Branched-Chain Amino Acids Supplementation Does Not Accelerate Recovery after a Change of Direction Sprinting Exercise Protocol
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Experimental Protocol
2.4. Exercise Protocol
2.5. Supplementation Strategy
2.6. Anthropometric Evaluation
2.7. Muscle Damage and Inflammatory Biomarkers
2.8. Muscle Soreness
2.9. Neuromuscular Performance
2.10. Vascular Health
2.11. Statistical Analysis
3. Results
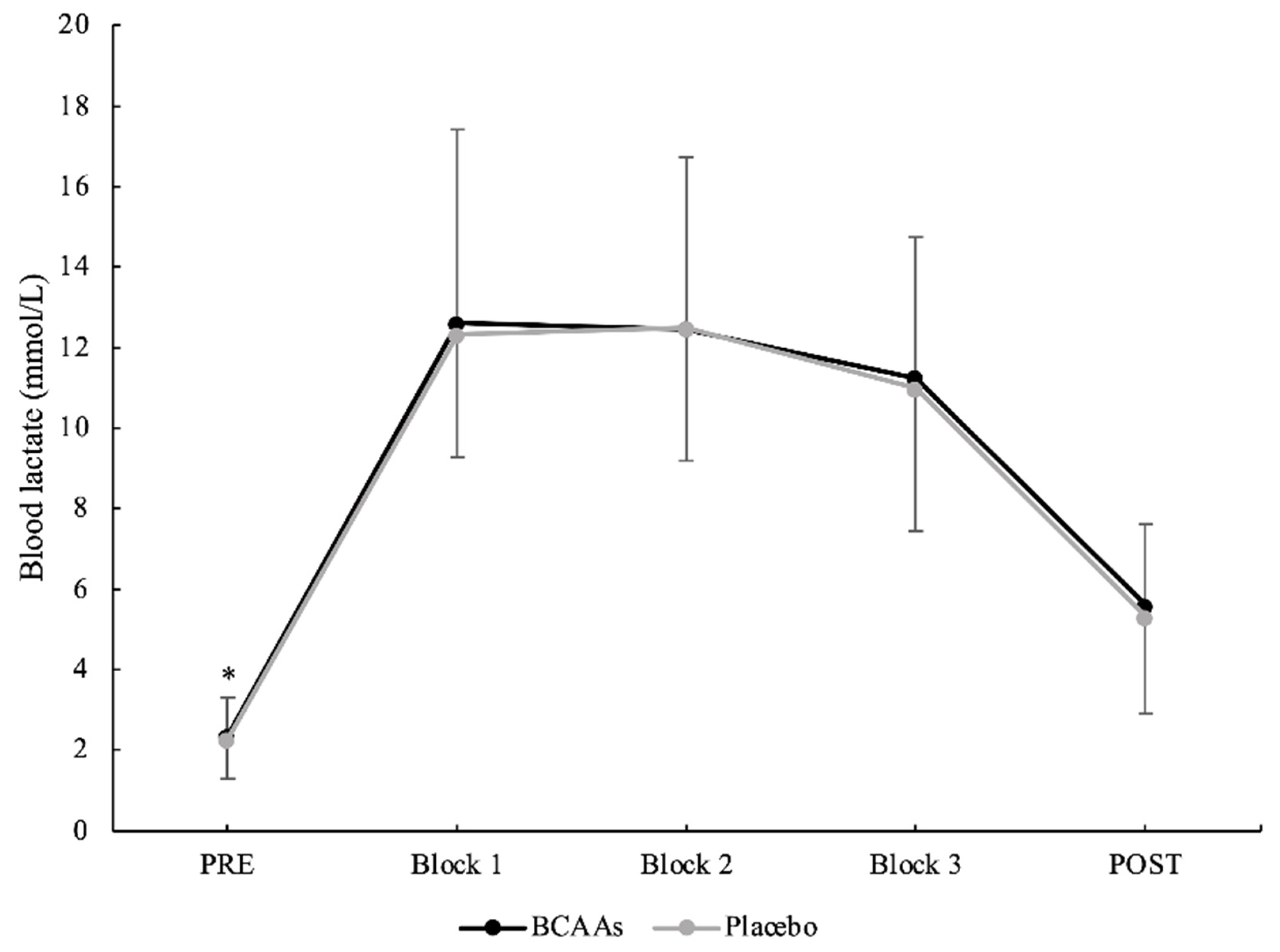
3.1. Exercise Intensity
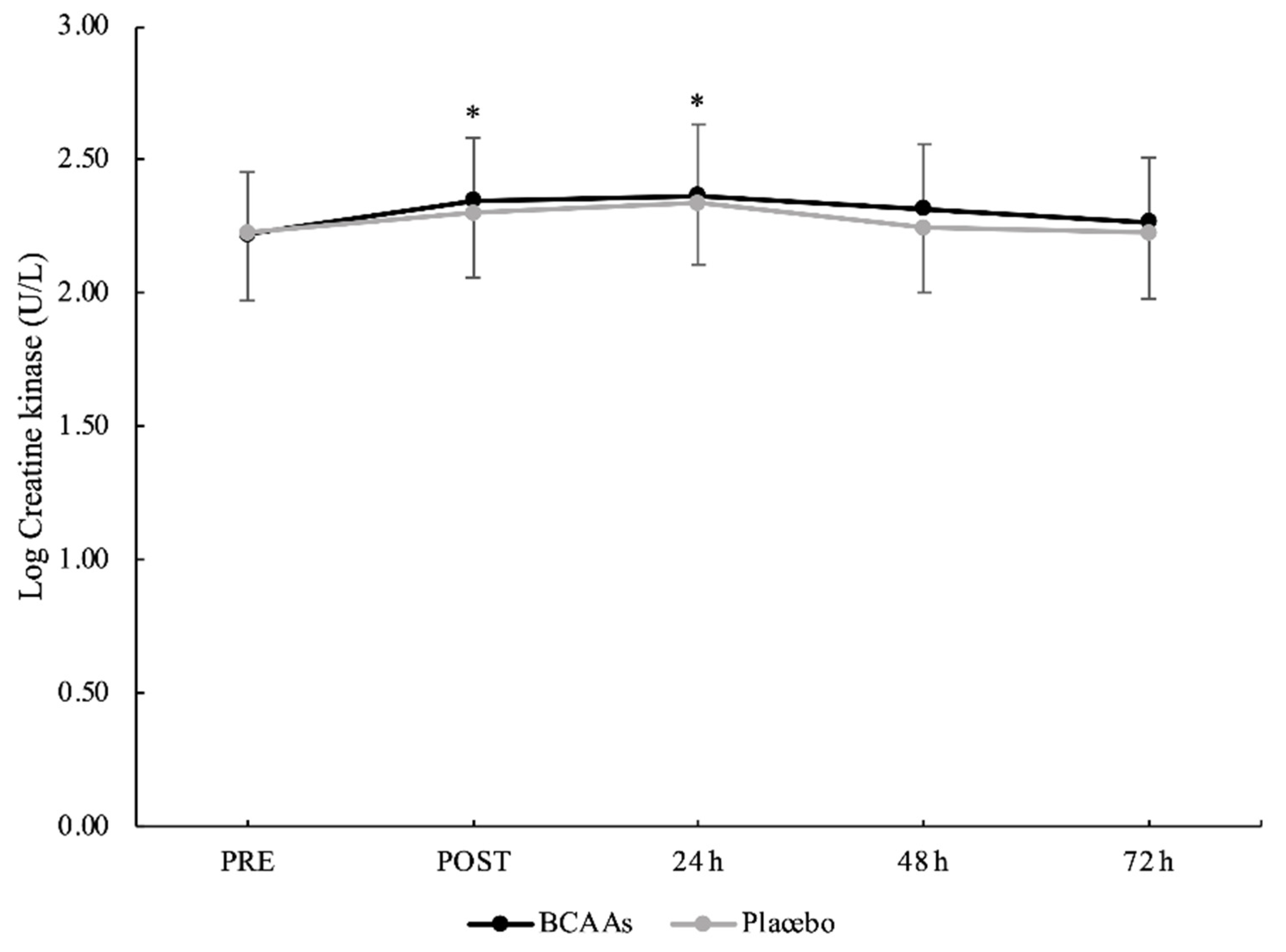
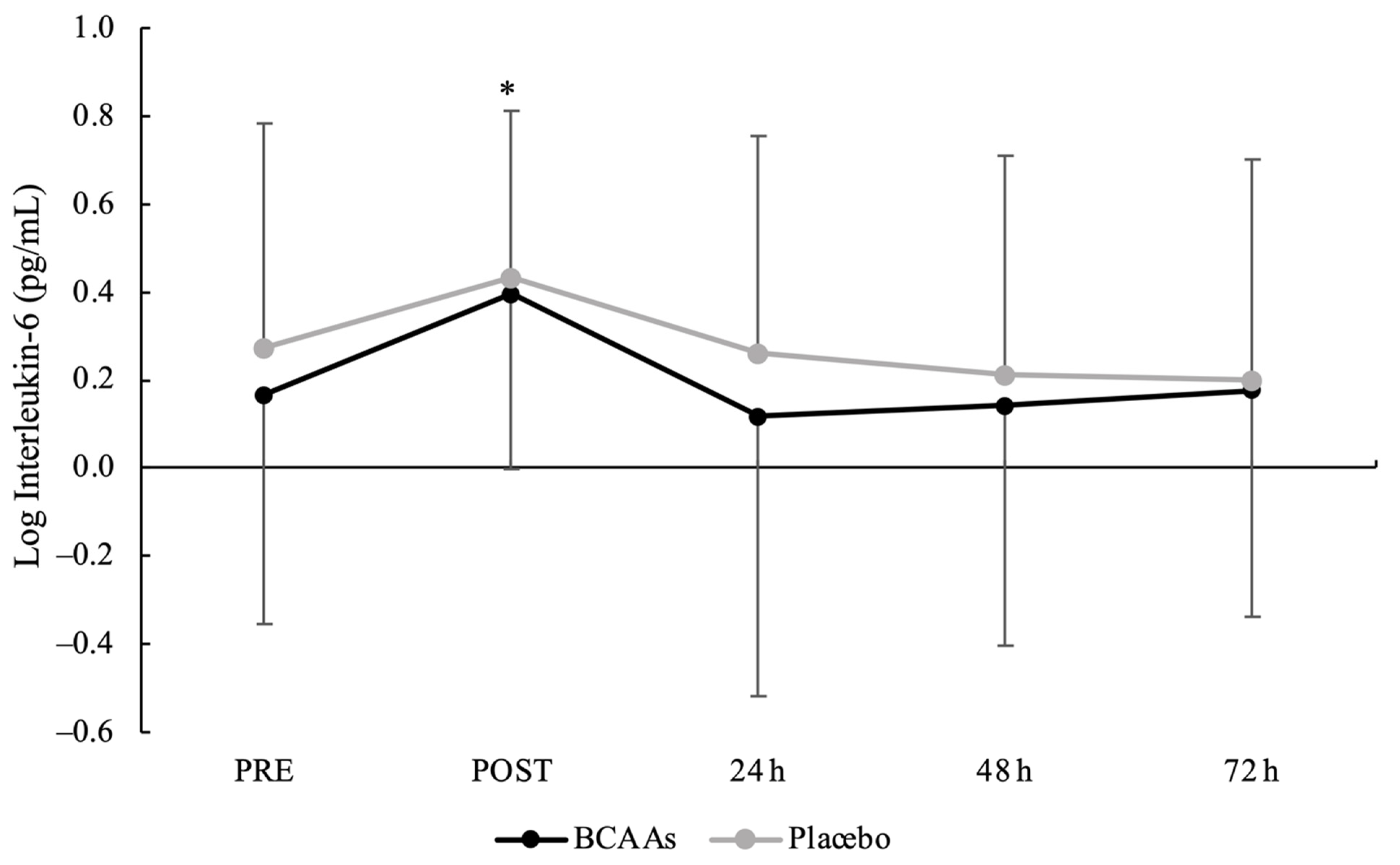
3.2. Muscle Damage and Inflammatory Biomarkers
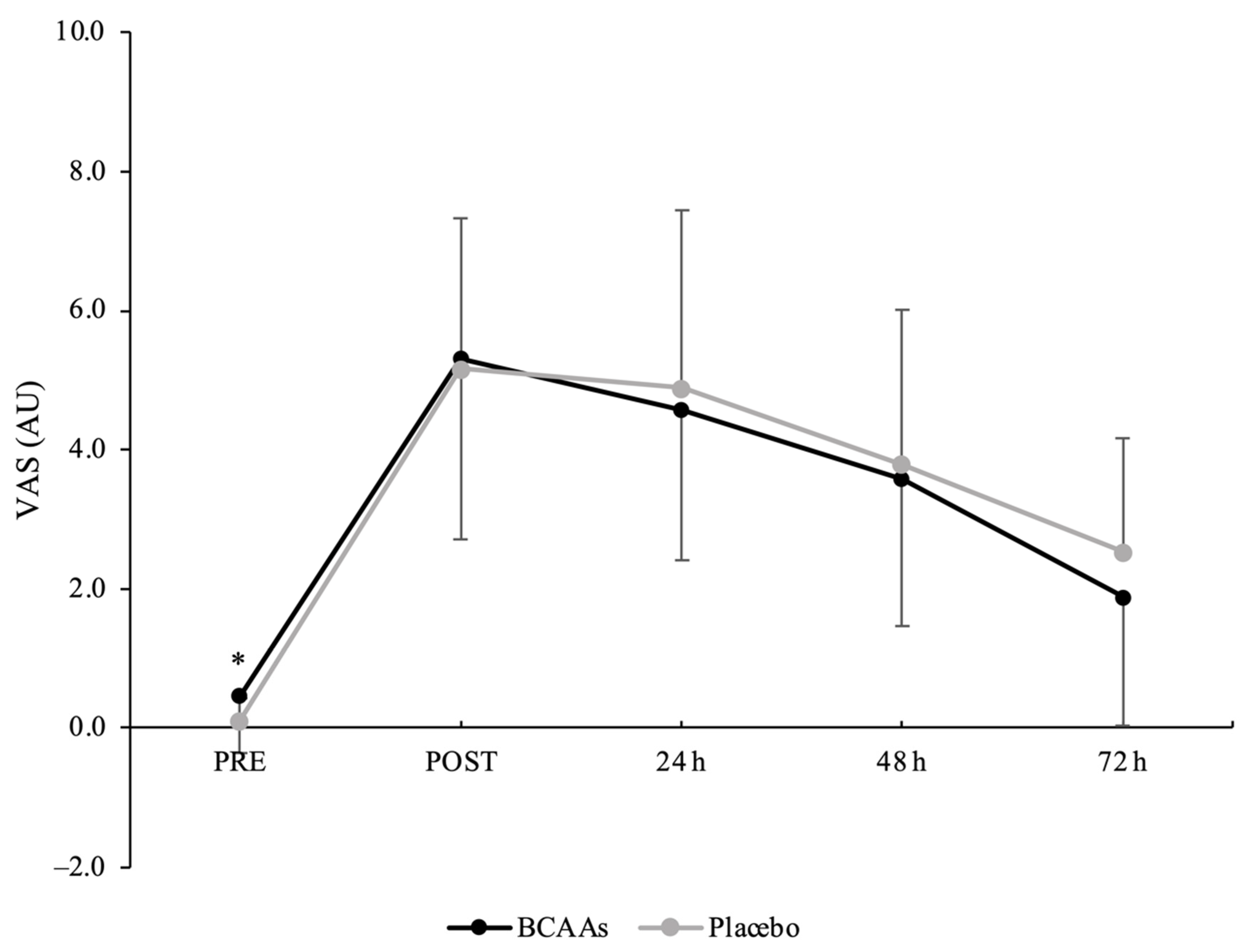
3.3. Muscle Soreness
3.4. Neuromuscular Performance
3.5. Vascular Health
3.6. Analysis of Associations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheppard, J.M.; Young, W.B. Agility literature review: Classifications, training and testing. J. Sports Sci. 2006, 24, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Chaabene, H.; Prieske, O.; Negra, Y.; Granacher, U. Change of direction speed: Toward a strength training approach with accentuated eccentric muscle actions. Sports Med. 2018, 48, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Chaabene, H. Change of direction tasks: Does the eccentric muscle contraction really matter? Sci. Pages Sports Med. 2017, 1, 1–2. [Google Scholar] [CrossRef]
- Byrne, C.; Twist, C.; Eston, R. Neuromuscular function after exercise-induced muscle damage: Theoretical and applied implications. Sports Med. 2004, 34, 49–69. [Google Scholar] [CrossRef]
- Howatson, G.; van Someren, K.A. The prevention and treatment of exercise-induced muscle damage. Sports Med. 2008, 38, 483–503. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Eston, R. Maximal-intensity isometric and dynamic exercise performance after eccentric muscle actions. J. Sports Sci 2002, 20, 951–959. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002, 81, S52–S69. [Google Scholar] [CrossRef]
- Paulsen, G.; Mikkelsen, U.R.; Raastad, T.; Peake, J.M. Leucocytes, cytokines and satellite cells: What role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Rev. 2012, 18, 42–97. [Google Scholar]
- Stankov, S. Definition of Inflammation, causes of Inflammation and possible anti-inflammatory strategies. Open Inflamm. J. 2012, 5, 1–9. [Google Scholar] [CrossRef]
- Davies, R.C.; Eston, R.G.; Poole, D.C.; Rowlands, A.V.; DiMenna, F.; Wilkerson, D.P.; Twist, C.; Jones, A.M. Effect of eccentric exercise-induced muscle damage on the dynamics of muscle oxygenation and pulmonary oxygen uptake. J. Appl. Physiol. 2008, 105, 1413–1421. [Google Scholar] [CrossRef]
- Caldwell, J.T.; Wardlow, G.C.; Branch, P.A.; Ramos, M.; Black, C.D.; Ade, C.J. Effect of exercise-induced muscle damage on vascular function and skeletal muscle microvascular deoxygenation. Physiol. Rep. 2016, 4, e13032. [Google Scholar] [CrossRef] [PubMed]
- Burt, D.G.; Twist, C. The effects of exercise-induced muscle damage on cycling time-trial performance. J. Strength Cond. Res. 2011, 25, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Hosios, A.M.; Hecht, V.C.; Danai, L.V.; Johnson, M.O.; Rathmell, J.C.; Steinhauser, M.L.; Manalis, S.R.; Vander Heiden, M.G. Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev. Cell 2016, 36, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.M.; Phillips, S.M. Branched-chain amino acids (leucine, isoleucine, and valine) and skeletal muscle. In Nutrition and Skeletal Muscle; Walrand, S., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 283–298. [Google Scholar]
- da Luz, C.R.; Nicastro, H.; Zanchi, N.E.; Chaves, D.F.; Lancha, A.H., Jr. Potential therapeutic effects of branched-chain amino acids supplementation on resistance exercise-based muscle damage in humans. J. Int. Soc. Sports Nutr. 2011, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.H.; Shab-Bidar, S.; Mollahosseini, M.; Djafarian, K. Branched-chain amino acid supplementation and exercise-induced muscle damage in exercise recovery: A meta-analysis of randomized clinical trials. Nutrition 2017, 42, 30–36. [Google Scholar] [CrossRef]
- Hormoznejad, R.; Zare Javid, A.; Mansoori, A. Effect of BCAA supplementation on central fatigue, energy metabolism substrate and muscle damage to the exercise: A systematic review with meta-analysis. Sport Sci. Health 2019, 15, 265–279. [Google Scholar] [CrossRef]
- Rahimlou, M.; Ramezani, A.; Mahdipour, M.; Palimi, E.; Moradipoodeh, B. Reduction of muscle injuries and improved post-exercise recovery by branched-chain amino acid supplementation: A systematic review and meta-analysis. J. Nutr. Fasting Health 2020, 8, 1–16. [Google Scholar] [CrossRef]
- Khemtong, C.; Kuo, C.H.; Chen, C.Y.; Jaime, S.J.; Condello, G. Does branched-chain amino acids (BCAAs) supplementation attenuate muscle damage markers and soreness after resistance exercise in trained males? A meta-analysis of randomized controlled trials. Nutrients 2021, 13, 1880. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G * Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998; p. 41. [Google Scholar]
- Dorrell, H.F.; Gee, T.I. The acute effects different quantities of branched-chain amino acids have on recovery of muscle function. Sports Nutr. Ther. 2016, 1, 115. [Google Scholar] [CrossRef]
- Cheng, I.S.; Wang, Y.W.; Chen, I.F.; Hsu, G.S.; Hsueh, C.F.; Chang, C.K. The supplementation of branched-chain amino acids, arginine, and citrulline improves endurance exercise performance in two consecutive days. J. Sports Sci. Med. 2016, 15, 509–515. [Google Scholar]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Ostrowski, K.; Schjerling, P. Exercise and cytokines with particular focus on muscle-derived IL-6. Exerc. Immunol. Rev. 2001, 7, 18–31. [Google Scholar]
- Sriwatanakul, K.; Kelvie, W.; Lasagna, L. The quantification of pain: An analysis of words used to describe pain and analgesia in clinical trials. Clin. Pharmacol. Ther. 1982, 32, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Nimphius, S.; Callaghan, S.J.; Spiteri, T.; Lockie, R.G. Change of direction deficit: A more isolated measure of change of direction performance than total 505 Time. J. Strength Cond. Res. 2016, 30, 3024–3032. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Munakata, M.; Kawano, Y.; Ohishi, M.; Shoji, T.; Sugawara, J.; Tomiyama, H.; Yamashina, A.; Yasuda, H.; Sawayama, T.; et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J. Hypertens. 2009, 27, 2022–2027. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 1988. [Google Scholar]
- Sammoud, S.; Bouguezzi, R.; Negra, Y.; Chaabene, H. The reliability and sensitivity of change of direction deficit and its association with linear sprint speed in prepubertal male soccer players. J. Funct. Morphol. Kinesiol. 2021, 6, 41. [Google Scholar] [CrossRef]
- VanDusseldorp, T.A.; Escobar, K.A.; Johnson, K.E.; Stratton, M.T.; Moriarty, T.; Cole, N.; McCormick, J.J.; Kerksick, C.M.; Vaughan, R.A.; Dokladny, K.; et al. Effect of branched-chain amino acid supplementation on recovery following acute eccentric exercise. Nutrients 2018, 10, 1389. [Google Scholar] [CrossRef]
- Howatson, G.; Hoad, M.; Goodall, S.; Tallent, J.; Bell, P.G.; French, D.N. Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: A randomized, double-blind, placebo controlled study. J. Int. Soc. Sports Nutr. 2012, 9, 20. [Google Scholar] [CrossRef]
- Jackman, S.R.; Witard, O.C.; Jeukendrup, A.E.; Tipton, K.D. Branched-chain amino acid ingestion can ameliorate soreness from eccentric exercise. Med. Sci. Sports Exerc. 2010, 42, 962–970. [Google Scholar] [CrossRef]
- Foure, A.; Nosaka, K.; Gastaldi, M.; Mattei, J.P.; Boudinet, H.; Guye, M.; Vilmen, C.; Le Fur, Y.; Bendahan, D.; Gondin, J. Effects of branched-chain amino acids supplementation on both plasma amino acids concentration and muscle energetics changes resulting from muscle damage: A randomized placebo controlled trial. Clin. Nutr. 2016, 35, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Grazioli, R.; Lopez, P.; Machado, C.L.F.; Farinha, J.B.; Fagundes, A.O.; Voser, R.; Reischak-Oliveira, A.; Setuain, I.; Izquierdo, M.; Pinto, R.S.; et al. Moderate volume of sprint bouts does not induce muscle damage in well-trained athletes. J. Bodyw. Mov. Ther. 2020, 24, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Steensberg, A.; Schjerling, P. Muscle-derived interleukin-6: Possible biological effects. J. Physiol. 2001, 536, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, S.; Handschin, C. Skeletal muscle as an endocrine organ: PGC-1alpha, myokines and exercise. Bone 2015, 80, 115–125. [Google Scholar] [CrossRef]
- Castell, L.M.; Poortmans, J.R.; Leclercq, R.; Brasseur, M.; Duchateau, J.; Newsholme, E.A. Some aspects of the acute phase response after a marathon race, and the effects of glutamine supplementation. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 47–53. [Google Scholar] [CrossRef]
- Ostrowski, K.; Rohde, T.; Zacho, M.; Asp, S.; Pedersen, B.K. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J. Physiol. 1998, 508, 949–953. [Google Scholar] [CrossRef]
- Ostrowski, K.; Rohde, T.; Asp, S.; Schjerling, P.; Pedersen, B.K. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J. Physiol. 1999, 515, 287–291. [Google Scholar] [CrossRef]
- Barnes, J.N.; Trombold, J.R.; Dhindsa, M.; Lin, H.F.; Tanaka, H. Arterial stiffening following eccentric exercise-induced muscle damage. J. Appl. Physiol. 2010, 109, 1102–1108. [Google Scholar] [CrossRef]
- Zhenyukh, O.; Civantos, E.; Ruiz-Ortega, M.; Sanchez, M.S.; Vazquez, C.; Peiro, C.; Egido, J.; Mas, S. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic. Biol. Med. 2017, 104, 165–177. [Google Scholar] [CrossRef]
- Nosaka, K.; Newton, M.; Sacco, P. Muscle damage and soreness after endurance exercise of the elbow flexors. Med. Sci. Sports Exerc. 2002, 34, 920–927. [Google Scholar] [CrossRef]
- Wilke, J.; Behringer, M. Is “delayed onset muscle soreness” a false friend? The potential implication of the fascial connective tissue in post-exercise discomfort. Int. J. Mol. Sci. 2021, 22, 9482. [Google Scholar] [CrossRef] [PubMed]
- Fridén, J.; Kjörell, U.; Thornell, L.E. Delayed muscle soreness and cytoskeletal alterations: An immunocytological study in man. Int. J. Sports Med. 1984, 5, 15–18. [Google Scholar] [CrossRef]
- Wilke, J.; Hespanhol, L.; Behrens, M. Is it all about the fascia? A systematic review and meta-analysis of the prevalence of extramuscular connective tissue lesions in muscle strain injury. Orthop. J. Sports Med. 2019, 7, 2325967119888500. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.D.C.; Comfort, P.; Jones, P.A. Comparison of change of direction speed performance and asymmetries between team-sport athletes: Application of change of direction deficit. Sports 2018, 6, 174. [Google Scholar] [CrossRef]
- Nimphius, S.; Geib, G.; Spiteri, T.; Carlisle, D. “Change of direction deficit” measurement in Division I American football players. J. Aust. Strength Cond. 2013, 21, 115–117. [Google Scholar]
- Aboyans, V.; Criqui, M.H.; Abraham, P.; Allison, M.A.; Creager, M.A.; Diehm, C.; Fowkes, F.G.; Hiatt, W.R.; Jonsson, B.; Lacroix, P.; et al. Measurement and interpretation of the ankle-brachial index: A scientific statement from the American Heart Association. Circulation 2012, 126, 2890–2909. [Google Scholar] [CrossRef] [PubMed]
- Engvall, J.; Nylander, E.; Wranne, B. Arm and ankle blood pressure response to treadmill exercise in normal people. Clin. Physiol. 1989, 9, 517–524. [Google Scholar] [CrossRef] [PubMed]
- King, L.T.; Strandness, D.E., Jr.; Bell, J.W. The hemodynamic response of the lower extremities exercise. J. Surg. Res. 1965, 5, 167–171. [Google Scholar] [CrossRef]
- Sugawara, J.; Hayashi, K.; Yokoi, T.; Cortez-Cooper, M.Y.; DeVan, A.E.; Anton, M.A.; Tanaka, H. Brachial-ankle pulse wave velocity: An index of central arterial stiffness? J. Hum. Hypertens. 2005, 19, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Burr, J.F.; Boulter, M.; Beck, K. Arterial stiffness results from eccentrically biased downhill running exercise. J. Sci. Med. Sport 2015, 18, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Kobayashi, R.; Hashimoto, Y.; Nosaka, K. Changes in arterial stiffness after eccentric versus concentric cycling. Appl. Physiol. Nutr. Metab. 2019, 44, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Pierce, D.R.; Doma, K.; Leicht, A.S. Acute effects of exercise mode on arterial stiffness and wave reflection in healthy young adults: A systematic review and meta-analysis. Front. Physiol. 2018, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, M.H.; Yang, H.T.; Tharp, D.L.; Rector, R.S.; Padilla, J.; Bowles, D.K. Vascular cell transcriptomic changes to exercise training differ directionally along and between skeletal muscle arteriolar trees. Microcirculation 2017, 24, e12336. [Google Scholar] [CrossRef]
- Buckwalter, J.B.; Clifford, P.S. The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exerc. Sport Sci. Rev. 2001, 29, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Pierce, D.R.; Doma, K.; Raiff, H.; Golledge, J.; Leicht, A.S. Influence of exercise mode on post-exercise arterial stiffness and pressure wave measures in healthy adult males. Front. Physiol. 2018, 9, 1468. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.; Coats, A.J.; Adamopoulos, S.; Bernardi, L.; Feng, Y.H.; Conway, J.; Sleight, P. Persistent peripheral vasodilation and sympathetic activity in hypotension after maximal exercise. J. Appl. Physiol. 1993, 75, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Khera, R.; Corrales-Medina, V.F.; Townsend, R.R.; Chirinos, J.A. Inflammation and arterial stiffness in humans. Atherosclerosis 2014, 237, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Sartori, T.; Santos, A.C.A.; Oliveira da Silva, R.; Kodja, G.; Rogero, M.M.; Borelli, P.; Fock, R.A. Branched chain amino acids improve mesenchymal stem cell proliferation, reducing nuclear factor kappa B expression and modulating some inflammatory properties. Nutrition 2020, 78, 110935. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Relationship between plasma endothelin-1 concentration and cardiovascular responses during high-intensity eccentric and concentric exercise. Clin. Physiol. Funct. Imaging 2008, 28, 43–48. [Google Scholar] [CrossRef]

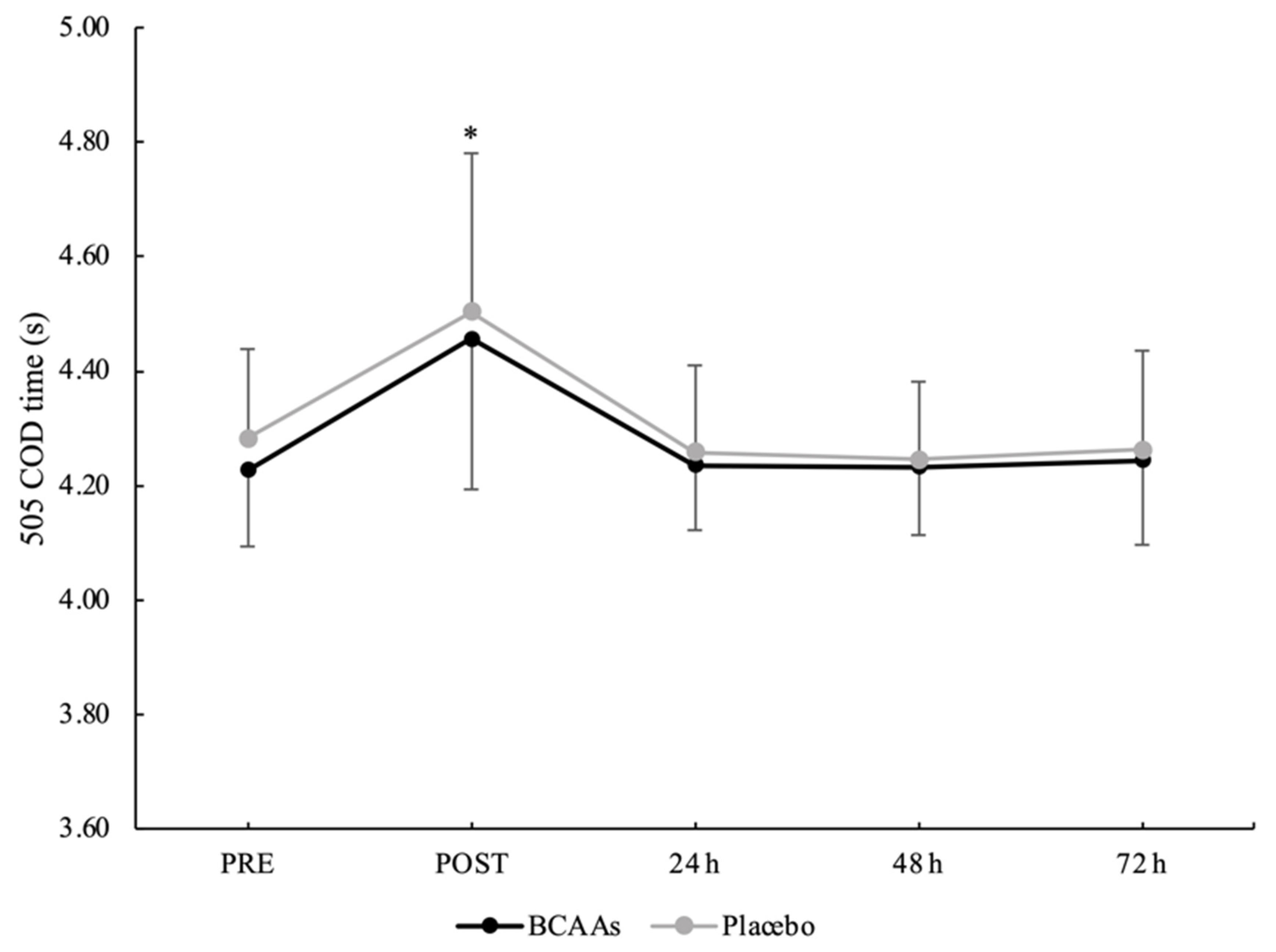
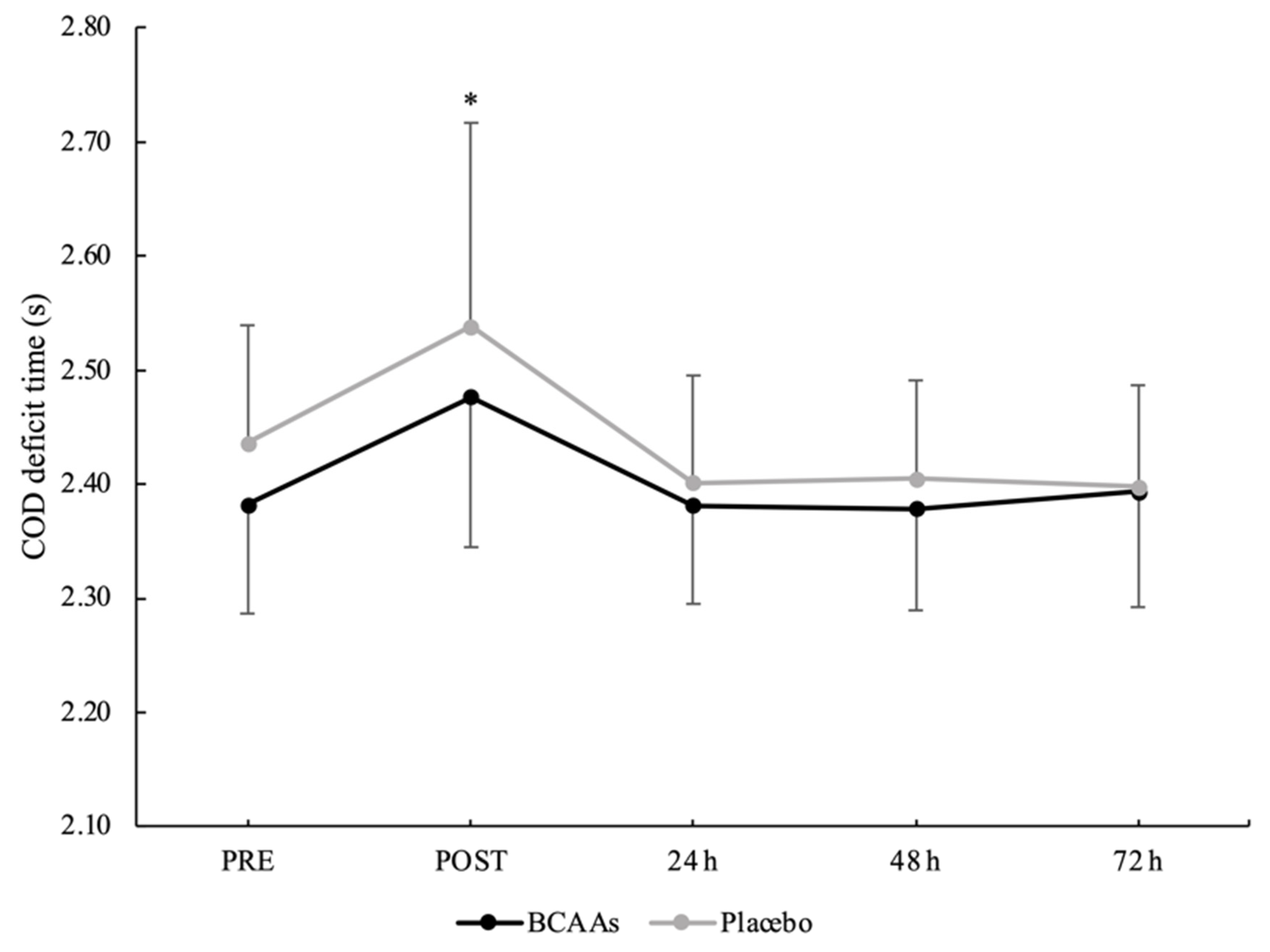
| Parameters | Condition | PRE | POST | 24 h | 48 h | 72 h |
|---|---|---|---|---|---|---|
| ABI | Placebo BCAAs | 1.06 ± 0.06 1.04 ± 0.05 | 0.95 ± 0.09 0.92 ± 0.05 | 1.06 ± 0.05 1.06 ± 0.07 | 1.06 ± 0.05 1.06 ± 0.06 | 1.07 ± 0.06 1.07 ± 0.06 |
| baPWV (cm/s) | Placebo BCAAs | 1064 ± 80 1098 ± 73 | 1023 ± 94 1049 ± 97 | 1049 ± 67 1052 ± 65 | 1050 ± 67 1047 ± 62 | 1061 ± 67 1068 ± 61 |
| Ankle SBP (mmHg) | Placebo BCAAs | 124 ± 11 127 ± 12 | 111 ± 15 113 ± 11 | 126 ± 12 126 ± 11 | 125 ± 11 124 ± 11 | 127 ± 11 128 ± 10 |
| Brachial DBP (mmHg) | Placebo BCAAs | 61 ± 6 65 ± 8 | 62 ± 8 64 ± 8 | 61 ± 5 61 ± 7 | 61 ± 6 61 ± 6 | 62 ± 6 62 ± 6 |
| Brachial SBP (mmHg) | Placebo BCAAs | 116 ± 6 121 ± 11 | 117 ± 10 121 ± 11 | 117 ± 8 117 ± 8 | 116 ± 8 116 ± 10 | 118 ± 8 118 ± 8 |
| CK | VAS | baPWV | Ankle SBP | ABI | |
|---|---|---|---|---|---|
| CK | 1 | 0.694 1 | −0.409 | 0.202 | −0.102 |
| VAS | 0.694 1 | 1 | −0.477 1 | 0.012 | −0.074 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khemtong, C.; Tessitore, A.; Jaime, S.J.; Gobbi, G.; Jensen, J.; Yang, A.-L.; Kuo, C.-H.; Condello, G. Branched-Chain Amino Acids Supplementation Does Not Accelerate Recovery after a Change of Direction Sprinting Exercise Protocol. Nutrients 2022, 14, 4331. https://doi.org/10.3390/nu14204331
Khemtong C, Tessitore A, Jaime SJ, Gobbi G, Jensen J, Yang A-L, Kuo C-H, Condello G. Branched-Chain Amino Acids Supplementation Does Not Accelerate Recovery after a Change of Direction Sprinting Exercise Protocol. Nutrients. 2022; 14(20):4331. https://doi.org/10.3390/nu14204331
Chicago/Turabian StyleKhemtong, Chutimon, Antonio Tessitore, Salvador J. Jaime, Giuliana Gobbi, Jørgen Jensen, Ai-Lun Yang, Chia-Hua Kuo, and Giancarlo Condello. 2022. "Branched-Chain Amino Acids Supplementation Does Not Accelerate Recovery after a Change of Direction Sprinting Exercise Protocol" Nutrients 14, no. 20: 4331. https://doi.org/10.3390/nu14204331
APA StyleKhemtong, C., Tessitore, A., Jaime, S. J., Gobbi, G., Jensen, J., Yang, A.-L., Kuo, C.-H., & Condello, G. (2022). Branched-Chain Amino Acids Supplementation Does Not Accelerate Recovery after a Change of Direction Sprinting Exercise Protocol. Nutrients, 14(20), 4331. https://doi.org/10.3390/nu14204331











