Neuronal Nitric Oxide Synthase Regulates Depression-like Behaviors in Shortening-Induced Obese Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
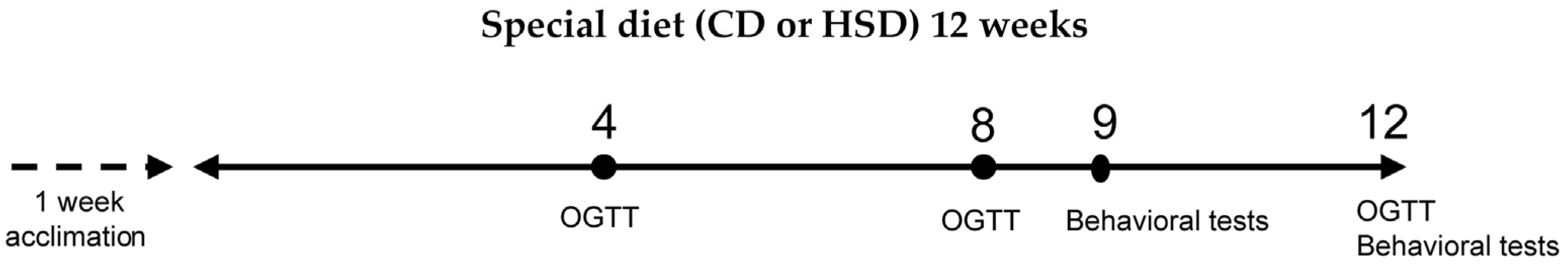
2.2. Study Design
2.3. Fasting Blood Glucose Test and Oral Glucose Tolerance Test (OGTT)
2.4. Behavioral Tests
2.4.1. Y-Maze
2.4.2. Open Field Test
2.4.3. Elevated Plus Maze
2.4.4. Sucrose Preference Test (SPT)
2.4.5. Forced Swimming Test (FST)
2.5. Blood Lipid Measurements
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
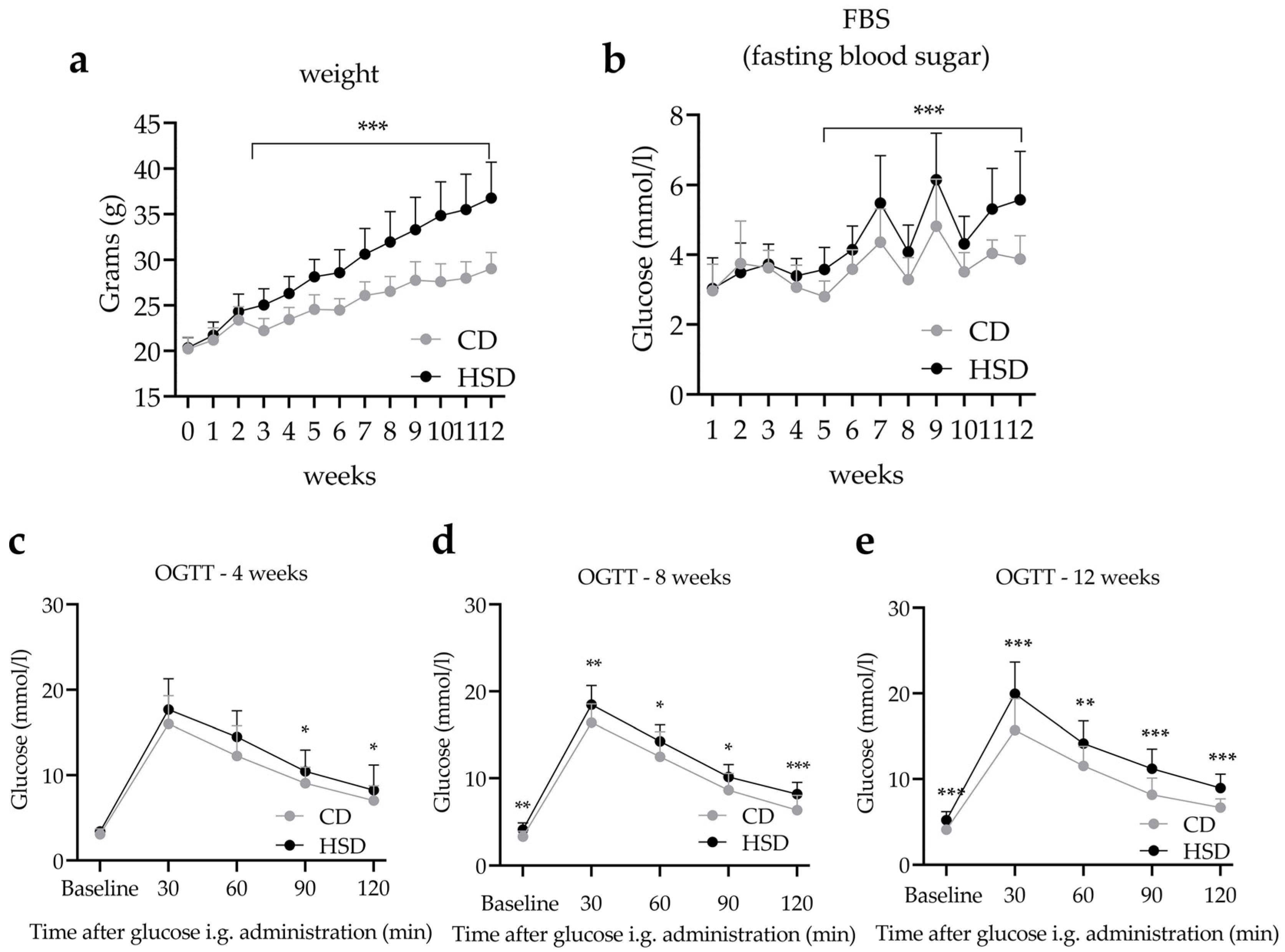
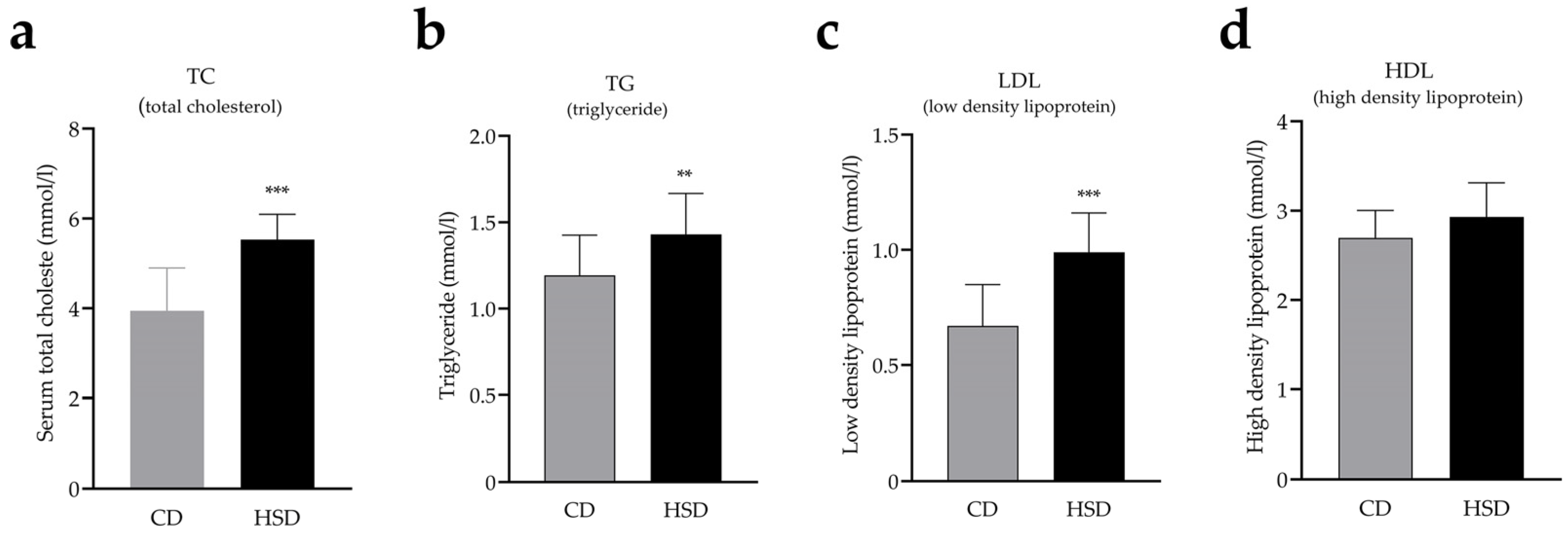
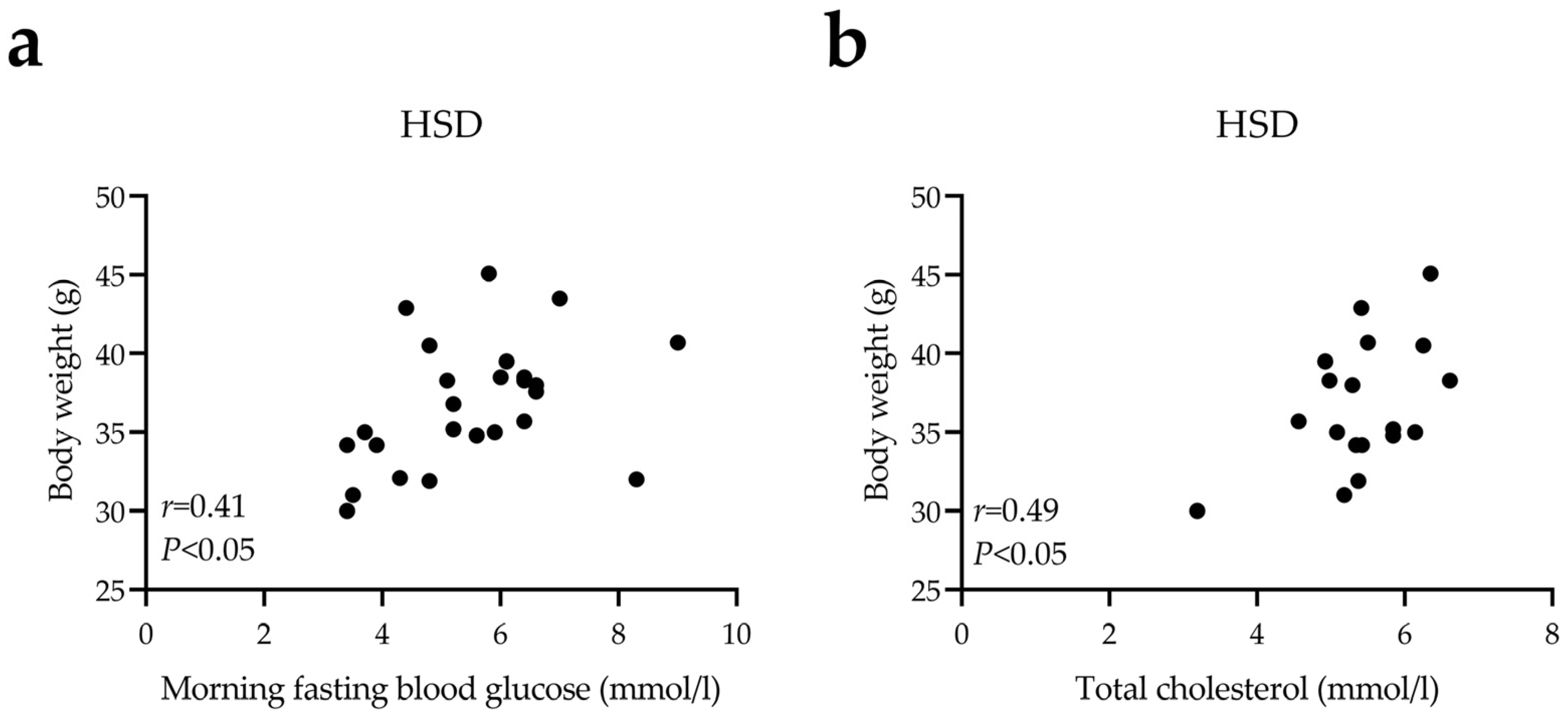
3.1. HSD Exposure Increases Bodyweight and Leads to Abnormal Glucose and Lipid Metabolism
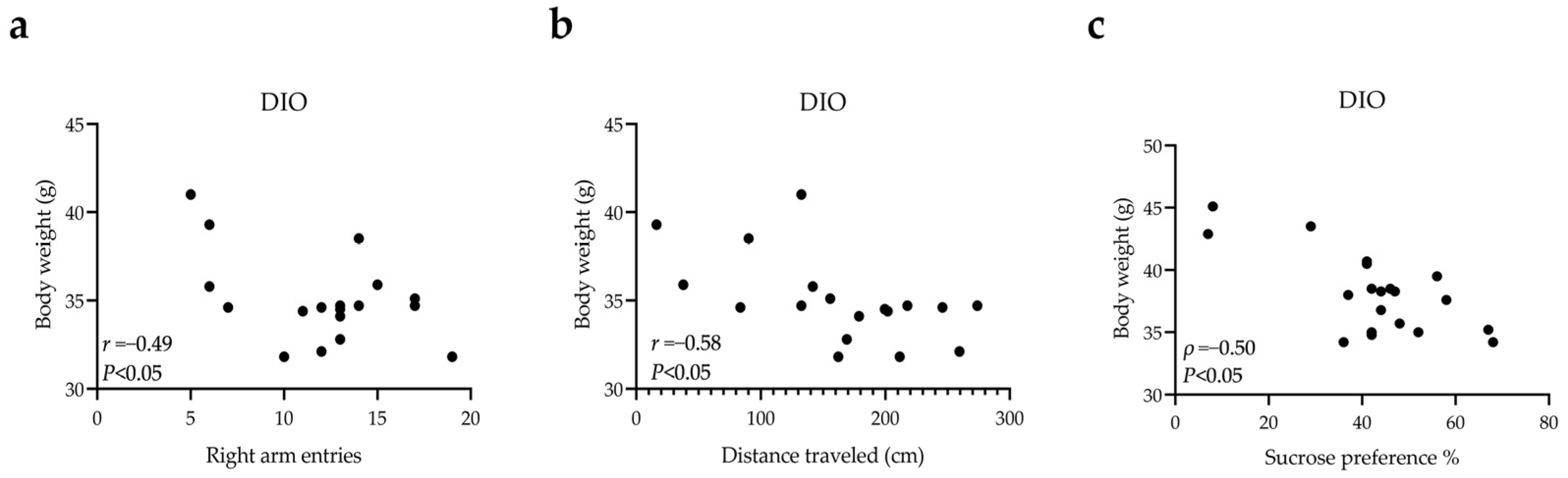
3.2. Short-Term Spatial Working Memory Impairment, Anxiety-like, and Depression-like Behaviors in DIO Mice
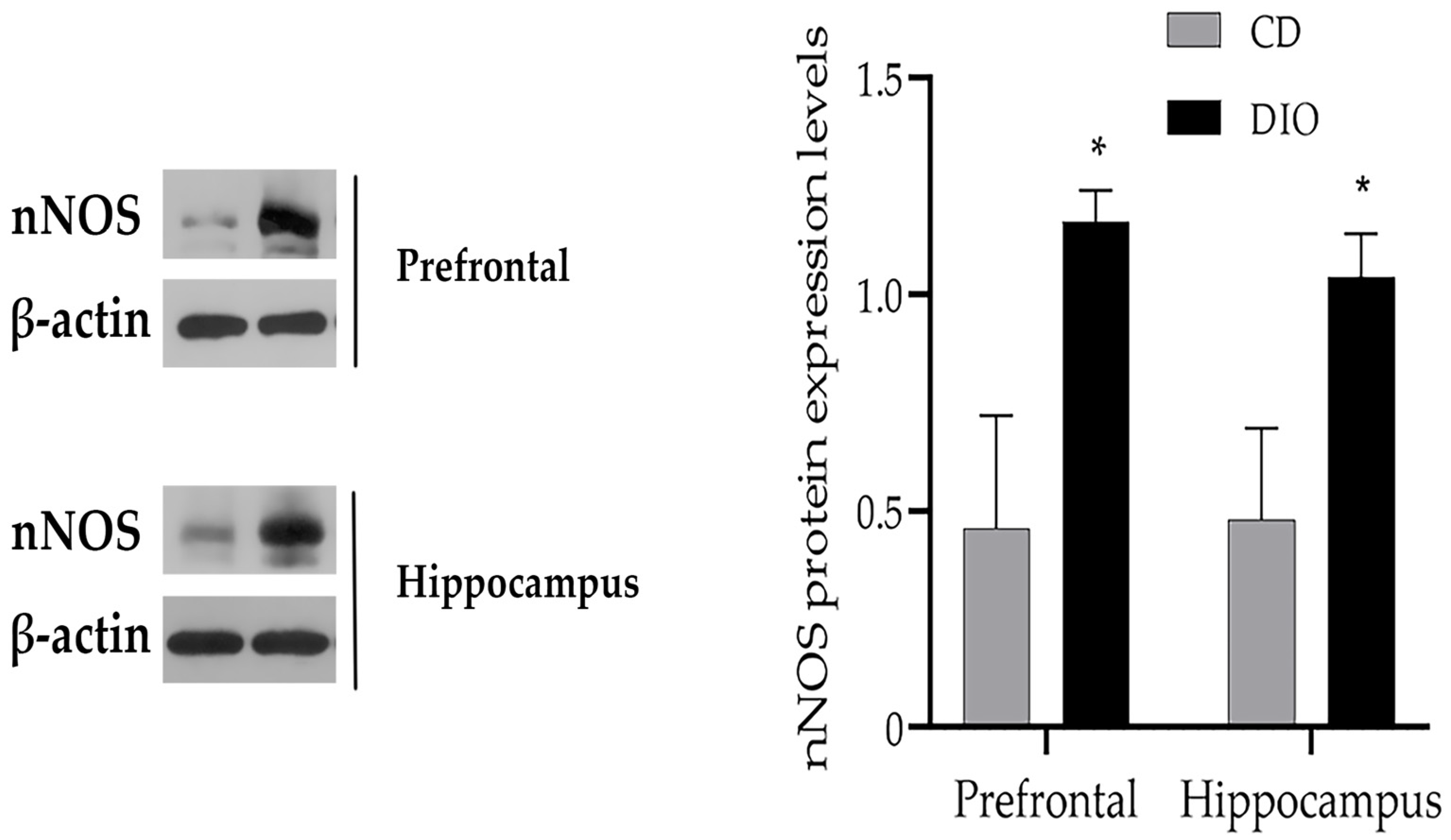
3.3. Changes of nNOS Protein Levels in the Brain of DIO Mice
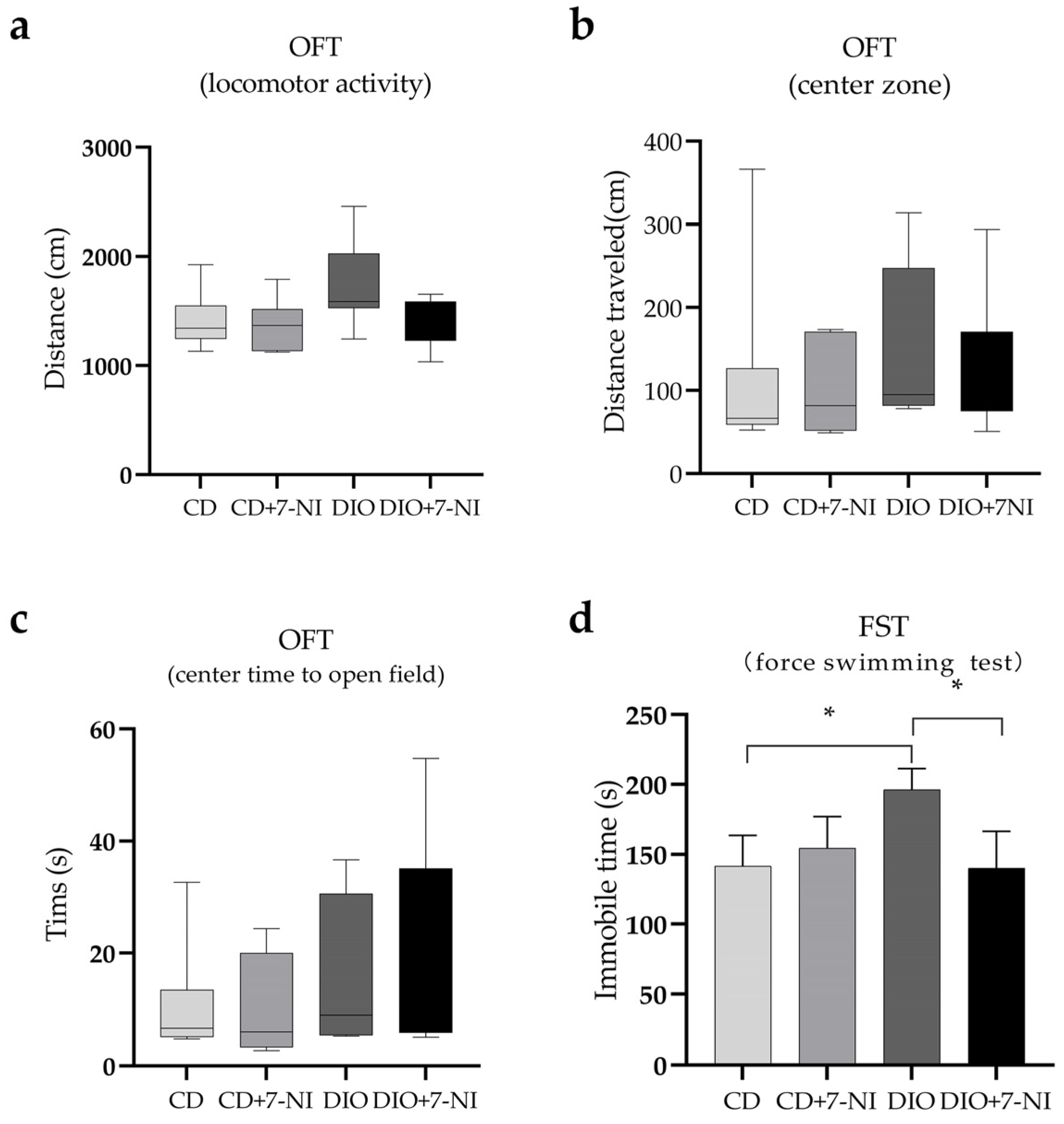
3.4. Depression-like Behavior Improved by 7-NI in DIO Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, N.; Kagiono, S.; Yoshinaga, K.; Mizobe, H.; Nagai, T.; Yoshida, A.; Beppu, F.; Nagao, K. Study of Trans Fatty Acid Formation in Oil by Heating Using Model Compounds. J. Oleo Sci. 2018, 67, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Ginter, E.; Simko, V. New Data on Harmful Effects of Trans-Fatty Acids. Bratisl. Med. J. 2016, 117, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Kuhnt, K.; Baehr, M.; Rohrer, C.; Jahreis, G. Trans Fatty Acid Isomers and the Trans-9/Trans-11 Index in Fat Containing Foods. Eur. J. Lipid Sci. Technol. EJLST 2011, 113, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Judd, J.T.; Baer, D.J.; Clevidence, B.A.; Kris-Etherton, P.; Muesing, R.A.; Iwane, M. Dietary Cis and Trans Monounsaturated and Saturated FA and Plasma Lipids and Lipoproteins in Men. Lipids 2002, 37, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Katan, M.B. Effect of Dietary Trans Fatty Acids on High-Density and Low-Density Lipoprotein Cholesterol Levels in Healthy Subjects. N. Engl. J. Med. 1990, 323, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Mannan, M.; Mamun, A.; Doi, S.; Clavarino, A. Is There a Bi-Directional Relationship between Depression and Obesity among Adult Men and Women? Systematic Review and Bias-Adjusted Meta Analysis. Asian J. Psychiatry 2016, 21, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.J.H.; Zitman, F.G. Overweight, Obesity, and Depression: A Systematic Review and Meta-Analysis of Longitudinal Studies. Arch. Gen. Psychiatry 2010, 67, 220. [Google Scholar] [CrossRef] [PubMed]
- Mannan, M.; Mamun, A.; Doi, S.; Clavarino, A. Prospective Associations between Depression and Obesity for Adolescent Males and Females—A Systematic Review and Meta-Analysis of Longitudinal Studies. PLoS ONE 2016, 11, e0157240. [Google Scholar] [CrossRef]
- Jacka, F.N.; Pasco, J.A.; Mykletun, A.; Williams, L.J.; Hodge, A.M.; O’Reilly, S.L.; Nicholson, G.C.; Kotowicz, M.A.; Berk, M. Association of Western and Traditional Diets with Depression and Anxiety in Women. Am. J. Psychiatry 2010, 167, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Villegas, A.; Verberne, L.; De Irala, J.; Ruíz-Canela, M.; Toledo, E.; Serra-Majem, L.; Martínez-González, M.A. Dietary Fat Intake and the Risk of Depression: The SUN Project. PLoS ONE 2011, 6, e16268. [Google Scholar] [CrossRef] [PubMed]
- André, C.; Dinel, A.-L.; Ferreira, G.; Layé, S.; Castanon, N. Diet-Induced Obesity Progressively Alters Cognition, Anxiety-like Behavior and Lipopolysaccharide-Induced Depressive-like Behavior: Focus on Brain Indoleamine 2,3-Dioxygenase Activation. Brain. Behav. Immun. 2014, 41, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Dutheil, S.; Ota, K.T.; Wohleb, E.S.; Rasmussen, K.; Duman, R.S. High-Fat Diet Induced Anxiety and Anhedonia: Impact on Brain Homeostasis and Inflammation. Neuropsychopharmacology 2016, 41, 1874–1887. [Google Scholar] [CrossRef] [PubMed]
- Décarie-Spain, L.; Sharma, S.; Hryhorczuk, C.; Issa-Garcia, V.; Barker, P.A.; Arbour, N.; Alquier, T.; Fulton, S. Nucleus Accumbens Inflammation Mediates Anxiodepressive Behavior and Compulsive Sucrose Seeking Elicited by Saturated Dietary Fat. Mol. Metab. 2018, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Fulton, S. Diet-Induced Obesity Promotes Depressive-like Behaviour That Is Associated with Neural Adaptations in Brain Reward Circuitry. Int. J. Obes. 2013, 37, 382–389. [Google Scholar] [CrossRef]
- Russo, S.J.; Nestler, E.J. The Brain Reward Circuitry in Mood Disorders. Nat. Rev. Neurosci. 2013, 14, 609–625. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Tomasi, D.; Baler, R.D. The Addictive Dimensionality of Obesity. Biol. Psychiatry 2013, 73, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Oteng, A.-B.; Kersten, S. Mechanisms of Action of Trans Fatty Acids. Adv. Nutr. 2020, 11, 697–708. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Simmons, W.K.; van Rossum, E.F.C.; Penninx, B.W. Depression and Obesity: Evidence of Shared Biological Mechanisms. Mol. Psychiatry 2019, 24, 18–33. [Google Scholar] [CrossRef]
- Bredt, D.S.; Snyder, S.H. Nitric Oxide, a Novel Neuronal Messenger. Neuron 1992, 8, 3–11. [Google Scholar] [CrossRef]
- Oliveira, R.M.W.; Guimarães, F.S.; Deakin, J.F.W. Expression of Neuronal Nitric Oxide Synthase in the Hippocampal Formation in Affective Disorders. Braz. J. Med. Biol. Res. 2008, 41, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, A.; Yaman, G.B.; Demirdas, A.; Onal, S. Possible Role of Adrenomedullin and Nitric Oxide in Major Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, M.S.; Stang, P.; Makadia, R. Depression Is Associated With High Levels of C-Reactive Protein and Low Levels of Fractional Exhaled Nitric Oxide: Results from the 2007–2012 National Health and Nutrition Examination Surveys. J. Clin. Psychiatry 2016, 77, 1666–1671. [Google Scholar] [CrossRef]
- Zhou, Q.-G.; Hu, Y.; Hua, Y.; Hu, M.; Luo, C.-X.; Han, X.; Zhu, X.-J.; Wang, B.; Xu, J.-S.; Zhu, D.-Y. Neuronal Nitric Oxide Synthase Contributes to Chronic Stress-Induced Depression by Suppressing Hippocampal Neurogenesis. J. Neurochem. 2007, 103, 1843–1854. [Google Scholar] [CrossRef]
- Anaeigoudari, A.; Soukhtanloo, M.; Shafei, M.N.; Sadeghnia, H.R.; Reisi, P.; Beheshti, F.; Behradnia, S.; Mousavi, S.M.; Hosseini, M.; Anaeigoudarai, A. Neuronal Nitric Oxide Synthase Has a Role in the Detrimental Effects of Lipopolysaccharide on Spatial Memory and Synaptic Plasticity in Rats. Pharmacol. Rep. 2016, 68, 243–249. [Google Scholar] [CrossRef]
- Xing, G.; Chavko, M.; Zhang, L.-X.; Yang, S.; Post, R.M. Decreased Calcium-Dependent Constitutive Nitric Oxide Synthase (CNOS) Activity in Prefrontal Cortex in Schizophrenia and Depression. Schizophr. Res. 2002, 58, 21–30. [Google Scholar] [CrossRef]
- Lim, D.W.; Kim, M.; Yoon, M.; Lee, J.; Lee, C.; Um, M.Y. 1,3-Dicaffeoylquinic Acid as an Active Compound of Arctium Lappa Root Extract Ameliorates Depressive-like Behavior by Regulating Hippocampal Nitric Oxide Synthesis in Ovariectomized Mice. Antioxid. Basel Switz. 2021, 10, 1281. [Google Scholar] [CrossRef]
- Tomiga, Y.; Yoshimura, S.; Ito, A.; Nakashima, S.; Kawanaka, K.; Uehara, Y.; Tanaka, H.; Higaki, Y. Exercise Training Rescues High Fat Diet-Induced Neuronal Nitric Oxide Synthase Expression in the Hippocampus and Cerebral Cortex of Mice. Nitric Oxide 2017, 66, 71–77. [Google Scholar] [CrossRef]
- Nagy, C.; Einwallner, E. Study of In Vivo Glucose Metabolism in High-Fat Diet-Fed Mice Using Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT). J. Vis. Exp. JoVE 2018, 2018, 56672. [Google Scholar] [CrossRef]
- Goshen, I.; Kreisel, T.; Ben-Menachem-Zidon, O.; Licht, T.; Weidenfeld, J.; Ben-Hur, T.; Yirmiya, R. Brain Interleukin-1 Mediates Chronic Stress-Induced Depression in Mice via Adrenocortical Activation and Hippocampal Neurogenesis Suppression. Mol. Psychiatry 2008, 13, 717–728. [Google Scholar] [CrossRef]
- Da Silva Leal, V.M.; Bonassoli, V.T.; Soares, L.M.; Milani, H.; de Oliveira, R.M.W. Depletion of 5 Hydroxy-Triptamine (5-HT) Affects the Antidepressant-like Effect of Neuronal Nitric Oxide Synthase Inhibitor in Mice. Neurosci. Lett. 2017, 656, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Lempesis, I.G.; Liu, J.; Dalamaga, M. Of Mice and Men: Considerations on Adipose Tissue Physiology in Animal Models of Obesity and Human Studies. Metab. Open 2022, 15, 100208. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schürmann, A.; et al. Animal Models of Obesity and Diabetes Mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef]
- Winzell, M.S.; Ahrén, B. The High-Fat Diet-Fed Mouse: A Model for Studying Mechanisms and Treatment of Impaired Glucose Tolerance and Type 2 Diabetes. Diabetes 2004, 53 (Suppl. S3), S215–S219. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Yasoshima, A.; Doi, K.; Nakayama, H.; Uetsuka, K. Involvement of Sex, Strain and Age Factors in High Fat Diet-Induced Obesity in C57BL/6J and BALB/cA Mice. Exp. Anim. 2007, 56, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Parks, B.W.; Sallam, T.; Mehrabian, M.; Psychogios, N.; Hui, S.T.; Norheim, F.; Castellani, L.W.; Rau, C.D.; Pan, C.; Phun, J.; et al. Genetic Architecture of Insulin Resistance in the Mouse. Cell Metab. 2015, 21, 334–347. [Google Scholar] [CrossRef]
- Ronchi, J.A.; Figueira, T.R.; Ravagnani, F.G.; Oliveira, H.C.F.; Vercesi, A.E.; Castilho, R.F. A Spontaneous Mutation in the Nicotinamide Nucleotide Transhydrogenase Gene of C57BL/6J Mice Results in Mitochondrial Redox Abnormalities. Free Radic. Biol. Med. 2013, 63, 446–456. [Google Scholar] [CrossRef]
- Takase, K.; Tsuneoka, Y.; Oda, S.; Kuroda, M.; Funato, H. High-Fat Diet Feeding Alters Olfactory-, Social-, and Reward-Related Behaviors of Mice Independent of Obesity: High-Fat Diet-Induced Behavioral Changes. Obesity 2016, 24, 886–894. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Asai, M.; Hara, T. High-Fat Diet Enhances Working Memory in the Y-Maze Test in Male C57BL/6J Mice with Less Anxiety in the Elevated Plus Maze Test. Nutrients 2020, 12, 2036. [Google Scholar] [CrossRef]
- Leffa, D.D.; Valvassori, S.S.; Varela, R.B.; Lopes-Borges, J.; Daumann, F.; Longaretti, L.M.; Dajori, A.L.F.; Quevedo, J.; Andrade, V.M. Effects of Palatable Cafeteria Diet on Cognitive and Noncognitive Behaviors and Brain Neurotrophins’ Levels in Mice. Metab. Brain Dis. 2015, 30, 1073–1082. [Google Scholar] [CrossRef]
- Papazoglou, I.K.; Jean, A.; Gertler, A.; Taouis, M.; Vacher, C.-M. Hippocampal GSK3β as a Molecular Link Between Obesity and Depression. Mol. Neurobiol. 2015, 52, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Fernandes, M.F.; Fulton, S. Adaptations in Brain Reward Circuitry Underlie Palatable Food Cravings and Anxiety Induced by High-Fat Diet Withdrawal. Int. J. Obes. 2013, 37, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Claunch, J.; Niu, K. Pathologic Role of Nitrergic Neurotransmission in Mood Disorders. Prog. Neurobiol. 2019, 173, 54–87. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.T.; Demady, D.R.; Osawa, Y. Ubiquitination of Neuronal Nitric-Oxide Synthase In Vitro and In Vivo. J. Biol. Chem. 2000, 275, 17407–17411. [Google Scholar] [CrossRef]
- Francis, H.M.; Mirzaei, M.; Pardey, M.C.; Haynes, P.A.; Cornish, J.L. Proteomic Analysis of the Dorsal and Ventral Hippocampus of Rats Maintained on a High Fat and Refined Sugar Diet. Proteomics 2013, 13, 3076–3091. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric Oxide Synthases: Structure, Function and Inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Noguchi, S.; Jianmongkol, S.; Bender, A.T.; Kamada, Y.; Demady, D.R.; Osawa, Y. Guanabenz-Mediated Inactivation and Enhanced Proteolytic Degradation of Neuronal Nitric-Oxide Synthase. J. Biol. Chem. 2000, 275, 2376–2380. [Google Scholar] [CrossRef]
- Panda, K.; Rosenfeld, R.J.; Ghosh, S.; Meade, A.L.; Getzoff, E.D.; Stuehr, D.J. Distinct Dimer Interaction and Regulation in Nitric-Oxide Synthase Types I, II, and III. J. Biol. Chem. 2002, 277, 31020–31030. [Google Scholar] [CrossRef]
- Lucki, I. The Forced Swimming Test as a Model for Core and Component Behavioral Effects of Antidepressant Drugs. Behav. Pharmacol. 1997, 8, 523–532. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral Despair in Mice: A Primary Screening Test for Antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar] [PubMed]
- The Forced Swim Test as a Model of Depressive-like Behavior. J. Vis. Exp. 2015, 2015, 52587. Available online: https://sci-hub.st/10.3791/52587 (accessed on 26 January 2022).
- Smith, J.C.E.; Whitton, P.S. Nitric Oxide Modulates N -Methyl- d -Aspartate-Evoked Serotonin Release in the Raphe Nuclei and Frontal Cortex of the Freely Moving Rat. Neurosci. Lett. 2000, 291, 5–8. [Google Scholar] [CrossRef]
- Wegener, G.; Volke, V.; Rosenberg, R. Endogenous Nitric Oxide Decreases Hippocampal Levels of Serotonin and Dopamine in Vivo: NO and Hippocampal 5-HT/DA. Br. J. Pharmacol. 2000, 130, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Sansbury, B.E.; Hill, B.G. Regulation of Obesity and Insulin Resistance by Nitric Oxide. Free Radic. Biol. Med. 2014, 73, 383–399. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, H.J.; Lee, Y.-S.; Kang, G.M.; Lim, H.S.; Lee, S.-H.; Song, D.K.; Kwon, O.; Hwang, I.; Son, M.; et al. Hypothalamic Macrophage Inducible Nitric Oxide Synthase Mediates Obesity-Associated Hypothalamic Inflammation. Cell Rep. 2018, 25, 934–946.e5. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Park, S.J.; Beyak, M.J. Inducible Nitric Oxide Synthase-Derived Nitric Oxide Reduces Vagal Satiety Signalling in Obese Mice. J. Physiol. 2019, 597, 1487–1502. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cobb, L.K.; Vesper, H.W.; Asma, S. Global Surveillance of Trans-Fatty Acids. Prev. Chronic. Dis. 2019, 16, 190121. [Google Scholar] [CrossRef]

| CD | HSD | |
|---|---|---|
| Fat source | Soybean oil | Partially hydrogenated palm oil |
| Fat (g/kg) | 70 | 270 |
| Casein (g/kg) | 200 | 200 |
| L-Cystine (g/kg) | 3 | 3 |
| Sucrose (g/kg) | 100 | 100 |
| Cornstarch (g/kg) | 397.5 | 197.5 |
| Dyetrose (g/kg) | 132 | 132 |
| Mineral Mix (g/kg) | 35 | 35 |
| Vitamine Mix (g/kg) | 10 | 10 |
| Choline Bitartrate | 2.5 | 2.5 |
| Cellulose | 50 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Kong, F.-Z.; Hong, X.-H.; Zhang, L.; Zhao, W.-H.; Yang, J.-C.; Zhang, H. Neuronal Nitric Oxide Synthase Regulates Depression-like Behaviors in Shortening-Induced Obese Mice. Nutrients 2022, 14, 4302. https://doi.org/10.3390/nu14204302
Wang P, Kong F-Z, Hong X-H, Zhang L, Zhao W-H, Yang J-C, Zhang H. Neuronal Nitric Oxide Synthase Regulates Depression-like Behaviors in Shortening-Induced Obese Mice. Nutrients. 2022; 14(20):4302. https://doi.org/10.3390/nu14204302
Chicago/Turabian StyleWang, Ping, Fan-Zhi Kong, Xiao-Hong Hong, Li Zhang, Wan-Hong Zhao, Jin-Cui Yang, and Heng Zhang. 2022. "Neuronal Nitric Oxide Synthase Regulates Depression-like Behaviors in Shortening-Induced Obese Mice" Nutrients 14, no. 20: 4302. https://doi.org/10.3390/nu14204302
APA StyleWang, P., Kong, F.-Z., Hong, X.-H., Zhang, L., Zhao, W.-H., Yang, J.-C., & Zhang, H. (2022). Neuronal Nitric Oxide Synthase Regulates Depression-like Behaviors in Shortening-Induced Obese Mice. Nutrients, 14(20), 4302. https://doi.org/10.3390/nu14204302





