; Hizuka, N.; Muraoka, T.; Ichihara, A. Adult growth hormone deficiency: Current concepts. Neurol. Med. Chir. 2014, 54, 599–605. [Google Scholar] [CrossRef] [Green Version]
- Lubrano, C.; Saponara, M.; Barbaro, G.; Specchia, P.; Addessi, E.; Costantini, D.; Tenuta, M.; Di Lorenzo, G.; Genovesi, G.; Donini, L.M.; et al. Relationships between body fat distribution, epicardial fat and obstructive sleep apnea in obese patients with and without metabolic syndrome. PLoS ONE 2012, 7, e47059. [Google Scholar] [CrossRef] [Green Version]
- Schwendicke, F.; Samek, W.; Krois, J. Artificial Intelligence in Dentistry: Chances and Challenges. J. Dent. Res. 2020, 99, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Challen, R.; Denny, J.; Pitt, M.; Gompels, L.; Edwards, T.; Tsaneva-Atanasova, K. Artificial intelligence, bias and clinical safety. BMJ Qual. Saf. 2019, 28, 231–237. [Google Scholar] [CrossRef]
- Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [CrossRef]
- White, A.; Witty, K. Men’s under use of health services—Finding alternative approaches. J. Mens. Health 2009, 6, 95–97. [Google Scholar] [CrossRef]
- Hunt, K.; Adamson, J.; Hewitt, C.; Nazareth, I. Do women consult more than men? A review of gender and consultation for back pain and headache. J. Health Serv. Res. Policy 2011, 16, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Gayoso-Diz, P.; Otero-González, A.; Rodriguez-Alvarez, M.X.; Gude, F.; García, F.; De Francisco, A.; Quintela, A.G. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr. Disord. 2013, 13, 47. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Gayoso-Diz, P.; Otero-Gonzalez, A.; Rodriguez-Alvarez, M.X.; Gude, F.; Cadarso-Suarez, C.; García, F.; De Francisco, A. Insulin resistance index (HOMA-IR) levels in a general adult population: Curves percentile by gender and age. The EPIRCE study. Diabetes Res. Clin. Pract. 2011, 94, 146–155. [Google Scholar] [CrossRef]
- Hildrum, B.; Mykletun, A.; Hole, T.; Midthjell, K.; Dahl, A.A. Age-specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: The Norwegian HUNT 2 study. BMC Public Health 2007, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patni, R.; Mahajan, A. The Metabolic Syndrome and Menopause. J. Midlife. Health 2018, 9, 111–112. [Google Scholar]
- Christakis, M.K.; Hasan, H.; De Souza, L.R.; Shirreff, L. The effect of menopause on metabolic syndrome: Cross-sectional results from the Canadian Longitudinal Study on Aging. Menopause 2020, 27, 999–1009. [Google Scholar] [CrossRef]
- Eshtiaghi, R.; Esteghamati, A.; Nakhjavani, M. Menopause is an independent predictor of metabolic syndrome in Iranian women. Maturitas 2010, 65, 262–266. [Google Scholar] [CrossRef]
- Osei, K.; Rhinesmith, S.; Gaillard, T.; Schuster, D. Is Glycosylated Hemoglobin A1c a Surrogate for Metabolic Syndrome in Nondiabetic, First-Degree Relatives of African-American Patients with Type 2 Diabetes? J. Clin. Endocrinol. Metab. 2003, 88, 4596–4601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geva, M.; Shlomai, G.; Berkovich, A.; Maor, E.; Leibowitz, A.; Tenenbaum, A.; Grossman, E. The association between fasting plasma glucose and glycated hemoglobin in the prediabetes range and future development of hypertension. Cardiovasc. Diabetol. 2019, 18, 53. [Google Scholar] [CrossRef] [Green Version]
- Sung, K.C.; Rhee, E.J. Glycated haemoglobin as a predictor for metabolic syndrome in non-diabetic Korean adults. Diabet. Med. 2007, 24, 848–854. [Google Scholar] [CrossRef] [Green Version]
- Tsushima, Y.; Nishizawa, H.; Tochino, Y.; Nakatsuji, H.; Sekimoto, R.; Nagao, H.; Shirakura, T.; Kato, K.; Imaizumi, K.; Takahashi, H.; et al. Uric acid secretion from adipose tissue and its increase in obesity. J. Biol. Chem. 2013, 288, 27138–27149. [Google Scholar] [CrossRef] [Green Version]
- Risi, R.; Tuccinardi, D.; Mariani, S.; Lubrano, C.; Manfrini, S.; Donini, L.M.; Watanabe, M. Liver disease in obesity and underweight: The two sides of the coin. A narrative review. Eat. Weight Disord. 2021, 26, 2097–2107. [Google Scholar] [CrossRef]
- Watanabe, M.; Risi, R.; Camajani, E.; Contini, S.; Persichetti, A.; Tuccinardi, D.; Ernesti, I.; Mariani, S.; Lubrano, C.; Genco, A.; et al. Baseline HOMA IR and Circulating FGF21 Levels Predict NAFLD Improvement in Patients Undergoing a Low Carbohydrate Dietary Intervention for Weight Loss: A Prospective Observational Pilot Study. Nutrients 2020, 12, 2141. [Google Scholar] [CrossRef]
- EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef]
- Jensen, M.D. Role of Body Fat Distribution and the Metabolic Complications of Obesity. J. Clin. Endocrinol. Metab. 2008, 93, s57–s63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solmaz, S.; Duksal, F.; Ganidağlı, S. Is obstructive sleep apnoea syndrome really one of the causes of secondary polycythaemia? Hematology 2015, 20, 108–111. [Google Scholar] [CrossRef]
- Mardi, T.; Toker, S.; Melamed, S.; Shirom, A.; Zeltser, D.; Shapira, I.; Berliner, S.; Rogowski, O. Increased erythropoiesis and subclinical inflammation as part of the metabolic syndrome. Diabetes Res. Clin. Pract. 2005, 69, 249–255. [Google Scholar] [CrossRef]
- Kotani, K.; Sakane, N.; Kurozawa, Y. Increased red blood cells in patients with metabolic syndrome. Endocr. J. 2006, 53, 711–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Festa, A.; D’Agostino, R.; Howard, G.; Mykkänen, L.; Tracy, R.P.; Haffner, S.M. Chronic Subclinical Inflammation as Part of the Insulin Resistance Syndrome. Circulation 2000, 102, 42–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kannel, W.B.; Anderson, K.; Wilson, P.W.F. White Blood Cell Count and Cardiovascular Disease: Insights from the Framingham Study. JAMA 1992, 267, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Lin, S.-Y.; Liu, P.-H.; Cheung, B.M.H.; Lai, W.-A. Association between hematological parameters and metabolic syndrome components in a Chinese population. J. Diabetes Complicat. 2004, 18, 322–327. [Google Scholar] [CrossRef]
- Watanabe, M.; Risi, R.; Tuccinardi, D.; Baquero, C.J.; Manfrini, S.; Gnessi, L. Obesity and SARS-CoV-2: A population to safeguard. Diabetes. Metab. Res. Rev. 2020, 36, e3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.; Caruso, D.; Tuccinardi, D.; Risi, R.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Tarallo, M.; Strigari, L.; Manfrini, S.; et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism 2020, 111, 154319. [Google Scholar] [CrossRef]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes. Metab. Res. Rev. 2021, 38, e3465. [Google Scholar] [CrossRef]
- Maddaloni, E.; D’Onofrio, L.; Alessandri, F.; Mignogna, C.; Leto, G.; Pascarella, G.; Mezzaroma, I.; Lichtner, M.; Pozzilli, P.; Agrò, F.E.; et al. Cardiometabolic multimorbidity is associated with a worse Covid-19 prognosis than individual cardiometabolic risk factors: A multicentre retrospective study (CoViDiab II). Cardiovasc. Diabetol. 2020, 19, 164. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.-L.; Yang, C.-Y.; Yen, B.L.; Ho, Y.-L.; Cheng, W.-C.; Bai, C.-H. Increased high sensitivity C-reactive protein and neutrophil count are related to increased standard cardiovascular risk factors in healthy Chinese men. Int. J. Cardiol. 2006, 110, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, C.; Tenuta, M.; Costantini, D.; Specchia, P.; Barbaro, G.; Basciani, S.; Mariani, S.; Pontecorvi, A.; Lenzi, A.; Gnessi, L. Severe growth hormone deficiency and empty sella in obesity: A cross-sectional study. Endocrine 2015, 49, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, C.; Masi, D.; Risi, R.; Balena, A.; Watanabe, M.; Mariani, S.; Gnessi, L. Is Growth Hormone Insufficiency the Missing Link Between Obesity, Male Gender, Age, and COVID-19 Severity? Obesity 2020, 28, 2038–2039. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, D.R.; Moses, A.C.; McKay, M.J.; Sommer, A.; Rosen, D.M.; Ruckle, J. The Combination of Insulin-Like Growth Factor I and Insulin-Like Growth Factor-Binding Protein-3 Reduces Insulin Requirements in Insulin-Dependent Type 1 Diabetes: Evidence for in VivoBiological Activity1. J. Clin. Endocrinol. Metab. 2000, 85, 1518–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillespie, C.M.; Merkel, A.L.; Martin, A.A. Effects of insulin-like growth factor-I and LR3IGF-I on regional blood flow in normal rats. J. Endocrinol. 1997, 155, 351–358. [Google Scholar] [CrossRef]
- Fornari, R.; Marocco, C.; Francomano, D.; Fittipaldi, S.; Lubrano, C.; Bimonte, V.M.; Donini, L.M.; Nicolai, E.; Aversa, A.; Lenzi, A.; et al. Insulin growth factor-1 correlates with higher bone mineral density and lower inflammation status in obese adult subjects. Eat. Weight Disord. 2018, 23, 375–381. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Lubrano, C.; Gnessi, L.; Mariani, S.; Lenzi, A.; Donini, L.M. Fatty Liver Index Associates with Relative Sarcopenia and GH/IGF- 1 Status in Obese Subjects. PLoS ONE 2016, 11, e0145811. [Google Scholar] [CrossRef]
- Teeratakulpisarn, N.; Charoensri, S.; Theerakulpisut, D.; Pongchaiyakul, C. FRAX score with and without bone mineral density: A comparison and factors affecting the discordance in osteoporosis treatment in Thais. Arch. Osteoporos. 2021, 16, 44. [Google Scholar] [CrossRef]
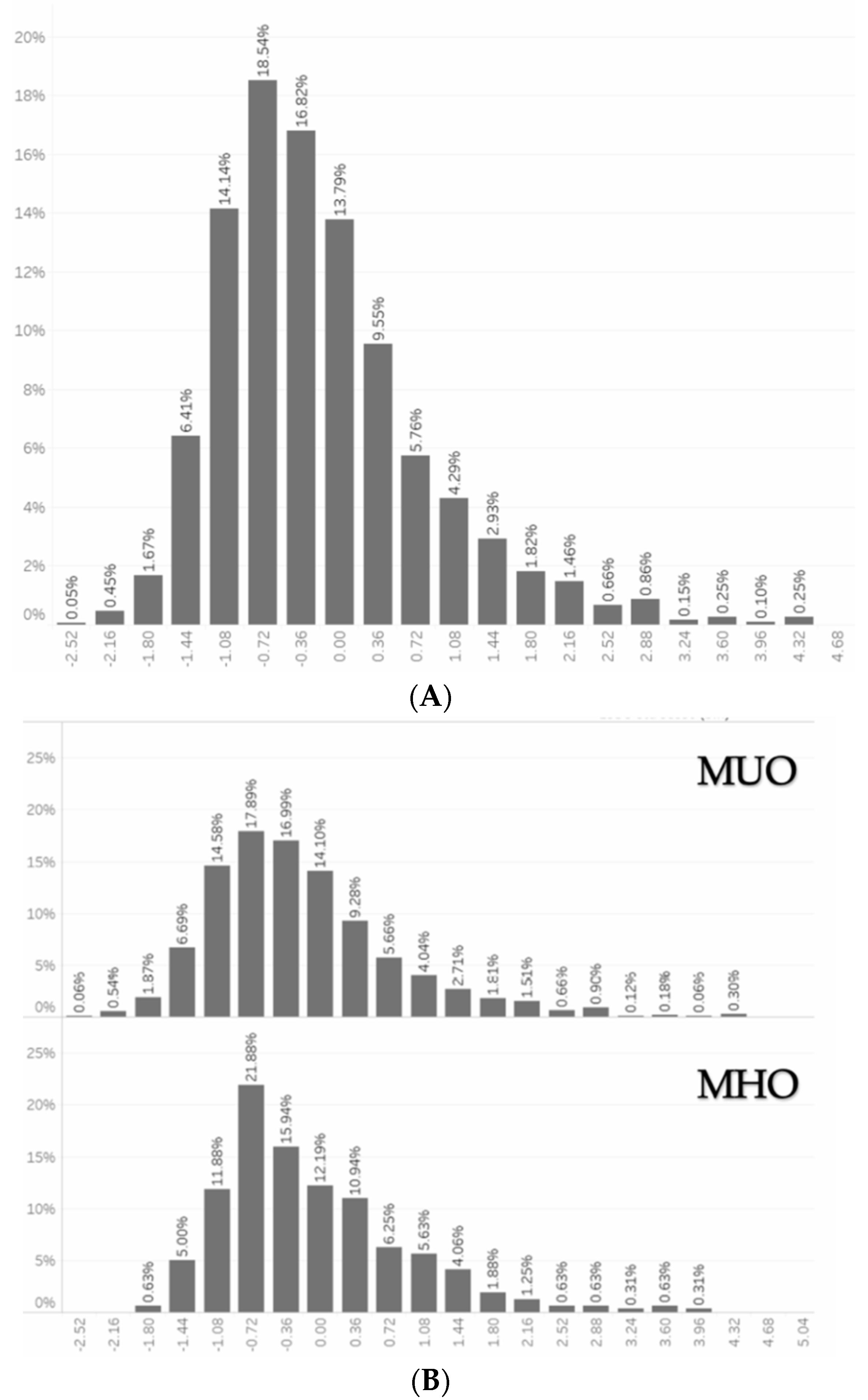
| MHO (n = 695) | MUO (n = 1872) | Overall (n = 2567) | |
|---|---|---|---|
| Age (yrs) | 45.9 ± 13.5 | 47.6 ± 13.5 ** | 47.1 ± 13.4 |
| Gender (%F) | 82.3% | 74.6% * | 76.7% |
| Obesity duration (yrs) | 25.5 ± 15.4 | 26.4 ± 15.1 | 26.1 ± 15.2 |
| BMI (kg/m2) | 38.0 ± 6.1 | 39.8 ± 6.8 *** | 39.3 ± 6.6 |
| WC (cm) | 116.6 ± 15.3 | 121.9 ± 15.4 ** | 120.5 ± 15.4 |
| HC (cm) | 121.5 ± 14.5 | 122.4 ± 14.9 | 122.2 ± 14.7 |
| WHR | 0.95 ± 0.12 | 0.99 ± 0.09 | 1.0 ± 0.1 |
| SBP (mmHg) | 126.4 ± 10.9 | 131.9 ± 16.3 * | 130.4 ± 15.2 |
| DBP (mmHg) | 79.3 ± 10.8 | 83.1 ± 11.1 ** | 82.1 ± 11.0 |
| IGF-1 (ng/mL) | 165.2 ± 77.2 | 154.4 ± 74.5 * | 157.3 ± 76.1 |
| IGF-1 zSDS | −0.96 ± 2.3 | −1.1 ± 1.96 | −1.1 ± 2.1 |
| AST (U/L) | 19.5 ± 7.5 | 22.1 ± 12.1 *** | 21.4 ± 8.7 |
| ALT (U/L) | 23.7 ± 16.4 | 30.3 ± 22.1 *** | 28.5 ± 21.3 |
| γ GT (U/L) | 23.4 ± 24.4 | 28.9 ± 16.5 * | 27.4 ± 19.4 |
| Uric acid (mg/dL) | 4.9 ± 1.3 | 5.5 ± 1.5 *** | 5.3 ± 1.4 |
| HOMA-IR | 3.5 ± 3.2 | 5.7 ± 5.4 *** | 5.1 ± 4.5 |
| HbA1c (%) | 5.7 ± 1.1 | 6.2 ± 1.1 | 6.1 ± 1.1 |
| Vitamin D (ng/mL) | 21.9 ± 10.2 | 20.5 ± 10.3 ** | 20.9 ± 10.3 |
| Folate (ng/mL) | 7.9 ± 23.2 | 8.8 ± 35.3 | 8.6 ± 28.4 |
| TG (mg/dL) | 91.6 ± 27.2 | 150 ± 80.1 *** | 134.2 ± 62.7 |
| TC (mg/dL) | 144 ± 33.3 | 195.1 ± 41 *** | 181,3 ± 37.2 |
| HDLC (mg/dL) | 59.6 ± 11.3 | 45.2 ± 10.6 ** | 49.1 ± 10.9 |
| LDLC (mg/dL) | 116.5 ± 30.7 | 120.1 ± 30.2 ** | 119.1 ± 30.5 |
| Creatinine (mg/dL) | 0.7 ± 0.16 | 0.8 ± 0.23 | 0.8 ± 0.19 |
| Ca (mg/dL) | 9.32 ± 0.44 | 9.34 ± 0.44 | 9.3 ± 0.44 |
| Ph (mg/dL) | 3.5 ± 0.5 | 3.5 ± 0.6 | 3.5 ± 0.6 |
| Na (mmol/L) | 141.5 ± 2.6 | 140.9 ± 2.5 | 141.1 ± 2.5 |
| K (mmol/L) | 4.2 ± 0.3 | 4.2 ± 0.4 | 4.2 ± 0.4 |
| Albumin (g/dL) | 4.3 ± 0.4 | 4.3 ± 0.4 | 4.3 ± 0.4 |
| CRP (µg/L) | 0.5 ± 0.5 | 0.7 ± 0.6 ** | 0.6 ± 0.6 |
| ESR (mm/h) | 26.1 ± 16.4 | 27.9 ± 17.2 * | 27.4 ± 16.8 |
| Body fat (%) | 41.6 ± 6.3 | 40.7 ± 6.7 ** | 40.9 ± 6.5 |
| Lean mass (%) | 58.4 ± 6.4 | 59.3 ± 6.7 ** | 59.1 ± 6.6 |
| Trunk fat (%) | 39.1 ± 6.5 | 39.4 ± 6.5 | 39.3 ± 6.5 |
| Upper/legs fat | 1.62 ± 0.3 | 1.97 ± 0.36 *** | 1.9 ± 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masi, D.; Risi, R.; Biagi, F.; Vasquez Barahona, D.; Watanabe, M.; Zilich, R.; Gabrielli, G.; Santin, P.; Mariani, S.; Lubrano, C.; et al. Application of a Machine Learning Technology in the Definition of Metabolically Healthy and Unhealthy Status: A Retrospective Study of 2567 Subjects Suffering from Obesity with or without Metabolic Syndrome. Nutrients 2022, 14, 373. https://doi.org/10.3390/nu14020373
Masi D, Risi R, Biagi F, Vasquez Barahona D, Watanabe M, Zilich R, Gabrielli G, Santin P, Mariani S, Lubrano C, et al. Application of a Machine Learning Technology in the Definition of Metabolically Healthy and Unhealthy Status: A Retrospective Study of 2567 Subjects Suffering from Obesity with or without Metabolic Syndrome. Nutrients. 2022; 14(2):373. https://doi.org/10.3390/nu14020373
Chicago/Turabian StyleMasi, Davide, Renata Risi, Filippo Biagi, Daniel Vasquez Barahona, Mikiko Watanabe, Rita Zilich, Gabriele Gabrielli, Pierluigi Santin, Stefania Mariani, Carla Lubrano, and et al. 2022. "Application of a Machine Learning Technology in the Definition of Metabolically Healthy and Unhealthy Status: A Retrospective Study of 2567 Subjects Suffering from Obesity with or without Metabolic Syndrome" Nutrients 14, no. 2: 373. https://doi.org/10.3390/nu14020373
APA StyleMasi, D., Risi, R., Biagi, F., Vasquez Barahona, D., Watanabe, M., Zilich, R., Gabrielli, G., Santin, P., Mariani, S., Lubrano, C., & Gnessi, L. (2022). Application of a Machine Learning Technology in the Definition of Metabolically Healthy and Unhealthy Status: A Retrospective Study of 2567 Subjects Suffering from Obesity with or without Metabolic Syndrome. Nutrients, 14(2), 373. https://doi.org/10.3390/nu14020373










