Prevalence of Vitamin D and Calcium Deficiency and Insufficiency in Women of Childbearing Age and Associated Risk Factors: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Databases
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
- Studies that provide data on the prevalence of serum calcium and/or vitamin D deficiency in women of childbearing age (15–49 years or menarche and menopause).
- Studies with representative population-based samples in hospitals, health centers, or outpatient clinics.
- Prevalence data in women of different age groups, such as adolescents, pregnant women, lactating women, and premenopausal adult women.
- Studies with a cross-sectional design and data from longitudinal studies (cohort studies) or intervention studies, such as clinical trials or community trials, provided they had prevalence information for a specific time. Articles in English, Portuguese, and Spanish were included.
2.2.2. Exclusion Criteria
- ○
- Opinion articles, comments, or editorials.
- ○
- Duplicate articles, i.e., the same study found in different databases.
- ○
- Articles with the same database/population/sample, in which case the study with the largest sample size was considered.
- ○
- Articles with primary data not accessible even after request to the authors.
- ○
- Case-control articles, narrative reviews, and case series.
- ○
- Studies conducted among female athletes of any sport.
- ○
- Studies conducted among women with the following specific diseases: autoimmune diseases such as lupus, psoriasis, thyroiditis, rheumatoid arthritis, and multiple sclerosis; eating disorders such as anorexia and bulimia; hematological diseases such as thalassemia and sickle cell disease; respiratory diseases such as chronic obstructive pulmonary disease, asthma, pneumonia, respiratory infections, and tuberculosis; chronic diseases such as heart failure, kidney failure, liver disease, chronic kidney disease, heart disease, nephrotic syndrome, AIDS, inflammatory bowel disease, hypo- or hyperthyroidism, sepsis, and cancer; genetic diseases and syndromes such as vitamin D receptor mutation, cystic fibrosis, and Prader–Willi syndrome; neurological or psychiatric disorders such as epilepsy (or antiepileptic medication use), attention deficit hyperactivity disorder, and schizophrenia.
- ○
- Studies conducted among post-surgical patients, patients with trauma or burns, or patients undergoing recent treatment for fractures or orthopedic/osteoarticular diseases.
- ○
- Studies conducted among patients undergoing intensive, urgency or emergency, or palliative care.
- ○
- Studies with fewer than 50 participants.
- ○
- Studies conducted among indigenous women.
2.3. Reviewer Training
2.4. Review Process
2.5. Data Extraction and Risk of Bias Assessment
2.6. Statistical Analysis
3. Results
3.1. Prevalence of Vitamin D Deficiency and Insufficiency
3.2. Factors Associated with Vitamin D Deficiency and Insufficiency
3.3. Prevalence of Calcium Deficiency and Associated Risk Factors
3.4. Quality Analysis of the Evidence
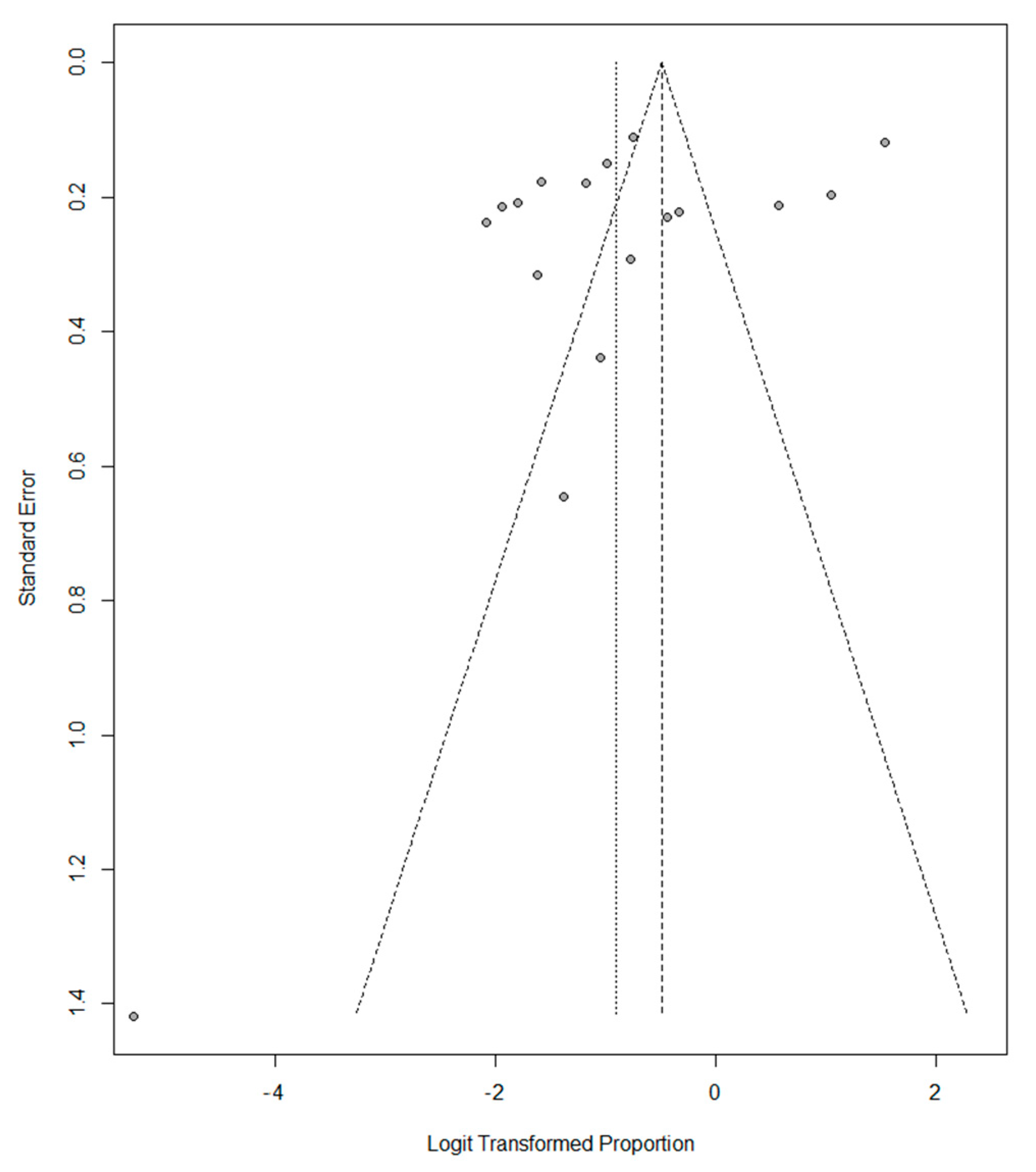
3.5. Meta-Analysis of the Prevalence of Vitamin D Deficiency and Insufficiency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global consensus recommendations on prevention and management of nutritional rickets. J. Clin. Endocrinol. Metab. 2016, 101, 94–415. [Google Scholar] [CrossRef]
- Barger, M.K. Maternal nutrition and perinatal outcomes. J. Midwifery Women’s Health. 2010, 55, 502–511. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. New insights into the vitamin D requirements during pregnancy. Bone Res. 2017, 5, 17030. [Google Scholar] [CrossRef] [Green Version]
- Suchdev, P.S.; Peña-Rosas, J.P.; De-Regil, L.M. Multiple micronutrient powders for home (point-of-use) fortification of foods in pregnant women. Cochrane Database Syst. Rev. 2014, 2014, CD011158. [Google Scholar]
- Pang, Y.; Kim, O.; Choi, J.-A.; Jung, H.; Kim, J.; Lee, H.; Lee, H. Vitamin D deficiency and associated factors in south Korean childbearing women: A cross-sectional study. BMC Nurs. 2021, 20, 4–11. [Google Scholar] [CrossRef]
- Amouzegar, A.; Azizi, F.; Ashrafivand, S.; Ahi, Z.; Saleh, M.; Mohaghegh, S.; Gargari, S.S. Prevalence of calcium and vitamin D deficiency and their association with feto-maternal outcomes in a sample of Iranian pregnant women. Hum. Antibodies. 2020, 28, 305–312. [Google Scholar] [CrossRef]
- Pilz, S.; Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; Keppel, M.H.; Grübler, M.R.; März, W.; Pandis, M. Vitamin D testing and treatment: A narrative review of current evidence. Endocr. Connect. 2019, 8, 27–43. [Google Scholar] [CrossRef] [Green Version]
- Zittermann, A.; Pilz, S. Vitamin D in Klinik und Praxis. Dtsch Med. Wochenschr. 2017, 142, 601–616. [Google Scholar] [CrossRef]
- Mendes, M.M.; Hart, K.H.; Botelho, P.B.; Lanham-New, S.A. Vitamin D status in the tropics: Is sunlight exposure the main determinant? Nutr. Bull. 2018, 43, 428–434. [Google Scholar] [CrossRef]
- Shen, Q.; Xu, Q.; Li, G.; Ren, L.; Zhang, Z.; Zhang, Y.; Zhong, Z.; Li, X.; Wang, Q. Joint effect of 25-hydroxyvitamin D and secondhand smoke exposure on hypertension in non-smoking women of childbearing age: NHANES 2007-2014. Environ. Health A Glob. Access Sci. Source. 2021, 20, 1–10. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, H.; Yang, J.; Huang, Z.; Xu, H.; Jin, J.; Xu, K.; Tong, Y.; Dong, Q.; Zheng, J. The relationship between maternal vitamin D deficiency and glycolipid metabolism and adverse pregnancy outcome. Clin. Endocrinol. 2020, 93, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.B.; Jorgensen, J.S.; Jensen, T.K.; Dalgård, C.; Barington, T.; Nielsen, J.; Beck-Nielsen, S.S.; Husby, S.; Abrahamsen, B.; Lamont, R.F.; et al. Vitamin D insufficiency is associated with increased risk of first-trimester miscarriage in the Odense Child Cohort. Am. J. Clin. Nutr. 2015, 102, 633–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arima, K.; Mizukami, S.; Nishimura, T.; Tomita, Y.; Nakashima, H.; Abe, Y.; Aoyagi, K. Epidemiology of the association between serum 25-hydroxyvitamin D levels and musculoskeletal conditions among elderly individuals: A literature review. J. Physiol. Anthropol. 2020, 39, 38. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma. 2019, 10, 1082–1093. [Google Scholar] [CrossRef]
- Wanigatunga, A.A.; Sternberg, A.L.; Blackford, A.L.; Cai, Y.; Mitchell, C.M.; Roth, D.L.; Miller, E.R., 3rd; Szanton, S.L.; Juraschek, S.P.; Michos, E.D.; et al. The effects of vitamin D supplementation on types of falls. J. Am. Geriatr. Soc. 2021, 69, 2851–2864. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef]
- Hong-Bi, S.; Yin, X.; Xiaowu, Y.; Ying, W.; Yang, X.; Ting, C.; Na, W. High prevalence of vitamin D deficiency in pregnant women and its relationship with adverse pregnancy outcomes in Guizhou, China. J. Int. Med. Res. 2018, 46, 4500–4505. [Google Scholar] [CrossRef] [PubMed]
- Mosavat, M.; Arabiat, D.; Smyth, A.; Newnham, J.; Whitehead, L. Second-trimester maternal serum vitamin D and pregnancy outcome: The Western Australian Raine cohort study. Diabetes Res. Clin. Pract. 2021, 175, 108779. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Y.; Xu, Y. Vitamin D deficiency in pregnant women: Influenced by multiple risk factors and increase the risks of spontaneous abortion and small-for-gestational age. Medicine. 2021, 100, e27505. [Google Scholar] [CrossRef] [PubMed]
- Elsori, D.H.; Hammoud, M.S. Vitamin D deficiency in mothers, neonates and children. J. Steroid. Biochem. Mol. Biol. 2018, 175, 195–199. [Google Scholar] [CrossRef]
- Ferrillo, M.; Migliario, M.; Roccuzzo, A.; Molinero-Mourelle, P.; Falcicchio, G.; Umano, G.R.; Pezzotti, F.; Foglio Bonda, P.L.; Calafiore, D.; de Sire, A. Periodontal Disease and Vitamin D Deficiency in Pregnant Women: Which Correlation with Preterm and Low-Weight Birth? J. Clin. Med. 2021, 10, 4578. [Google Scholar] [CrossRef] [PubMed]
- Kulda, V. Vitamin D metabolism. Vnitr. Lek. 2012, 58, 400–404. [Google Scholar] [PubMed]
- Reid, I.R.; Bolland, M.J. Controversies in medicine: The role of calcium and vitamin D supplements in adults. Med. J. Aust. 2019, 211, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Bolland, M.J. Calcium and/or vitamin D supplementation for the prevention of fragility fractures: Who needs it? Nutrients. 2020, 12, 1011. [Google Scholar] [CrossRef] [Green Version]
- Song, L. Calcium and Bone Metabolism Indices. Adv. Clin. Chem. 2017, 82, 1–46. [Google Scholar]
- Herrera-Cuenca, M.; Previdelli, A.; Koletzko, B.; Hernandez, P.; Landaeta-Jimenez, M.; Sifontes, Y.; Gómez, G.; Kovalskys, I.; García, M.; Pareja, R.; et al. Childbearing age women characteristics in latin america. Building evidence bases for early prevention. results from the elans study. Nutrients. 2021, 13, 45. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Brandi, M.L.; Cusano, N.E.; Mannstadt, M.; Rejnmark, L.; Rizzoli, R.; Rubin, M.R.; Winer, K.K.; Liberman, U.A.; Potts, J.T. Management of Hypoparathyroidism Present and Future. J. Clin. Endocrinol. Metab. 2016, 101, 2313–2324. [Google Scholar] [CrossRef]
- Roth, D.E.; Abrams, S.A.; Aloia, J.; Bergeron, G.; Bourassa, M.W.; Brown, K.H.; Calvo, M.S.; Cashman, K.D.; Combs, G.; De-Regil, L.M.; et al. Global prevalence and disease burden of vitamin D deficiency: A roadmap for action in low-and middle-income countries. Ann. N. Y. Acad. Sci. 2018, 1430, 44–79. [Google Scholar] [CrossRef] [Green Version]
- Hofmeyr, G.J.; Atallah, Á.N.; Duley, L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst. Rev. 2018, 10, CD001059. [Google Scholar] [CrossRef]
- Lucchetta, R.C.; Lemos, I.H.; Luísa, A.; Gini, R.; De Andrade Cavicchioli, S.; Forgerini, M.; Varallo, F.R.; de Nadai, M.N.; Fernandez-Llimos, F.; Mastroianni, P.D. Deficiency and Insufficiency of Vitamin D in Women of Childbearing Age: A Systematic Review and Meta-Analysis Deficiência e Insuficiência de Vitamina D em Mulheres na Idade Reprodutiva: Uma Revisão Sistemática e Meta-Análise. Rev. Bras. Ginecol. Obs. 2022, 44, 409–424. Available online: https://creativecommons.org/licenses/by/4.0/ (accessed on 22 June 2022).
- Silveira, E.A.; Moura, L.d.A.N.; Castro, M.C.R.; Kac, G.; Noll, P.R.E.S.; de Oliveira, C.; Noll, M. Prevalence of vitamin D and calcium deficiencies and their health impacts on women of childbearing age: A protocol for systematic review and meta-analysis. BMJ Open. 2022, 12, e049731. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.S.; Borba, V.Z.C.; Camargo, M.B.R.; Silva, D.M.W.; Borges, J.L.C.; Bandeira, F.; Lazaretti-Castro, M. Recommendations of the Brazilian Society of Endocrinology and Metabology (SBEM) for the diagnosis and treatment of hypovitaminosis D. Arq. Bras. Endocrinol. Metabol. 2014, 58, 411–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrisostomo, K.R.; Skare, T.L.; Kulak, J.; Urbanetz, A.A.; Chrisostomo, E.R.; Nisihara, R. The prevalence and clinical associations of hypovitaminosis D in pregnant women from Brazil. Int. J. Gynecol. Obstet. 2018, 143, 66–70. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.R.D.J.L.; De Azevedo Silva, T.S.; Figueredo, E.D. Hypovitaminosis d in pregnancy: Is it a public health issue? Rev. Bras. Saude Matern. Infant. 2019, 19, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Santos, M.; Queiroz Carvalho, G.; David Couto, R.; Barbosa dos Santos, D.; Marlucia Oliveira, A. Vitamin D deficiency and associated factors among pregnant women of a sunny city in Northeast of Brazil. Clin. Nutr. ESPEN. 2018, 23, 240–244. [Google Scholar] [CrossRef]
- Araújo, E.P.D.S.; Queiroz, D.J.M.; Neves, J.P.R.; De Lacerda, L.M.; Gonçalves, M.D.C.R.; De Carvalho, A.T. Prevalencia de hipovitaminosis D y factores asociados en adolescentes de una ciudad capital del noroeste de Brasil. Nutr. Hosp. 2017, 34, 1416–1423. [Google Scholar]
- Fonseca Valle, D.; Giannini, D.T. Correlation between vitamin D and blood pressure in adolescents. Int. J. Adolesc. Med. Health. 2019, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Segheto, K.J.; Juvanhol, L.L.; da Silva, D.C.G.; de Carvalho, C.J.; Hansen, F.; Gabiatti, M.P.; Kakehasi, A.M.; Longo, G.Z. Does the relationship between 25-hydroxyvitamin D status and bone mass vary according to skin color in adults? Results of a Brazilian population-based study. Arch. Osteoporos. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Peters, B.S.E.; Roque, J.P.; Fisberg, M.; Martini, L.A. Metabólitos séricos da vitamina D não se correlacionam com pressão arterial em adolescentes. Arq. Bras. Endocrinol. Metabol. 2009, 53, 416–424. [Google Scholar] [CrossRef] [Green Version]
- Santos, B.R.; Mascarenhas, L.P.G.; Satler, F.; Boguszewski, M.C.S.; Spritzer, P.M. Vitamin D deficiency in girls from South Brazil: A cross-sectional study on prevalence and association with vitamin D receptor gene variants. BMC Pediatr. 2012, 12, 62. [Google Scholar] [CrossRef] [Green Version]
- Lopes, V.M.; Lopes, J.R.C.; Brasileiro, J.P.B.; de Oliveira, I.; Lacerda, R.P.; Andrade, M.R.D.; Tierno, N.I.Z.; de Souza, R.C.C.; da Motta, L.A.C.R. Highly prevalence of vitamin D deficiency among Brazilian women of reproductive age. Arch. Endocrino. Metab. 2017, 61, 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, B.R.; Costa, N.C.; Silva, T.R.; Oppermann, K.; Magalhães, J.A.; Casanova, G.; Spritzer, P.M. Prevalence of Vitamin D deficiency in women from southern Brazil and association with Vitamin D-binding protein levels and GC-DBP gene polymorphisms. PLoS ONE 2019, 14, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, M.R.M.C.D.; Oliveira, F.D.C.C.; Assis, K.F.; Ribeiro, S.A.V.; Junior, P.P.D.P.; Sant’Ana, L.F.D.R.; Priore, S.E.; Franceschini, S.D.C.C. Prevalence of Vitamin D deficiency and associated factors in women and newborns in the immediate postpartum period. Rev. Paul Pediatr. 2015, 33, 287–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, A.C.C.; Cocate, P.; Adegboye, A.R.A.; Franco-Sena, A.B.; Farias, D.; De Castro, M.B.T.; Brito, A.; Allen, L.H.; Mokhtar, R.; Holick, M.F.; et al. Changes in plasma concentrations of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D during pregnancy: A Brazilian cohort. Eur. J. Nutr. 2017, 57, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.S.; Rocha, T.M.; Klein, M.R.S.T.; Sanjuliani, A.F. Vitamin D deficiency is associated with insulin resistance independent of intracellular calcium, dietary calcium and serum levels of parathormone, calcitriol and calcium in premenopausal women. Nutr. Hosp. 2015, 31, 1491–1498. [Google Scholar]
- Vivan, M.A.; Kops, N.L.; Fülber, E.R.; de Souza, A.C.; Fleuri, M.A.S.B.; Friedman, R. Prevalence of Vitamin D Depletion, and Associated Factors, among Patients Undergoing Bariatric Surgery in Southern Brazil. Obes. Surg. 2019, 29, 3179–3187. [Google Scholar]
- Lopes, M.P.; Giudici, K.V.; Marchioni, D.M.; Fisberg, R.M.; Martini, L.A. Relationships between n-3 polyunsaturated fatty acid intake, serum 25 hydroxyvitamin D, food consumption, and nutritional status among adolescents. Nutr. Res. 2015, 35, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Maciel, D.G.; de Abreu Reis, M.J. Frequência de hipovitaminose D em mulheres adultas. J. Health Sci. Inst. 2017, 35, 25–28. [Google Scholar]
- Mendes, M.M.; Hart, K.H.; Lanham-New, S.A.; Botelho, P.B. Exploring the impact of individual uvb radiation levels on serum 25-hydroxyvitamin D in women living in high versus low latitudes: A cross-sectional analysis from the D-sol study. Nutrients. 2020, 12, 3805. [Google Scholar] [CrossRef]
- Martínez Torres, J.; Barajas Lizarazo, M.A.; Cárdenas Malpica, P.A.; Escobar-Velásquez, K.; Carvajal Suárez, L.S.; Moreno-Bayona, J.A.; Rangel Navia, H.J. Prevalence of vitamin D deficiency and insufficiency and associated factors in Colombian women in 2015. Nutr. Hosp. 2022, 39, 843–851. [Google Scholar]
- Junaid, K.; Rehman, A.; Jolliffe, D.A.; Wood, K.; Martineau, A.R. High prevalence of vitamin D deficiency among women of child-bearing age in Lahore Pakistan, associating with lack of sun exposure and illiteracy. BMC Womens Health. 2015, 15, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerges, M.E.S.; Amin, G.E.A.; Andraous, F.; Abdel Hamid, D.M.; Allam, M.F. Vitamin D level in a sample of Egyptian females of childbearing age attending a family medicine center. Int. J. Clin. Pract. 2021, 75, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, A.; Sandström, H.; Stenlund, H.; Johansson, I.; Hultdin, J. Vitamin D status during pregnancy: A longitudinal study in Swedish women from early pregnancy to seven months postpartum. PLoS ONE 2016, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F. Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health. Anticancer Res. 2016, 36, 1345–1356. [Google Scholar] [PubMed]
- Mays, S.; Prowse, T.; George, M.; Brickley, M. Latitude, urbanization, age, and sex as risk factors for vitamin D deficiency disease in the Roman Empire. Am. J. Phys. Anthropol. 2018, 167, 484–496. [Google Scholar] [CrossRef]
- Chávez-Courtois, M.; Godínez-Martínez, E.; Muñoz-Manrique, C.; Negrete-Martínez, V.; González-Leyva, C.P.; Tolentino-Dolores, M.; Suárez-Rico, B.; Estrada-Gutierrez, G.; Perichart-Perera, O. Vitamin D status and its determinants in mexican pregnant women from a rural and an urban area: A comparative study. Int. J. Environ. Res. Public Health 2021, 18, 4571. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Laudisio, D.; Pugliese, G.; de Alteriis, G.; Colao, A.; Savastano, S. Influence of the Mediterranean Diet on 25-Hydroxyvitamin D Levels in Adults. Nutrients 2020, 12, 1439. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee; Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; del Valle, H.B. (Eds.) Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK56070/ (accessed on 25 July 2022).
- Herrera-Cuenca, M.; Kovalskys, I.; Gerardi, A.; Hernandez, P.; Sifontes, Y.; Gómez, G.; García, M.C.Y.; Méndez-Pérez, B.; Landaeta-Jimenez, M.; Pareja, R.; et al. Anthropometric Profile of Latin American Population: Results from the ELANS Study. Front. Nutr. 2021, 8, 740361. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Kechichian, E.; Ezzedine, K. Vitamin D and the Skin: An Update for Dermatologists. Am. J. Clin. Dermatol. 2018, 19, 223–235. [Google Scholar] [CrossRef]
- Passeron, T.; Bouillon, R.; Callender, V.; Cestari, T.; Diepgen, T.; Green, A.; van der Pols, J.; Bernard, B.; Ly, F.; Bernerd, F.; et al. Sunscreen photoprotection and vitamin D status. Br. J. Dermatol. 2019, 181, 916–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cashman, K.D. Vitamin D Deficiency: Defining, Prevalence, Causes, and Strategies of Addressing. Calcif. Tissue Int. 2020, 106, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, D.E.; Al-Khashan, H.I.; Mishriky, A.M. Comparison of vitamin D deficiency in Saudi married couples. Eur. J. Clin. Nutr. 2012, 66, 742–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Jiang, L.; Zhang, Y.; Chai, J.; Li, J.; Song, X.; Pei, L. Socioeconomic status and vitamin D deficiency among women of childbearing age: A population-based, case-control study in rural northern China. BMJ Open. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- van der Pligt, P.; Willcox, J.; Szymlek-Gay, E.A.; Murray, E.; Worsley, A.; Daly, R.M. Associations of maternal vitamin D deficiency with pregnancy and neonatal complications in developing countries: A systematic review. Nutrients. 2018, 10, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savastano, S.; Barrea, L.; Savanelli, M.C.; Nappi, F.; Di Somma, C.; Orio, F.; Colao, A. Low vitamin D status and obesity: Role of nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 215–225. [Google Scholar] [CrossRef]
- Barrea, L.; Frias-Toral, E.; Pugliese, G.; Garcia-Velasquez, E.; de Los Angeles Carignano, M.; Savastano, S.; Colao, A.; Muscogiuri, G. Vitamin D in obesity and obesity-related diseases: An overview. Minerva Endocrinol. 2021, 46, 177–192. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Somma, C.D.; Laudisio, D.; Salzano, C.; Pugliese, G.; de Alteriis, G.; Colao, A.; Savastano, S. Sex Differences of Vitamin D Status across BMI Classes: An Observational Prospective Cohort Study. Nutrients. 2019, 11, 3034. [Google Scholar] [CrossRef]
- Autier, P.; Gandini, S.; Mullie, P. A systematic review: Influence of vitamin D supplementation on serum 25-hydroxyvitamin D concentration. J. Clin. Endocrinol. Metab. 2012, 97, 2606–2613. [Google Scholar] [CrossRef] [Green Version]
- Silveira, E.A.; Cardoso, C.; Moura, L.; Dos Santos Rodrigues, A.P.; de Oliveira, C. Serum and Dietary Vitamin D in Individuals with Class II and III Obesity: Prevalence and Association with Metabolic Syndrome. Nutrients 2021, 13, 2138. [Google Scholar] [CrossRef]
- Bu, F.X.; Armas, L.; Lappe, J.; Zhou, Y.; Gao, G.; Wang, H.W.; Recker, R.; Zhao, L.J. Comprehensive association analysis of nine candidate genes with serum 25-hydroxy vitamin D levels among healthy Caucasian subjects. Hum. Genet. 2010, 128, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Hibler, E.A.; Jurutka, P.W.; Egan, J.B.; Hu, C.; LeRoy, E.C.; Martinez, M.E.; Thompson, P.A.; Jacobs, E.T. Association between polymorphic variation in VDR and RXRA and circulating levels of vitamin D metabolites. J. Steroid. Biochem. Mol. Biol. 2010, 121, 438–441. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F. Vitamin D: A D-Lightful health perspective. Nutr. Rev. 2008, 66, S182–S194. [Google Scholar] [CrossRef] [PubMed]
- Ribas Filho, D.; Almeida, C.A.N.; Oliveira Filho, A.E. Posicionamento atual sobre vitamina D naprática clínica: Posicionamento da Associação Brasileira de Nutrologia (Abran). Int. J. Nutrol. 2020, 12, 82–96. [Google Scholar]






| Author/Year/Location | Investigated Variables | Summary of the Association of Vitamin D Deficiency or Insufficiency |
|---|---|---|
| Pregnant women | ||
| Pereira-Santos/2017 [35] San Antonio de Jesus-Bahia Northeast | Age, MFI, YS, skin color, MS, GA, number of weekly SEs, region of body exposed to sun, SY, means of transport | Association–risk factor to: Being married or in a relationship Face and hands on the sun Use of vehicle as transport Season-winter |
| Chrisostomo/2018 [33] Curitiba-Paraná South | Age, ethnic origin, skin phototype according to the Fitzpatrick classification, tobacco exposure, AI, YS, PCI, SY; clinical data: preeclampsia, DM, HIV, BMI, medication use, stage of pregnancy, parity, number of spontaneous abortions, NP | Association–risk factor to: Preeclampsia Serum vitamin D Increase when blood collection is in the summer |
| Souza/2019 [34] São Luís-Maranhão Northeast | Marital status, skin color, PCI, religion, sunscreen use, adolescence, NP, gestational trimester | Lower mean Vitamin D–risk factor to: Religion/protestant Primiparous Association hypovitaminosis-risk factor to: Adolescents Primiparous, Income |
| Prado/2015 [43] Viçosa-Minas Gerais Southeast | Women: age, skin color, place of residence, parity, supplementation, US, SE, education, MS, TD, AP, Ca, PTH, P | No association |
| Figueiredo/2017 [44] Rio de Janeiro Southeast | Age, skin color, YS, PCI, parity, smoking in the 1st trimester, AI in the 1st trimester, PA before pregnancy, SY, daily calcium and VD intake | No association Only increase of serum Vitamin D during the third trimester of pregnancy stated in winter or spring |
| Non-pregnant women | ||
| Ferreira/2015/Rio de Janeiro-RJ [45] Southeast | Age, skin color, AI, daily Ca consumption, creatinine, TPL, albumin, globulin, intracellular Ca, serum Ca, ionic Ca, urinary Ca/creatinine, PTH, W, BMI, BF, WC, HC, WC/HC ratio, WC/height ratio, glucose, insulin, HOMA-IR, TCL, HDL, LDL, TG, leptin, adiponectin, hs-CRP, RHI, SBP, DBP. | Association–risk factor to: Serum glucose, Homeostatic model assessment for insulin resistance |
| Araújo/2017 [36] João Pessoa-PB Northeast | Age, skin color, daily hours of sleep, SE, PA, daily VD intake, weight, height, BMI | Association–risk factor to: Calcium |
| Vivan **/2019 [46] Porto Alegre-RS South | Age, sex, skin color, educational level, SY, BMI, SAH, DM, multiple comorbidities, use of thiazide diuretics, use of Ca channel blocker, Hb1Ac, FBG | Association–risk factor to: Non-white skin Diabetes Use of calcium channel blocker Glycated hemoglobin Fasting blood glucose Autumn-winter Body mass index |
| Fonseca Valle/2019 [37] Rio de Janeiro-RJ Southeast | Age, weight, height, BMI, WC, waist-to-height ratio, CI, PTH, LDL, HDL, TCL, TG, SBP, DBS, FBG, Hb1Ac, SI, HOMA-IR, | Association–risk factor to: Weight Body mass index Waist circumference Waist-to-height ratio Systolic blood pressure Homeostatic model assessment for insulin resistance |
| Peters/2009 [39] Indaiatuba-SP Southeast | Sunscreen, physical exercise, sun exposure, DBS, SBP | No association |
| Santos **/2012 [40] Curitiba-PR, Porto Alegre-RS South | Age, height, BMI, age at menarche, thelarche, SY, genotype, WC | No association |
| Lopes/2017 [41] Brasilia DF Midwest | Infertility (low ovarian reserve, PCOS, tubal factors, endometriosis, multiple factors, unexplained infertility) | No association |
| Santos/2019 [42] Brazil South | BP, weight, height, WC, BMI, TCL, LDL, HDL, TG, HOMA-IR, estradiol, testosterone, SHBG, PTH, VDBP, albumin | No association |
| Segheto **/2021 [38] Viçosa-Minas Gerais Southeast | Bone mass, total bone mineral content | No association |
| Lopes/2015 [47] São Paulo-SP Southeast | Not investigated | |
| Maciel/2017 [48] Jacareí-SP Southeast | Not investigated | |
| Mendes/2020 [49] several cities in Brazil | Not investigated |
| Study (Year) | Conflict of Interests | Ethical Approval | Downs and Black Checklist | GRADE | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A/1 | B/2 | C/3 | D/5 | E/6 | F/7 | G/9 | H/10 | I/11 | J/12 | K/17 | L/18 | M/20 | N/21 | O/25 | P/26 | Total | Score # | ||||
| Segheto et al. (2021) [39] | * | YES | 1 | 1 | 1 | 2 | 1 | 1 | - | 1 | 1 | 1 | - | 1 | 1 | 1 | 13/13 | 100% | ●●● | ||
| Mendes et al. (2020) [49] | * | YES | 1 | 1 | 1 | 2 | 1 | 1 | - | 1 | 0 | 0 | - | 1 | 1 | - | 1 | - | 11/13 | 85% | ●●● |
| De Souza; Silva; Figueiredo (2019) [44] | * | YES | 1 | 1 | 1 | 0 | 1 | 1 | - | 1 | 0 | 0 | - | 1 | 1 | - | 0 | - | 8/13 | 62% | ●● |
| Valle; Giannini (2019) [37] | * | NO | 1 | 1 | 1 | 0 | 1 | 1 | - | 1 | 0 | 1 | - | 1 | 1 | - | 1 | - | 10/13 | 77% | ●● |
| Vivan et al. (2019) [46] | * | YES | 1 | 1 | 1 | 1 | 1 | 1 | - | 1 | 0 | 0 | - | 1 | 1 | - | 1 | - | 10/13 | 77% | ●●●● |
| Christostomo et al. (2018) [33] | * | YES | 1 | 1 | 1 | 1 | 1 | 1 | - | 1 | 0 | 1 | - | 1 | 1 | - | 1 | - | 11/13 | 85% | ●●● |
| Figueiredo et al. (2017) [44] | * | YES | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 15/17 | 88% | ●●● |
| Araújo et al. (2017) [36] | * | YES | 1 | 1 | 1 | 1 | 1 | 1 | - | 1 | 0 | 0 | - | 1 | 1 | - | 1 | - | 10/13 | 77% | ●●● |
| Pereira-Santos et al. (2017) [35] | * | YES | 1 | 1 | 1 | 1 | 1 | 1 | - | 1 | 1 | 1 | - | 1 | 1 | - | 1 | - | 12/13 | 92% | ●●● |
| Lopes et al. (2017) [41] | * | NO | 1 | 1 | 1 | 1 | 1 | 1 | - | 1 | 0 | 1 | - | 1 | 1 | - | 1 | - | 11/13 | 85% | ●●● |
| Maciel; Reis (2017) [48] | * | YES | 1 | 1 | 1 | 0 | 1 | 1 | - | 0 | 0 | 0 | - | 1 | 1 | - | 0 | - | 7/13 | 54% | ● |
| Ferreira et al. (2015) [45] | * | YES | 1 | 1 | 1 | 2 | 1 | 1 | - | 1 | 0 | 0 | - | 1 | 1 | - | 1 | - | 11/13 | 85% | ●●● |
| Prado et al. (2015) [43] | * | YES | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 12/13 | 92% | ●●● | ||||
| Lopes et al. (2015) [47] | * | YES | 1 | 1 | 1 | 1 | 1 | 1 | - | 1 | 0 | 1 | - | 1 | 1 | - | 1 | - | 11/13 | 85% | ●●● |
| Santos et al. (2012) [40] | * | YES | 1 | 1 | 1 | 2 | 1 | 1 | - | 1 | 0 | 0 | - | 1 | 1 | - | 1 | - | 11/13 | 85% | ●●● |
| Peters et al. (2009) [39] | * | YES | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 10/13 | 77% | ●● | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silveira, E.A.; Moura, L.d.A.N.e.; Castro, M.C.R.; Kac, G.; Hadler, M.C.C.M.; Noll, P.R.E.S.; Noll, M.; Rezende, A.T.d.O.; Delpino, F.M.; Oliveira, C.d. Prevalence of Vitamin D and Calcium Deficiency and Insufficiency in Women of Childbearing Age and Associated Risk Factors: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4351. https://doi.org/10.3390/nu14204351
da Silveira EA, Moura LdANe, Castro MCR, Kac G, Hadler MCCM, Noll PRES, Noll M, Rezende ATdO, Delpino FM, Oliveira Cd. Prevalence of Vitamin D and Calcium Deficiency and Insufficiency in Women of Childbearing Age and Associated Risk Factors: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(20):4351. https://doi.org/10.3390/nu14204351
Chicago/Turabian Styleda Silveira, Erika Aparecida, Letícia de Almeida Nogueira e Moura, Maria Clara Rezende Castro, Gilberto Kac, Maria Claret Costa Monteiro Hadler, Priscilla Rayanne E. Silva Noll, Matias Noll, Andréa Toledo de Oliveira Rezende, Felipe Mendes Delpino, and Cesar de Oliveira. 2022. "Prevalence of Vitamin D and Calcium Deficiency and Insufficiency in Women of Childbearing Age and Associated Risk Factors: A Systematic Review and Meta-Analysis" Nutrients 14, no. 20: 4351. https://doi.org/10.3390/nu14204351








