A Specific High-Protein Weight Loss Program Does Not Impair Renal Function in Patients Who Are Overweight/Obese
Abstract
:1. Introduction
2. Materials and Methods
2.1. RNPC© Program
2.2. Data Definitions
2.3. Population
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Population
3.2. Evolution of the Anthropometric Measures
3.3. Evolution of the MDRD Measures
3.4. Sensitivity Analyses
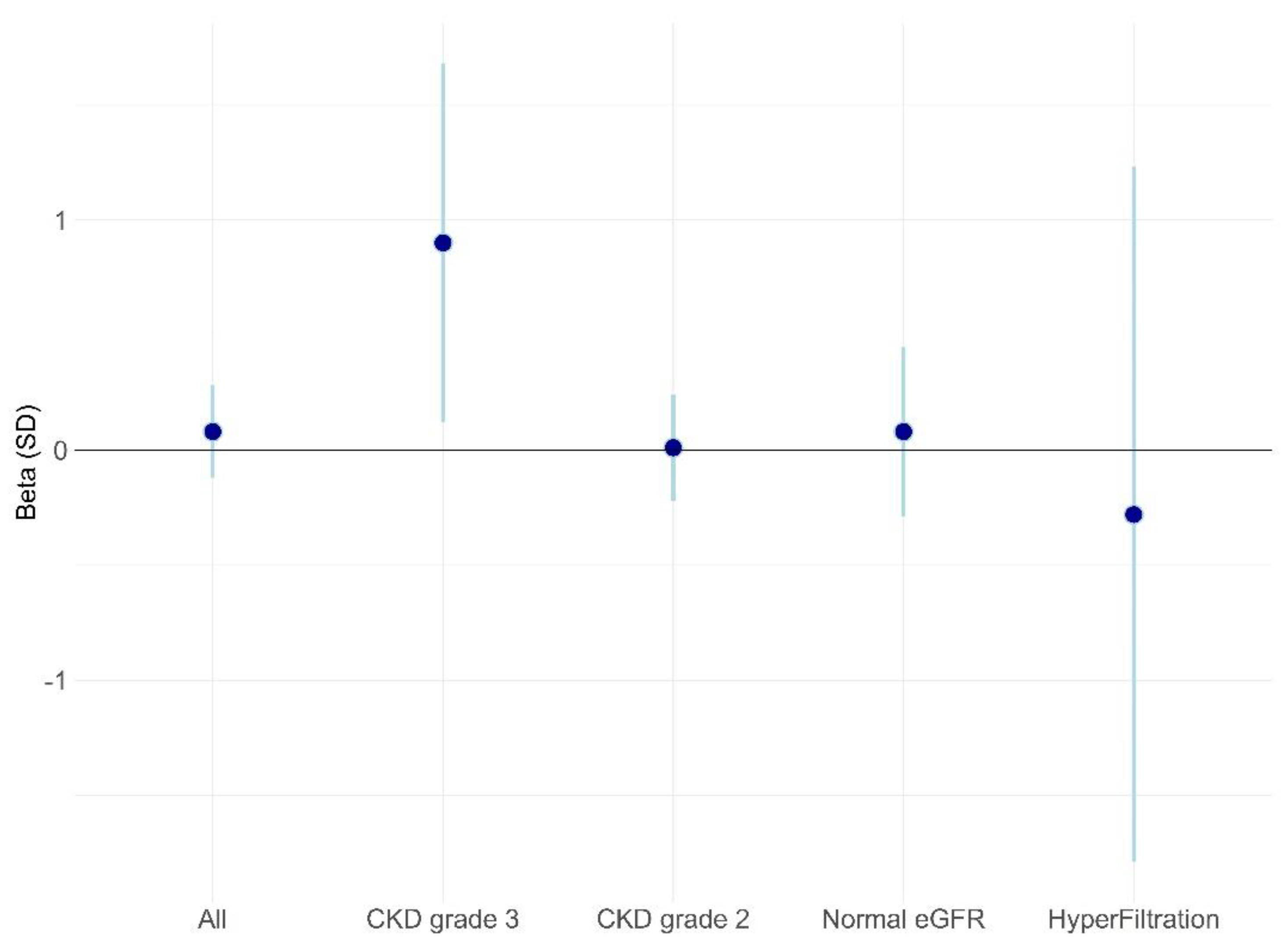
3.5. Multivariable Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 November 2021).
- Garofalo, C.; Borrelli, S.; Minutolo, R.; Chiodini, P.; De Nicola, L.; Conte, G. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 2017, 91, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- D’Agati, V.D.; Chagnac, A.; de Vries, A.P.; Levi, M.; Porrini, E.; Herman-Edelstein, M.; Praga, M. Obesity-related glomerulopathy: Clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 2016, 12, 453–471. [Google Scholar] [CrossRef]
- Group, C.W. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Haase, C.L.; Lopes, S.; Olsen, A.H.; Satylganova, A.; Schnecke, V.; McEwan, P. Weight loss and risk reduction of obesity-related outcomes in 0.5 million people: Evidence from a UK primary care database. Int. J. Obes. 2021, 45, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.S.; Sjödin, A.; Fabre, O.; Legrand, R.; Astrup, A.; Hjorth, M.F. Weight loss following an intensive dietary weight loss program in obese candidates for bariatric surgery: The retrospective RNPC® cohort. Obes. Med. 2019, 15, 100127. [Google Scholar] [CrossRef]
- de las Fuentes, L.; Waggoner, A.D.; Mohammed, B.S.; Stein, R.I.; Miller, B.V., 3rd; Foster, G.D.; Wyatt, H.R.; Klein, S.; Davila-Roman, V.G. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J. Am. Coll. Cardiol. 2009, 54, 2376–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L.; et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Avenell, A.; Bolland, M.; Hudson, J.; Stewart, F.; Robertson, C.; Sharma, P.; Fraser, C.; MacLennan, G. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: Systematic review and meta-analysis. BMJ 2017, 359, j4849. [Google Scholar] [CrossRef] [Green Version]
- Larsen, T.M.; Dalskov, S.M.; van Baak, M.; Jebb, S.A.; Papadaki, A.; Pfeiffer, A.F.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunesova, M.; Pihlsgard, M.; et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N. Engl. J. Med. 2010, 363, 2102–2113. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Kramer, H.M.; Fouque, D. High-protein diet is bad for kidney health: Unleashing the taboo. Nephrol. Dial. Transpl. 2020, 35, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Thorning, T.K.; Fabre, O.; Legrand, R.; Astrup, A.; Hjorth, M.F. Weight loss and weight loss maintenance efficacy of a novel weight loss program: The retrospective RNPC® cohort. Obes. Med. 2018, 10, 16–23. [Google Scholar] [CrossRef]
- Christensen, L.; Thorning, T.K.; Fabre, O.; Legrand, R.; Astrup, A.; Hjorth, M.F. Metabolic improvements during weight loss: The RNPC® cohort. Obes. Med. 2019, 14, 100085. [Google Scholar] [CrossRef]
- Black, A.E.; Coward, W.A.; Cole, T.J.; Prentice, A.M. Human energy expenditure in affluent societies: An analysis of 574 doubly-labelled water measurements. Eur. J. Clin. Nutr. 1996, 50, 72–92. [Google Scholar] [PubMed]
- Camara, N.O.; Iseki, K.; Kramer, H.; Liu, Z.H.; Sharma, K. Kidney disease and obesity: Epidemiology, mechanisms and treatment. Nat. Rev. Nephrol. 2017, 13, 181–190. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Song, Y.; Caballero, B.; Cheskin, L.J. Association between obesity and kidney disease: A systematic review and meta-analysis. Kidney Int. 2008, 73, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Piche, M.E.; Auclair, A.; Harvey, J.; Marceau, S.; Poirier, P. How to choose and use bariatric surgery in 2015. Can. J. Cardiol. 2015, 31, 153–166. [Google Scholar] [CrossRef]
- Bilha, S.C.; Nistor, I.; Nedelcu, A.; Kanbay, M.; Scripcariu, V.; Timofte, D.; Siriopol, D.; Covic, A. The Effects of Bariatric Surgery on Renal Outcomes: A Systematic Review and Meta-analysis. Obes. Surg. 2018, 28, 3815–3833. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, V.; Franzen, S.; Svensson, A.M.; Sattar, N.; Miftaraj, M.; Bjorck, S.; Ottosson, J.; Naslund, I.; Gudbjornsdottir, S.; Eliasson, B. Renal and Cardiovascular Outcomes After Weight Loss From Gastric Bypass Surgery in Type 2 Diabetes: Cardiorenal Risk Reductions Exceed Atherosclerotic Benefits. Diabetes Care 2020, 43, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, N.A.; Perez, S.; Mondestin, M.; Davis, S.S.; Lin, E.; Tata, S.; Kirk, A.D.; Larsen, C.P.; Pearson, T.C.; Sweeney, J.F. The impact of renal function on outcomes of bariatric surgery. J. Am. Soc. Nephrol. 2012, 23, 885–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Ryden, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jodar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.L.; Rorth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Kober, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Tsimihodimos, V.; Filippas-Ntekouan, S.; Elisaf, M. SGLT1 inhibition: Pros and cons. Eur. J. Pharm. 2018, 838, 153–156. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heerspink, H.J.L.; Stefansson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- van Bommel, E.J.M.; Muskiet, M.H.A.; van Baar, M.J.B.; Tonneijck, L.; Smits, M.M.; Emanuel, A.L.; Bozovic, A.; Danser, A.H.J.; Geurts, F.; Hoorn, E.J.; et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int. 2020, 97, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Afshinnia, F.; Wilt, T.J.; Duval, S.; Esmaeili, A.; Ibrahim, H.N. Weight loss and proteinuria: Systematic review of clinical trials and comparative cohorts. Nephrol. Dial. Transpl. 2010, 25, 1173–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navaneethan, S.D.; Yehnert, H.; Moustarah, F.; Schreiber, M.J.; Schauer, P.R.; Beddhu, S. Weight loss interventions in chronic kidney disease: A systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 2009, 4, 1565–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity, kidney dysfunction and hypertension: Mechanistic links. Nat. Rev. Nephrol. 2019, 15, 367–385. [Google Scholar] [CrossRef]
- Diaz-Lopez, A.; Becerra-Tomas, N.; Ruiz, V.; Toledo, E.; Babio, N.; Corella, D.; Fito, M.; Romaguera, D.; Vioque, J.; Alonso-Gomez, A.M.; et al. Effect of an Intensive Weight-Loss Lifestyle Intervention on Kidney Function: A Randomized Controlled Trial. Am. J. Nephrol. 2021, 52, 45–58. [Google Scholar] [CrossRef]
- Ko, G.J.; Rhee, C.M.; Kalantar-Zadeh, K.; Joshi, S. The Effects of High-Protein Diets on Kidney Health and Longevity. J. Am. Soc. Nephrol. 2020, 31, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.L.; Stampfer, M.J.; Hankinson, S.E.; Spiegelman, D.; Curhan, G.C. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann. Intern. Med. 2003, 138, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Esmeijer, K.; Geleijnse, J.M.; de Fijter, J.W.; Kromhout, D.; Hoogeveen, E.K. Dietary protein intake and kidney function decline after myocardial infarction: The Alpha Omega Cohort. Nephrol. Dial. Transpl. 2020, 35, 106–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhee, J.H.; Kee, Y.K.; Park, S.; Kim, H.; Park, J.T.; Han, S.H.; Kang, S.W.; Yoo, T.H. High-protein diet with renal hyperfiltration is associated with rapid decline rate of renal function: A community-based prospective cohort study. Nephrol. Dial. Transpl. 2020, 35, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.N.; Ogden, L.G.; Foster, G.D.; Klein, S.; Stein, R.; Miller, B.; Hill, J.O.; Brill, C.; Bailer, B.; Rosenbaum, D.R.; et al. Comparative effects of low-carbohydrate high-protein versus low-fat diets on the kidney. Clin. J. Am. Soc. Nephrol. 2012, 7, 1103–1111. [Google Scholar] [CrossRef] [Green Version]
- Porrini, E.; Ruggenenti, P.; Luis-Lima, S.; Carrara, F.; Jimenez, A.; de Vries, A.P.J.; Torres, A.; Gaspari, F.; Remuzzi, G. Estimated GFR: Time for a critical appraisal. Nat. Rev. Nephrol. 2019, 15, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Sebekova, K.; Gurecka, R.; Tothova, L.; Podracka, L. Creatinine-Based Formulae Poorly Match in the Classification of Hypofiltration or Hyperfiltration in a General Population of Adolescents: A Retrospective Analysis of a Cross-Sectional Study. Front. Pediatr. 2021, 9, 719997. [Google Scholar] [CrossRef]
- Fabbian, F.; Pala, M.; Monesi, M.; De Giorgi, A.; Mallozzi Menegatti, A.; Tomasi, F.; Portaluppi, F.; Manfredini, R. The estimation of glomerular filtration rate in type 2 diabetic patients may depend on the equation used. Eur. Rev. Med. Pharm. Sci. 2013, 17, 2791–2797. [Google Scholar]
- Wuerzner, G.; Bochud, M.; Giusti, V.; Burnier, M. Measurement of glomerular filtration rate in obese patients: Pitfalls and potential consequences on drug therapy. Obes. Facts 2011, 4, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Jesudason, D.R.; Clifton, P. Interpreting different measures of glomerular filtration rate in obesity and weight loss: Pitfalls for the clinician. Int. J. Obes. 2012, 36, 1421–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juraschek, S.P.; Appel, L.J.; Anderson, C.A.; Miller, E.R., 3rd. Effect of a high-protein diet on kidney function in healthy adults: Results from the OmniHeart trial. Am. J. Kidney Dis. 2013, 61, 547–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppini, L.Z.; Waitzberg, D.L.; Campos, A.C. Limitations and validation of bioelectrical impedance analysis in morbidly obese patients. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 329–332. [Google Scholar] [CrossRef] [PubMed]

| All (N = 4394) | CKD Grade 3 N = 221 (5%) | CKD Grade 2 N = 2383 (54.2%) | Normal eGFR N = 1579 (35.9%) | Hyperfiltration N = 210 (4.8%) | Overall p-Value | |
|---|---|---|---|---|---|---|
| Age | 57 (48; 65) | 68 (61; 74) | 60 (51; 67) | 54 (46; 62) | 48 (33; 58) | <0.01 |
| Sex (Woman) N (%) | 3379 (76.9) | 146 (66.1) | 1871 (78.5) | 1208 (76.5) | 154 (73.3) | <0.01 |
| Hypertension N (%) | 1731 (52.8) | 148 (72.5) | 993 (54.2) | 518 (47.3) | 71 (49.7) | <0.01 |
| Diabetes N (%) | 504 (15.4) | 48 (23.5) | 244 (13.3) | 174 (15.90) | 37 (25.9) | <0.01 |
| No hypertension No diabetes N (%) | 1383 (31.5) | 50 (22.6) | 761 (31.9) | 515 (32.6) | 57 (27.1) | 0.01 |
| Initial values | ||||||
| BMI (kg/m2) | 33.8 (30.6; 37.7) | 34.4 (31.3; 38.1) | 33.5 (30.4; 37.4) | 33.9 (30.7; 37.8) | 35.4 (31.0; 38.9) | <0.01 |
| Weight (kg) | 92.2 (81.2; 105.2) | 96.9 (81.4; 109.6) | 91.3 (80.8; 104.1) | 92.5 (81.5; 106.1) | 94.9 (83.1; 109.6) | <0.01 |
| Waist circumference (cm) | 110 (101; 120) | 114 (104; 123) | 109 (101; 118) | 110 (101; 120) | 112 (103; 123) | <0.01 |
| Muscle mass (%) | 28.9 (27.0; 31.9) | 28.0 (25.6; 31.0) | 28.5 (26.8; 31.5) | 29.3 (27.5; 32.3) | 30.5 (28.0; 33.1) | <0.01 |
| Fat mass (%) | 42.0 (38.6; 45.3) | 42.7 (39.4; 46.6) | 42.1 (38.8; 45.3) | 41.6 (38.2; 45.2) | 41.5 (37.9; 45.0) | <0.01 |
| Serum creatinine (mg/L) | 7.91 (7.0; 9.1) | 11.5 (10.4; 13.7) | 8.4 (7.8; 9.5) | 7.0 (6.5; 7.6) | 5.8 (5.3; 6.6) | <0.01 |
| MDRD (mL/min/1.73 m2) | 85.0 (74.0; 97.0) | 54.0 (49.0; 57.5) | 78.0 (71.0; 83.0) | 98.0 (93.0; 106.0) | 130.0 (124.0; 137.5) | <0.01 |
| Duration between two measures (days) | 238 (205; 282) | 234 (210; 276) | 237 (205; 281) | 239 (205; 284) | 236 (205; 282) | 0.89 |
| All (N = 4394) | CKD Grade 3 N = 221 (5%) | CKD Grade 2 N = 2383 (54.2%) | Normal eGFR N = 1579 (35.9%) | Hyperfiltration N = 210 (4.8%) | Overall p-Value | |
|---|---|---|---|---|---|---|
| Delta weight (kg) | −14.5 (−19.9; −10.1) | −13.3 (−19.5; −9.4) | −14.3 (−19.4; −10) | −14.8 (−20.7; −10.4) | −14.8 (−21.5; −11.4) | <0.01 |
| Delta BMI (kg/m2) | −5.3 (−7.2; −3.7) | −4.8 (−6.7; −3.4) | −5.3 (−7.1; −3.7) | −5.5 (−7.5; −3.8) | −5.4 (−7.7; −4.3) | <0.01 |
| Delta waist circumference (cm) | −16 (−20; −12) | −15 (−19; −12) | −15 (−20; −11) | −16 (−21; −12) | −16 (−21; −12) | <0.01 |
| Delta muscle mass (%) | 1.3 (0.5; 2.1) | 1.2 (0.4; 2.1) | 1.2 (0.5; 2.1) | 1.3 (0.6; 2.2) | 1.5 (0.6; 2.4) | 0.03 |
| Delta fat mass (%) | −4.4 (−6.5; −2.8) | −3.7 (−6.1; −2.1) | −4.3 (−6.4; −2.7) | −4.6 (−6.6; −2.9) | −4.7 (−6.7; −2.7) | 0.04 |
| All (N = 4394) | CKD Grade 3 N = 221 (5%) | CKD Grade 2 N = 2383 (54.2%) | Normal eGFR N = 1579 (35.9%) | Hyperfiltration N = 210 (4.8%) | Overall p-Value | |
|---|---|---|---|---|---|---|
| Initial MDRD | 85 (74; 97) | 54 (49; 58) | 78 (71; 83) | 98 (93; 106) | 130 (124; 138) | <0.01 * |
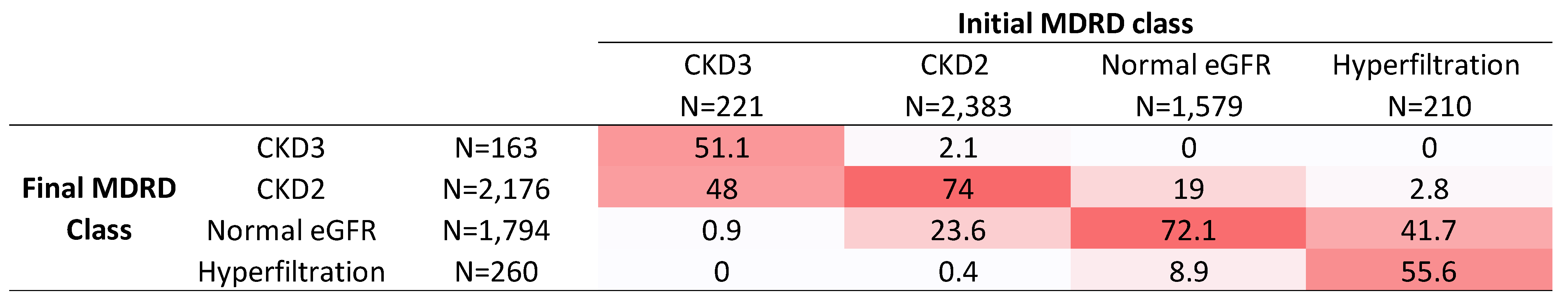
| Final MDRD | 88 (77; 101) | 59 (52; 65) | 81 (74; 89) | 100 (92; 110) | 122 (109; 136) | <0.01 * |
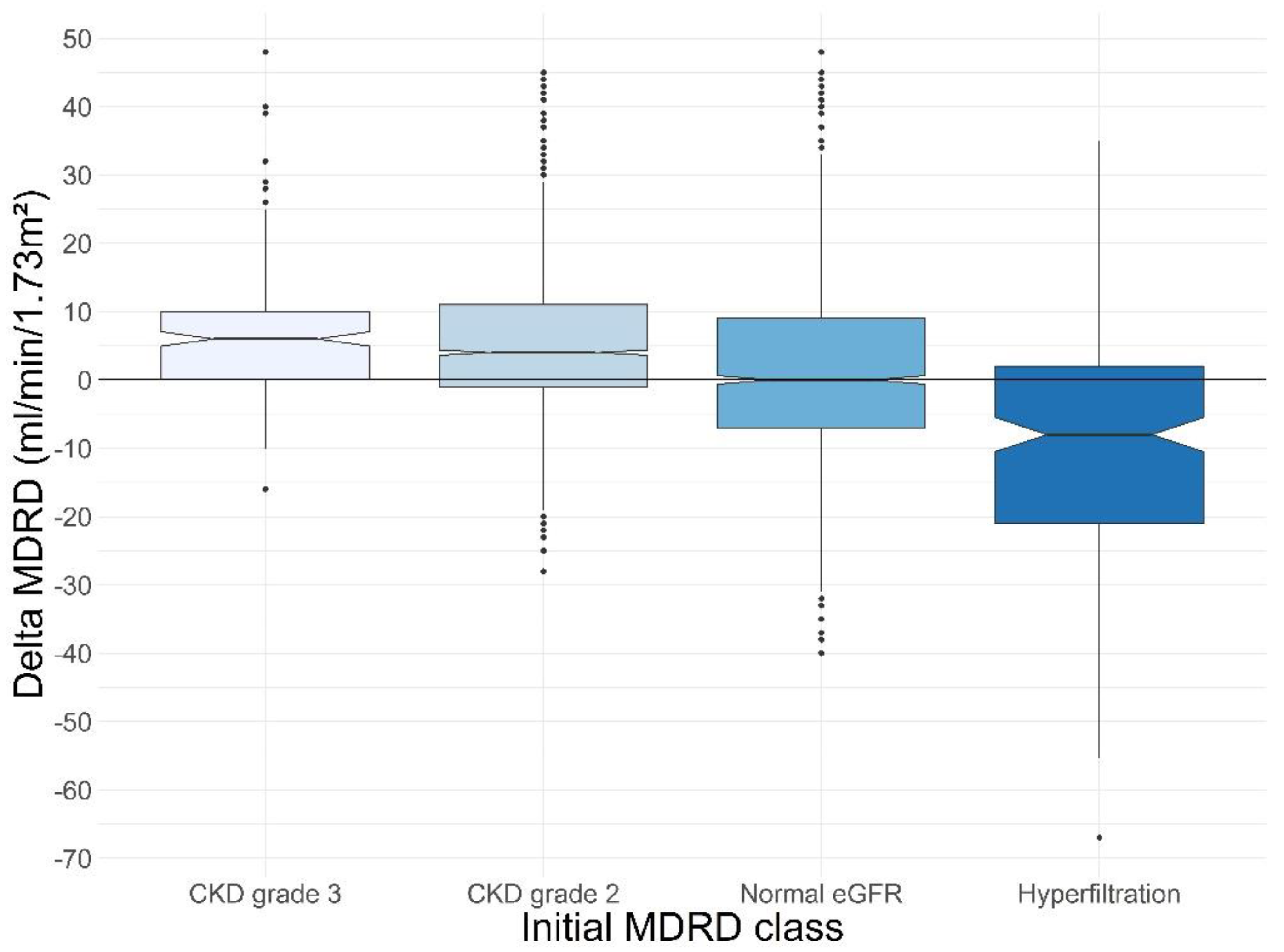
| Delta MDRD | 3 (−3; 10) | 6 (0; 10) | 4 (−1; 11) | 0 (−7; 9) | −8 (−21; 2) | <0.01 * |
| All | CKD Grade 3 | CKD Grade 2 | Normal eGFR | Hyperfiltration | Overall p-Value | |
|---|---|---|---|---|---|---|
| Diabetes N (%) | 503 (100) | 48 (9.5) | 244 (48.5) | 174 (34.6) | 37 (7.4) | |
| Initial MDRD | 86 (71; 100) | 52 (46.5; 55) | 77 (70; 83) | 100 (94; 108) | 132 (126; 136) | <0.01 |
| Final MDRD | 87 (74; 103) | 55 (46.5; 62.5) | 79 (72; 89) | 102 (89; 113) | 119 (110; 138) | <0.01 |
| Delta MDRD | 1 (−5; 10) | 2.5 (−1.5; 8.5) | 3 (−4; 10) | 0 (−10; 10) | −8 (−22; 5) | <0.01 |
| Hypertension N (%) | 1730 (100) | 148 (8.5) | 993 (57.3) | 518 (29.9) | 71 (4.1) | |
| Initial MDRD | 82 (70; 94) | 53 (48; 57) | 76 (69; 82.8) | 97 (93; 105) | 131 (124; 137) | <0.01 |
| Final MDRD | 85 (74; 98) | 57.5 (50; 64.5) | 80 (73; 88) | 101 (92; 110) | 125 (110; 138) | <0.01 |
| Delta MDRD | 3 (−3; 10) | 5 (0; 9) | 4 (−2; 11) | 0 (−6; 11) | −3 (−21; 3) | <0.01 |
| No diabetes and No hypertension N (%) | 1383 (100) | 50 (3.6) | 761 (55) | 515 (37.2) | 57 (4.1) | |
| Initial MDRD | 85 (76; 97) | 56 (53; 58) | 78 (72; 83) | 98 (93; 106) | 129 (123; 137) | <0.01 |
| Final MDRD | 89 (78; 101) | 63 (55; 67) | 82 (74; 89) | 100 (92; 110) | 118 (108; 132) | <0.01 |
| Delta MDRD | 3 (−4; 10) | 8.5 (1; 14) | 4 (−1; 11) | 0 (−7; 9) | −10 (−19; −1) | <0.01 |
| Weight loss period N (%) | 1243 (100) | 77 (6.2) | 647 (52) | 451 (36.2) | 70 (5.6) | |
| Initial MDRD | 86 (74; 98) | 55 (51; 58) | 78 (70; 83) | 99 (94; 107) | 130 (123; 136) | <0.01 |
| Final MDRD | 88 (77; 100) | 59 (52; 65) | 81 (73; 89) | 99 (91; 109) | 124 (114; 141) | <0.01 |
| Delta MDRD | 2 (−4; 10) | 5 (−1; 9) | 4 (−2; 10) | 0 (−8; 9) | −5 (−18; 4) | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truche, A.-S.; Bailly, S.; Fabre, O.; Legrand, R.; Zaoui, P. A Specific High-Protein Weight Loss Program Does Not Impair Renal Function in Patients Who Are Overweight/Obese. Nutrients 2022, 14, 384. https://doi.org/10.3390/nu14020384
Truche A-S, Bailly S, Fabre O, Legrand R, Zaoui P. A Specific High-Protein Weight Loss Program Does Not Impair Renal Function in Patients Who Are Overweight/Obese. Nutrients. 2022; 14(2):384. https://doi.org/10.3390/nu14020384
Chicago/Turabian StyleTruche, Anne-Sophie, Sébastien Bailly, Odile Fabre, Rémy Legrand, and Philippe Zaoui. 2022. "A Specific High-Protein Weight Loss Program Does Not Impair Renal Function in Patients Who Are Overweight/Obese" Nutrients 14, no. 2: 384. https://doi.org/10.3390/nu14020384
APA StyleTruche, A.-S., Bailly, S., Fabre, O., Legrand, R., & Zaoui, P. (2022). A Specific High-Protein Weight Loss Program Does Not Impair Renal Function in Patients Who Are Overweight/Obese. Nutrients, 14(2), 384. https://doi.org/10.3390/nu14020384







