Risk Stratification Based on a Pattern of Immunometabolic Host Factors Is Superior to Body Mass Index—Based Prediction of COVID-19-Associated Respiratory Failure
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Assessment of Body Composition and Immunonutritional Scores
2.3. Statistics
3. Results
3.1. Patient Characteristics
3.2. Patients with the Need for IMV Have More Adipose Tissue and Adverse Immunonutritional Scores
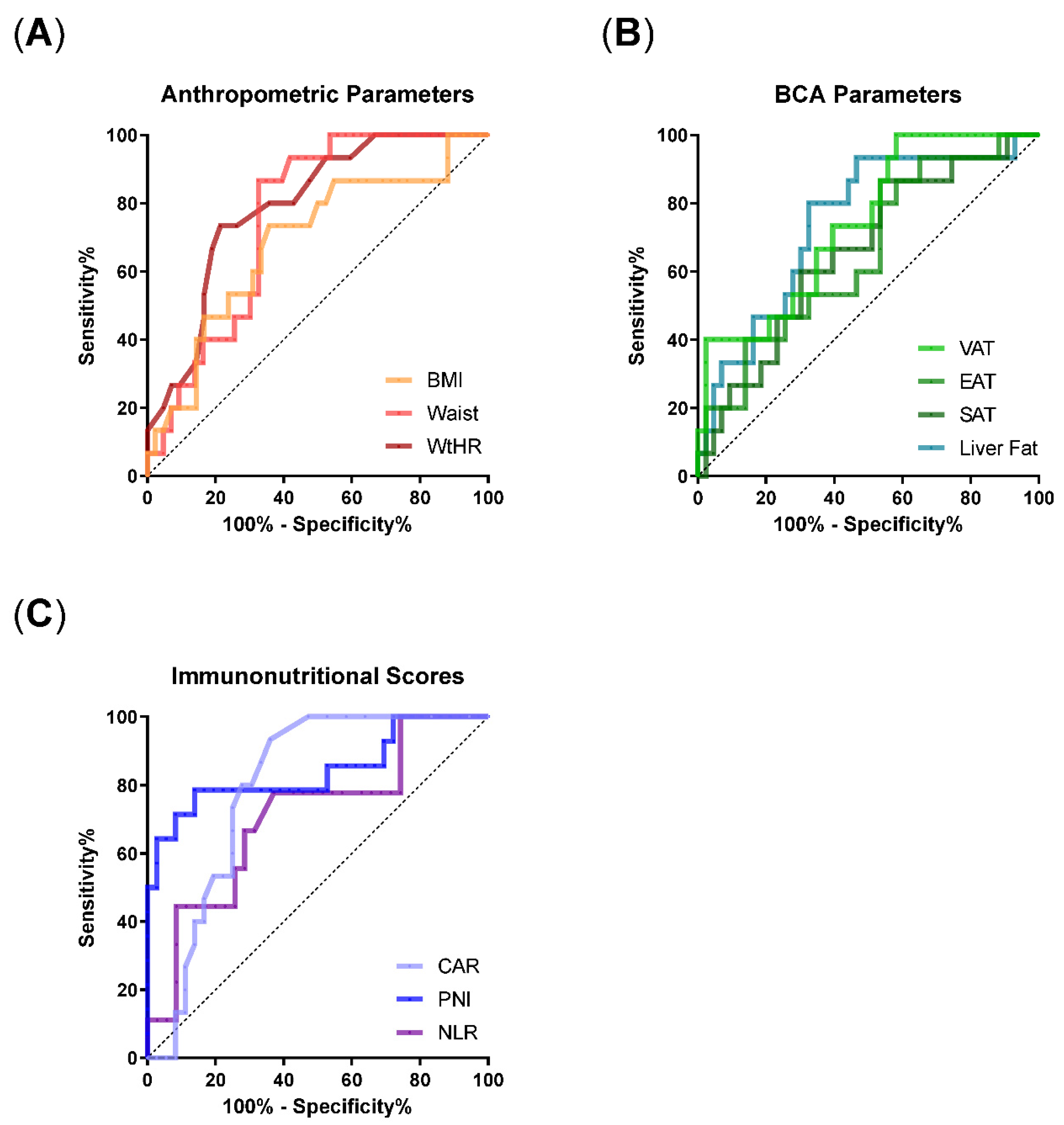
3.3. ROC Analyses Identify WtHR, VAT, Liver Fat, and Immunonutritional Scores as Risk Factors for the Requirement of IMV
3.4. Metabolically High-Risk Adipose Tissue Sites Correlate with Inflammatory Parameters and Immunonutritional Scores
3.5. Stepwise Multivariable Logistic Regression Identifies an Optimal Model for IMV Requirement including Liver Fat, WtHR, and CAR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Soltani, S.; Motlagh, S.Z.; Emadi, A.; Shahinfar, H.; Moosavi, H.; Shab-Bidar, S. Anthropometric and adiposity indicators and risk of type 2 diabetes: Systematic review and dose-response meta-analysis of cohort studies. BMJ 2022, 376, e067516. [Google Scholar] [CrossRef] [PubMed]
- Khaodhiar, L.; McCowen, K.C.; Blackburn, G.L. Obesity and its comorbid conditions. Clin. Cornerstone 1999, 2, 17–31. [Google Scholar] [CrossRef]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kompoti, M. Obesity and infection. Lancet Infect. Dis. 2006, 6, 438–446. [Google Scholar] [CrossRef]
- Sattar, N.; Valabhji, J. Obesity as a risk factor for severe COVID-19: Summary of the best evidence and implications for health care. Curr. Obes. Rep. 2021, 10, 282–289. [Google Scholar] [CrossRef]
- Sanoudou, D.; Hill, M.A.; Belanger, M.J.; Arao, K.; Mantzoros, C.S. Obesity, metabolic phenotypes and COVID-19. Metab. Clin. Exp. 2022, 128, 155121. [Google Scholar] [CrossRef]
- Martí, A.; Marcos, A.; Martínez, J.A. Obesity and immune function relationships. Obes. Rev. 2001, 2, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Kanneganti, T.-D.; Dixit, V.D. Immunological complications of obesity. Nat. Immunol. 2012, 13, 707–712. [Google Scholar] [CrossRef]
- Festa, A.; D’Agostino, R., Jr.; Williams, K.; Karter, A.J.; Mayer-Davis, E.J.; Tracy, R.P.; Haffner, S.M. The relation of body fat mass and distribution to markers of chronic inflammation. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1407–1415. [Google Scholar] [CrossRef]
- Park, H.S.; Park, J.Y.; Yu, R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes. Res. Clin. Pract. 2005, 69, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Bulló, M.; García-Lorda, P.; Megias, I.; Salas-Salvadó, J. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes. Res. 2003, 11, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Radigan, K.A.; Morales-Nebreda, L.; Soberanes, S.; Nicholson, T.; Nigdelioglu, R.; Cho, T.; Chi, M.; Hamanaka, R.B.; Misharin, A.V.; Perlman, H.; et al. Impaired clearance of influenza A virus in obese, leptin receptor deficient mice is independent of leptin signaling in the lung epithelium and macrophages. PLoS ONE 2014, 9, e108138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, B.; Yang, G.; Liu, L.; Lu, J.; Lu, Z.; Xue, Y. Delayed SARS-CoV-2 Clearance in Patients with Obesity. Infect. Drug. Resist. 2021, 14, 2823–2827. [Google Scholar] [CrossRef]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Smith, S.R.; Lovejoy, J.C.; Greenway, F.; Ryan, D.; de Jonge, L.; de la Bretonne, J.; Volafova, J.; Bray, G.A. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism 2001, 50, 425–435. [Google Scholar] [CrossRef]
- Misra, A.; Vikram, N.K. Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition 2003, 19, 457–466. [Google Scholar] [CrossRef]
- Lundbom, J.; Hakkarainen, A.; Lundbom, N.; Taskinen, M. Deep subcutaneous adipose tissue is more saturated than superficial subcutaneous adipose tissue. Int. J. Obes. 2013, 37, 620–622. [Google Scholar] [CrossRef] [Green Version]
- Iacobellis, G. Epicardial adipose tissue in endocrine and metabolic diseases. Endocrine 2014, 46, 8–15. [Google Scholar] [CrossRef]
- Ansaldo, A.M.; Montecucco, F.; Sahebkar, A.; Dallegri, F.; Carbone, F. Epicardial adipose tissue and cardiovascular diseases. Int. J. Cardiol. 2019, 278, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Duy, T.B.; Nichaman, M.Z.; Church, T.S.; Blair, S.N.; Ross, R. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E1065–E1071. [Google Scholar] [CrossRef] [PubMed]
- Proctor, M.J.; Morrison, D.S.; Talwar, D.; Balmer, S.M.; O’reilly, D.S.J.; Foulis, A.K.; Horgan, P.G.; McMillan, D.C. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: A Glasgow Inflammation Outcome Study. Br. J. Cancer 2011, 104, 726–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. [Google Scholar] [PubMed]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts—Rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek Listy 2001, 102, 5–14. [Google Scholar] [PubMed]
- Almasaudi, A.S.; Dolan, R.D.; Edwards, C.A.; McMillan, D.C. Hypoalbuminemia Reflects Nutritional Risk, Body Composition and Systemic Inflammation and Is Independently Associated with Survival in Patients with Colorectal Cancer. Cancers 2020, 12, 1986. [Google Scholar] [CrossRef]
- Karimi, A.; Shobeiri, P.; Kulasinghe, A.; Rezaei, N. Novel Systemic Inflammation Markers to Predict COVID-19 Prognosis. Front. Immunol. 2021, 12, 741061. [Google Scholar] [CrossRef]
- Kasymjanova, G.; MacDonald, N.; Agulnik, J.S.; Cohen, V.; Pepe, C.; Kreisman, H.; Small, D. The predictive value of pre-treatment inflammatory markers in advanced non-small-cell lung cancer. Curr. Oncol. 2010, 17, 52–58. [Google Scholar]
- Liu, S.L.; Wang, S.Y.; Sun, Y.F.; Jia, Q.Y.; Yang, C.L.; Cai, P.J.; Li, J.Y.; Wang, L.; Chen, Y. Expressions of SAA, CRP, and FERR in different severities of COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11386–11394. [Google Scholar]
- Stringer, D.; Braude, P.; Myint, P.K.; Evans, L.; Collins, J.T.; Verduri, A.; Quinn, T.J.; Moraga, A.; Stechman, M.J.; Pearce, L.; et al. The role of C-reactive protein as a prognostic marker in COVID-19. Int. J. Epidemiol. 2021, 50, 420–429. [Google Scholar] [CrossRef]
- Elshazli, R.M.; Toraih, E.A.; Elgaml, A.; El-Mowafy, M.; El-Mesery, M.; Amin, M.N.; Hussein, M.H.; Killackey, M.T.; Fawzy, M.S.; Kandil, E. Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: A meta-analysis of 6320 patients. PLoS ONE 2020, 15, e0238160. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, K.I.; Liu, S.; Yan, Z.; Xu, C.; Qiao, Z. Plasma CRP level is positively associated with the severity of COVID-19. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Mosquera-Sulbaran, J.A.; Pedreañez, A.; Carrero, Y.; Callejas, D. C-reactive protein as an effector molecule in Covid-19 pathogenesis. Rev. Med. Virol. 2021, 31, e2221. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.N.; Lu, H.Z.; Cao, B.; Du, B.; Shang, H.; Gan, J.H.; Lu, S.H.; Ynag, Y.D.; Fang, Q.; Shen, Y.Z.; et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N. Engl. J. Med. 2013, 368, 2277–2285. [Google Scholar] [CrossRef] [Green Version]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Mourtzakis, M.; Mulder, K.E.; Reiman, T.; Sawyer, M.B. Body composition as an independent determinant of 5-fluorouracil–based chemotherapy toxicity. Clin. Cancer Res. 2007, 13, 3264–3268. [Google Scholar] [CrossRef] [Green Version]
- Jayawardena, E.; Li, D.; Nakanishi, R.; Dey, D.; Dailing, C.; Qureshi, A.; Dickens, B.; Hathiramani, N.; Kim, M.; Flores, F.; et al. Non-contrast cardiac CT-based quantitative evaluation of epicardial and intra-thoracic fat in healthy, recently menopausal women: Reproducibility data from the Kronos Early Estrogen Prevention Study. J. Cardiovasc. Comput. Tomogr. 2020, 14, 55–59. [Google Scholar] [CrossRef]
- Kim, C.O.; Nam, C.M.; Lee, D.C.; Chang, J.; Lee, J.W. Is abdominal obesity associated with the 2009 influenza A (H1N1) pandemic in Korean school-aged children? Influenza Other Respir. Viruses 2012, 6, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Peters, S.A.; MacMahon, S.; Woodward, M. Obesity as a risk factor for COVID-19 mortality in women and men in the UK Biobank: Comparisons with influenza/pneumonia and coronary heart disease. Diabetes Obes. Metab. 2021, 23, 258–262. [Google Scholar] [CrossRef]
- Lasbleiz, A.; Gaborit, B.; Soghomonian, A.; Bartoli, A.; Ancel, P.; Jacquier, A.; Dutour, A. COVID-19 and obesity: Role of ectopic visceral and Epicardial adipose tissues in myocardial injury. Front. Endocrinol. 2021, 12, 726967. [Google Scholar] [CrossRef]
- Watanabe, M.; Caruso, D.; Tuccinardi, D.; Risi, R.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Tarallo, M.; Strigare, L.; Manfrini, S.; et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism 2020, 111, 154319. [Google Scholar] [CrossRef]
- Malavazos, A.E.; Goldberger, J.J.; Iacobellis, G. Does epicardial fat contribute to COVID-19 myocardial inflammation? Eur. Heart J. 2020, 41, 2333. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.; Bressem, K.; Albrecht, J.; Thieß, H.M.; Vahldiek, J.; Hamm, B.; Makowsik, M.R.; Niehues, A.; Niehues, S.F.; Adams, L.C. The role of visceral adiposity in the severity of COVID-19: Highlights from a unicenter cross-sectional pilot study in Germany. Metabolism 2020, 110, 154317. [Google Scholar] [CrossRef]
- Favre, G.; Legueult, K.; Pradier, C.; Raffaelli, C.; Ichai, C.; Iannelli, A.; Redheuil, A.; Lucidarme, O.; Esnault, V. Visceral fat is associated to the severity of COVID-19. Metabolism 2021, 115, 154440. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; Chrysohoou, C.; Skoumas, J.; Pitsavos, C.; Panagiotakos, D.B.; Mantzoros, C.S. The presence of NAFLD influences the transition of metabolically healthy to metabolically unhealthy obesity and the ten-year cardiovascular disease risk: A population-based cohort study. Metab. Clin. Exp. 2022, 128, 154893. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, P.J.; Váncsa, S.; Ocskay, K.; Dembrovszky, F.; Kiss, S.; Farkas, N.; Eross, B.; Szakacs, Z.; Hegyi, P.; Pár, G.; et al. Metabolic associated fatty liver disease is associated with an increased risk of severe COVID-19: A systematic review with meta-analysis. Front. Med. 2021, 8, 626425. [Google Scholar] [CrossRef]
- Chen, H.; Wang, D.; Zhong, Q.; Tao, Y.; Zhou, Y.; Shi, Y. Pretreatment body mass index and clinical outcomes in cancer patients following immune checkpoint inhibitors: A systematic review and meta-analysis. Cancer Immunol. Immunother. 2020, 69, 2413–2424. [Google Scholar] [CrossRef]
- Li, J.; Tian, A.; Zhu, H.; Chen, L.; Wen, J.; Liu, W.; Chen, P. Mendelian Randomization Analysis Reveals No Causal Relationship Between Nonalcoholic Fatty Liver Disease and Severe COVID-19. Clin. Gastroenterol. Hepatol. 2022, 20, 1553–1560.e78. [Google Scholar] [CrossRef]
- Mushtaq, K.; Khan, M.U.; Iqbal, F.; Alsoub, D.H.; Chaudhry, H.S.; Ata, F.; Iqbal, P.; Elfert, K.; Balaraju, G.; Almaslamani, M.; et al. NAFLD is a predictor of liver injury in COVID-19 hospitalized patients but not of mortality, disease severity on the presentation or progression—The debate continues. J. Hepatol. 2021, 74, 482–484. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, G.; Ren, J.; Ren, H.; Li, G.; Wu, X.; Gu, G.; Li, R.; Guo, K.; Deng, Y.; et al. Preoperative prognostic nutritional index predicts postoperative infectious complications and oncological outcomes after hepatectomy in intrahepatic cholangiocarcinoma. BMC Cancer 2021, 21, 708. [Google Scholar]
- Xiao, Y.; Wei, G.; Ma, M.; Liu, D.; Chen, P.; Quan, H.; Luo, J.; Xiao, H. Association among prognostic nutritional index, post-operative infection and prognosis of stage II/III gastric cancer patients following radical gastrectomy. Eur. J. Clin. Nutr. 2022, 76, 1449–1456. [Google Scholar] [CrossRef]
- Ushirozako, H.; Hasegawa, T.; Yamato, Y.; Yoshida, G.; Yasuda, T.; Banno, T.; Arima, H.; Oe, S.; Yamada, T.; Ide, K.; et al. Does preoperative prognostic nutrition index predict surgical site infection after spine surgery? Eur. Spine J. 2021, 30, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Inose, H.; Ushio, S.; Yuasa, M.; Hirai, T.; Yoshii, T.; Okawa, A. Body Mass Index and Modified Glasgow Prognostic Score Are Useful Predictors of Surgical Site Infection After Spinal Instrumentation Surgery: A Consecutive Series. Spine 2020, 45, E148–E154. [Google Scholar] [CrossRef] [PubMed]
- Bolat, I.; Biteker, M. Modified Glasgow Prognostic Score is a novel predictor of clinical outcome in heart failure with preserved ejection fraction. Scand. Cardiovasc. J. 2020, 54, 174–178. [Google Scholar] [CrossRef]
- Correa-Rodríguez, M.; Pocovi-Gerardino, G.; Callejas-Rubio, J.L.; Fernández, R.R.; Martín-Amada, M.; Cruz-Caparros, M.G.; Ortego-Centeno, N.; Rueda-Medina, B. The Prognostic Nutritional Index and Nutritional Risk Index Are Associated with Disease Activity in Patients with Systemic Lupus Erythematosus. Nutrients 2019, 11, 638. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.S.; Jung, S.M.; Song, J.J.; Park, Y.B.; Lee, S.W. Prognostic nutritional index is correlated with disease activity in patients with systemic lupus erythematosus. Lupus 2018, 27, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Isoda, K.; Tsuji, S.; Harada, Y.; Yoshimura, M.; Nakabayashi, A.; Sato, M.; Nagano, H.; Kim, D.; Hashimoto, J.; Ohshima, S. Potential of the prognostic nutritional index to determine the risk factor for severe infection in elderly patients with rheumatoid arthritis. Mod. Rheumatol. 2022. ahead of print. [Google Scholar] [CrossRef]
- Xue, G.; Gan, X.; Wu, Z.; Xie, D.; Xiong, Y.; Hua, L.; Zhou, B.; Zhou, N.; Xiang, J.; Li, J. Novel serological biomarkers for inflammation in predicting disease severity in patients with COVID-19. Int. Immunopharmacol. 2020, 89 Pt A, 107065. [Google Scholar] [CrossRef]
- Wang, R.; He, M.; Yin, W.; Liao, X.; Wang, B.; Jin, X.; Ma, Y.; Yue, J.; Bai, L.; Liu, D.; et al. The Prognostic Nutritional Index is associated with mortality of COVID-19 patients in Wuhan, China. J. Clin. Lab. Anal. 2020, 34, e23566. [Google Scholar] [CrossRef]
- Lockhart, S.M.; O’Rahilly, S. When two pandemics meet: Why is obesity associated with increased COVID-19 mortality? Medicine 2020, 18, 33–42. [Google Scholar] [CrossRef]
- Lanza, K.; Perez, L.G.; Costa, L.B.; Cordeiro, T.M.; Palmeira, V.A.; Ribeiro, V.T.; Simoes e Silva, A.C. Covid-19: The renin–angiotensin system imbalance hypothesis. Clin. Sci. 2020, 134, 1259–1264. [Google Scholar] [CrossRef]
- Xiao, L.; Sakagami, H.; Miwa, N. ACE2: The key Molecule for Understanding the Pathophysiology of Severe and Critical Conditions of COVID-19: Demon or Angel? Viruses 2020, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Al-Benna, S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes. Med. 2020, 19, 100283. [Google Scholar] [CrossRef] [PubMed]
- Sarver, D.C.; Wong, G.W. Obesity alters Ace2 and Tmprss2 expression in lung, trachea, and esophagus in a sex-dependent manner: Implications for COVID-19. Biochem. Biophys. Res. Commun. 2021, 538, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Zorita, S.; Milton-Laskibar, I.; García-Arellano, L.; González, M.; Portillo, M.P. An Overview of Adipose Tissue ACE2 Modulation by Diet and Obesity. Potential Implications in COVID-19 Infection and Severity. Int. J. Mol. Sci. 2021, 22, 7975. [Google Scholar] [CrossRef]
- Reiterer, M.; Rajan, M.; Gómez-Banoy, N.; Lau, J.D.; Gomez-Escobar, L.G.; Ma, L.; Gilani, A.; Alvarez-Mulett, S.; Sholle, E.E.; Chandar, V.; et al. Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metab. 2021, 33, 2174–2188.e5. [Google Scholar] [CrossRef]
- van der Voort, P.H.; Moser, J.; Zandstra, D.F.; Kobold, A.C.M.; Knoester, M.; Calkhoven, C.F.; Hamming, I.; van Meurs, M. Leptin levels in SARS-CoV-2 infection related respiratory failure: A cross-sectional study and a pathophysiological framework on the role of fat tissue. Heliyon 2020, 6, e04696. [Google Scholar] [CrossRef] [PubMed]
- Blot, M.; Masson, D.; Nguyen, M.; Bourredjem, A.; Binquet, C.; Piroth, L. Are adipokines the missing link between obesity, immune response, and outcomes in severe COVID-19? Int. J. Obes. 2021, 45, 2126–2131. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Marques-Vidal, P.; Bastardot, F.; von Känel, R.; Paccaud, F.; Preisig, M.; Waeber, G.; Vollenweider, P. Association between circulating cytokine levels, diabetes and insulin resistance in a population-based sample (CoLaus study). Clin. Endocrinol. 2013, 78, 232–241. [Google Scholar] [CrossRef]
- Um, J.Y.; Chung, H.S.; Song, M.Y.; Shin, H.D.; Kim, H.M. Association of interleukin-1β gene polymorphism with body mass index in women. Clin. Chem. 2004, 50, 647–650. [Google Scholar] [CrossRef]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pala, L.; De Pas, T.; Catania, C.; Giaccone, G.; Mantovani, A.; Minucci, S.; Viale, G.; Grlber, R.D.; Conforti, F. Sex and cancer immunotherapy: Current understanding and challenges. Cancer Cell 2022, 40, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Tong, B.; Sun, L.; Shi, S.; Zheng, B.; Wang, Z.; Dong, X.; Zheng, P. N501Y mutation of spike protein in SARS-CoV-2 strengthens its binding to receptor ACE2. eLife 2021, 10, e69091. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016.
- Fang, H.; Berg, E.; Cheng, X.; Shen, W. How to best assess abdominal obesity. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 360–365. [Google Scholar] [CrossRef]
- Anderson, M.R.; Udupa, J.K.; Edwin, E.; Diamond, J.M.; Singer, J.P.; Kukreja, J.; Hays, S.R.; Greenland, J.R.; Ferrante, A.; Lippel, M.; et al. Adipose tissue quantification and primary graft dysfunction after lung transplantation: The Lung Transplant Body Composition study. J. Heart Lung Transplant. 2019, 38, 1246–1256. [Google Scholar] [CrossRef]
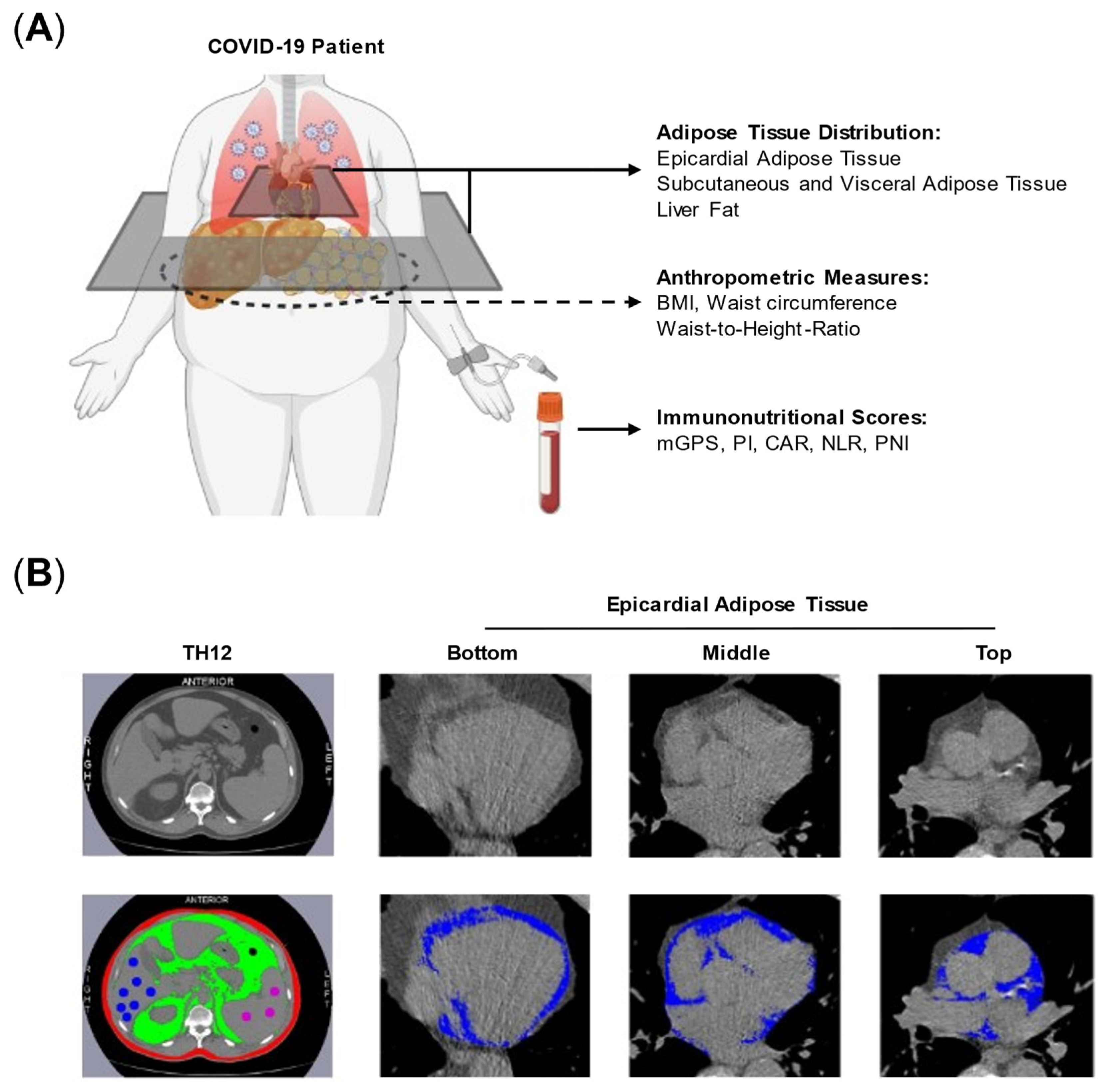

| Characteristic | Invasive Mechanical Ventilation | p-Values | ||
|---|---|---|---|---|
| All Patients (N = 58) | No (N = 43) | Yes (N = 15) | ||
| Age, median (range) [years] | 63 (32–91) | 61 (31–91) | 64 (47–82) | 0.66 |
| 30–50 years | 13 (22.4) | 12 (27.9) | 1 (6.7) | 0.13 |
| 51–70 years | 27 (46.6) | 17 (39.5) | 10 (66.7) | |
| >71 years | 18 (31) | 14 (32.6) | 4 (26.7) | |
| Female | 16 (27.6) | 14 (32.6) | 2 (13.3) | 0.19 |
| Comorbidities | ||||
| None | 33 (56.9) | 24 (55.8) | 9 (60) | 0.39 |
| 1 comorbidity | 18 (31) | 15 (34.9) | 3 (20) | |
| ≥2 comorbidities | 7 (12) | 4 (9.3) | 3 (20) | |
| Diabetes | 10 (27.2) | 6 (14) | 4 (26.7) | 0.75 |
| Coronary heart disease | 13 (22.4) | 10 (23.3) | 3 (20) | |
| COPD | 5 (8.6) | 4 (9.3) | 1 (6.7) | |
| Chronic kidney disease | 5 (8.6) | 4 (9.3) | 1 (6.7) | |
| Serum parameters | ||||
| Creatinine, median (range) [mg/dL] | 0.95 (0.4–6.0) | 0.9 (0.4–6.0) | 1.1 (0.8–2.1) | 0.006 |
| Troponin, median (range) [ng/mL] | 0 (0–0.18) | 0 (0–0.18) | 0.02 (0–0.04) | 0.002 |
| Invasive Mechanical Ventilation | ||||
|---|---|---|---|---|
| Characteristic | All Patients (N = 58) | No (N = 43) | Yes (N = 15) | p-Values |
| Anthropometric Parameters | ||||
| BMI [kg/m²] | 25.7 (17.7–45.8) | 24.8 (17.7–38.5) | 27.8 (20.4–45.8) | 0.03 |
| BMI ≥ 30, number (percent) [kg/m²] | 13 (22.8%) | 7 (16.7%) | 6 (40%) | |
| Waist circumference [cm] | 107.5 (77.7–150.4) | 103.4 (77.7–134) | 111.2 (103.2–150.4) | 0.003 |
| WtHR [rel.] | 0.61 (0.47–0.8) | 0.59 (0.47–0.71) | 0.66 (0.57–0.8) | 0.0006 |
| Adipose Tissue Distribution | ||||
| SAT [cm²] | 97 (8.5–383.6) | 92.9 (8.5–383.6) | 118 (40.8–343.7) | 0.07 |
| VAT [cm²] | 88.9 (7–300.3) | 84.6 (7–237.2) | 133.4 (64.7–300.3) | 0.005 |
| EAT [cm²] | 12.3 (3.4–32.3) | 11.9 (3.4–30.7) | 13.2 (5.9–32.3) | 0.08 |
| Liver Fat [HU] | 46.7 (28.6–61.2) | 48.6 (31.3–61.2) | 45 (28.6–57) | 0.0044 |
| Spleen [HU] | 44.4 (29–55.1) | 44.4 (29–55.1) | 45.7 (31.2–54.8) | 0.984 |
| Immunonutritional Scores | ||||
| NLR [rel.] | 4.3 (0.9–20.4) | 3.5 (0.9–19.1) | 5.8 (2.5–20.4) | 0.06 |
| PNI [rel.] | 42.6 (27.1–54.8) | 43.1 (36.4–54.8) | 36.6 (27.1–46.7) | <0.0001 |
| CAR [rel.] | 0.8 (0–9.8) | 0.5 (0–9.8) | 2.6 (0.6–4.9) | 0.0007 |
| mGPS | ||||
| – 0 | 45 | 35 | 10 | 0.007 |
| – 1 | 6 | 6 | 0 | |
| – 2 | 7 | 2 | 5 | |
| PI | ||||
| – 0 | 43 | 35 | 8 | 0.09 |
| – 1 | 12 | 6 | 6 | |
| – 2 | 3 | 2 | 1 | |
| AUC (95%CI) | p Value AUC | Discriminatory Value | OR (95%CI) | p-Value OR | |
|---|---|---|---|---|---|
| Anthropometric Parameters | |||||
| BMI | 0.69 (0.53–0.85) | 0.03 | 26.1 kg/m² | 1.13 (1.01–1.29) | 0.04 |
| Waist | 0.76 (0.63–0.88) | 0.003 | 109.3 cm | 1.09 (1.03–1.16) | 0.009 |
| WtHR | 0.79 (0.67–0.91) | 0.0009 | 0.635 cm/m² | 1.21 (1.09–1.4) | 0.002 |
| Adipose Tissue Distribution | |||||
| SAT | 0.66 (0.5– 0.82) | 0.07 | 86.7 cm² | 1.01 (1–1.01) | 0.16 |
| VAT | 0.74 (0.6–0.88) | 0.006 | 67.4 cm² | 1.01 (1.01–1.02) | 0.006 |
| EAT | 0.65 (0.49–0.81) | 0.08 | 9.7 cm² | 1.09 (1–1.2) | 0.048 |
| Liver fat | 0.74 (0.6–0.89) | 0.005 | 46.2 HU | 0.88 (0.79–0.97) | 0.01 |
| Inflammation Scores | |||||
| CAR | 0.79 (0.67–0.91) | 0.001 | 0.7 | 1.28 (0.97–1.76) | 0.1 |
| PNI | 0.84 (0.7–0.99) | 0.0002 | 38.7 | 1.15 (1.02–1.32) | 0.03 |
| NLR | 0.71 (0.51–0.9) | 0.057 | 4.75 | 1.17 (1.05–1.43) | 0.01 |
| Parameter | Discriminatory Threshold | Odds Ratio | 95%CI | p-Value |
|---|---|---|---|---|
| Liver Fat | < 46.2 HU | 5.6 | 1.03–38.3 | 0.02 |
| WtHR | > 0.635 | 5.6 | 1.11–35.5 | 0.07 |
| CAR | > 0.7 | 22.3 | 3.01–496.1 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordas dos Santos, D.M.; Liu, L.; Gerisch, M.; Hellmuth, J.C.; von Bergwelt-Baildon, M.; Kunz, W.G.; Theurich, S. Risk Stratification Based on a Pattern of Immunometabolic Host Factors Is Superior to Body Mass Index—Based Prediction of COVID-19-Associated Respiratory Failure. Nutrients 2022, 14, 4280. https://doi.org/10.3390/nu14204280
Cordas dos Santos DM, Liu L, Gerisch M, Hellmuth JC, von Bergwelt-Baildon M, Kunz WG, Theurich S. Risk Stratification Based on a Pattern of Immunometabolic Host Factors Is Superior to Body Mass Index—Based Prediction of COVID-19-Associated Respiratory Failure. Nutrients. 2022; 14(20):4280. https://doi.org/10.3390/nu14204280
Chicago/Turabian StyleCordas dos Santos, David M., Lian Liu, Melvin Gerisch, Johannes C. Hellmuth, Michael von Bergwelt-Baildon, Wolfgang G. Kunz, and Sebastian Theurich. 2022. "Risk Stratification Based on a Pattern of Immunometabolic Host Factors Is Superior to Body Mass Index—Based Prediction of COVID-19-Associated Respiratory Failure" Nutrients 14, no. 20: 4280. https://doi.org/10.3390/nu14204280
APA StyleCordas dos Santos, D. M., Liu, L., Gerisch, M., Hellmuth, J. C., von Bergwelt-Baildon, M., Kunz, W. G., & Theurich, S. (2022). Risk Stratification Based on a Pattern of Immunometabolic Host Factors Is Superior to Body Mass Index—Based Prediction of COVID-19-Associated Respiratory Failure. Nutrients, 14(20), 4280. https://doi.org/10.3390/nu14204280






