Effect of Taraxaci Herba on Bone Loss in an OVX-Induced Model through the Regulation of Osteoclast Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of TH
2.3. Liquid Chromatography–Mass Spectrometry (LC–MS) Analysis for Marker Components
2.4. Cell Culture and Cytotoxicity
2.5. Tartrate-Resistant Acid Phosphatase (TRAP) Staining and TRAP Activity
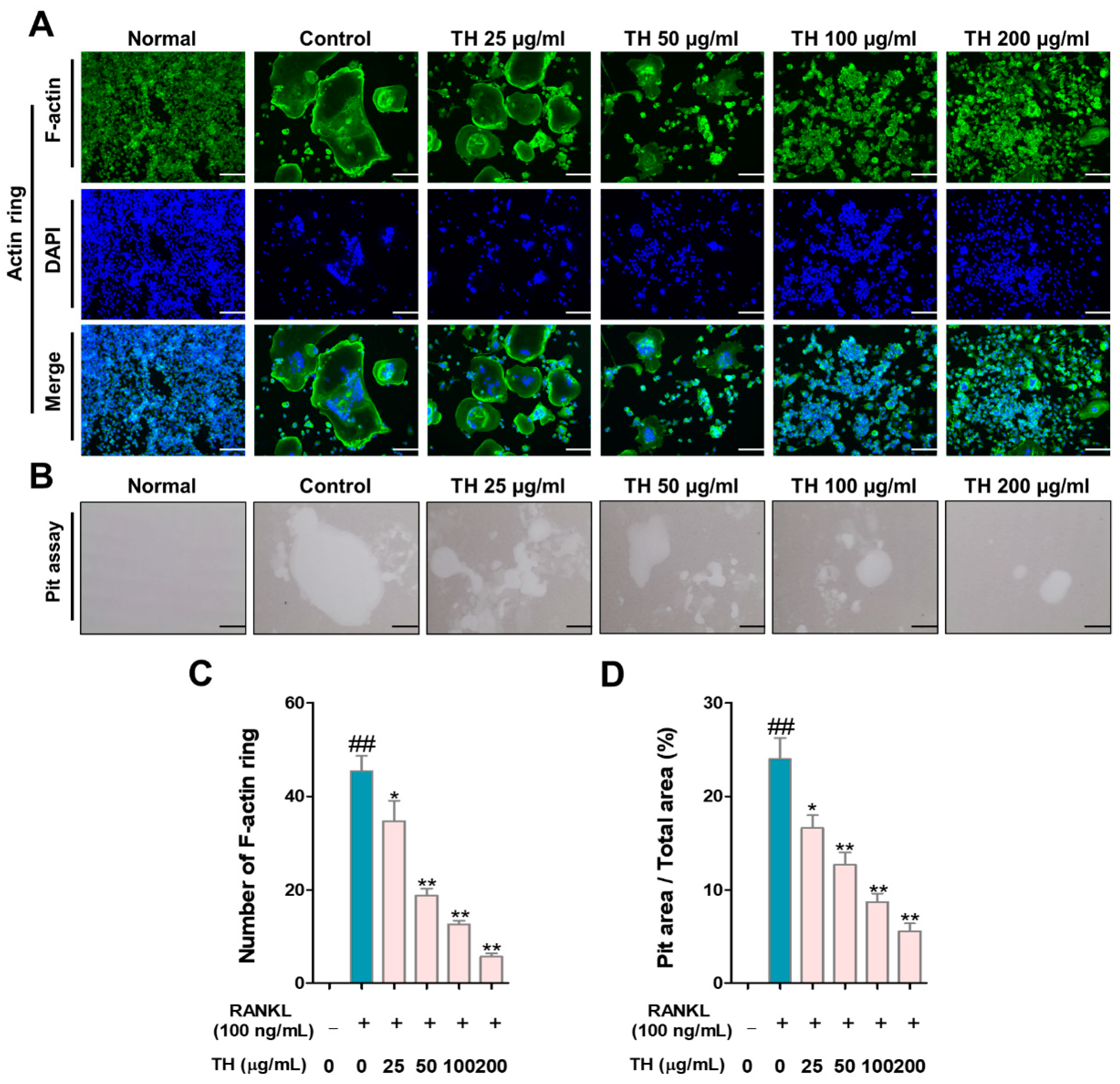
2.6. F-Actin Ring Formation and Pit Formation Assay
2.7. Western Blot
2.8. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
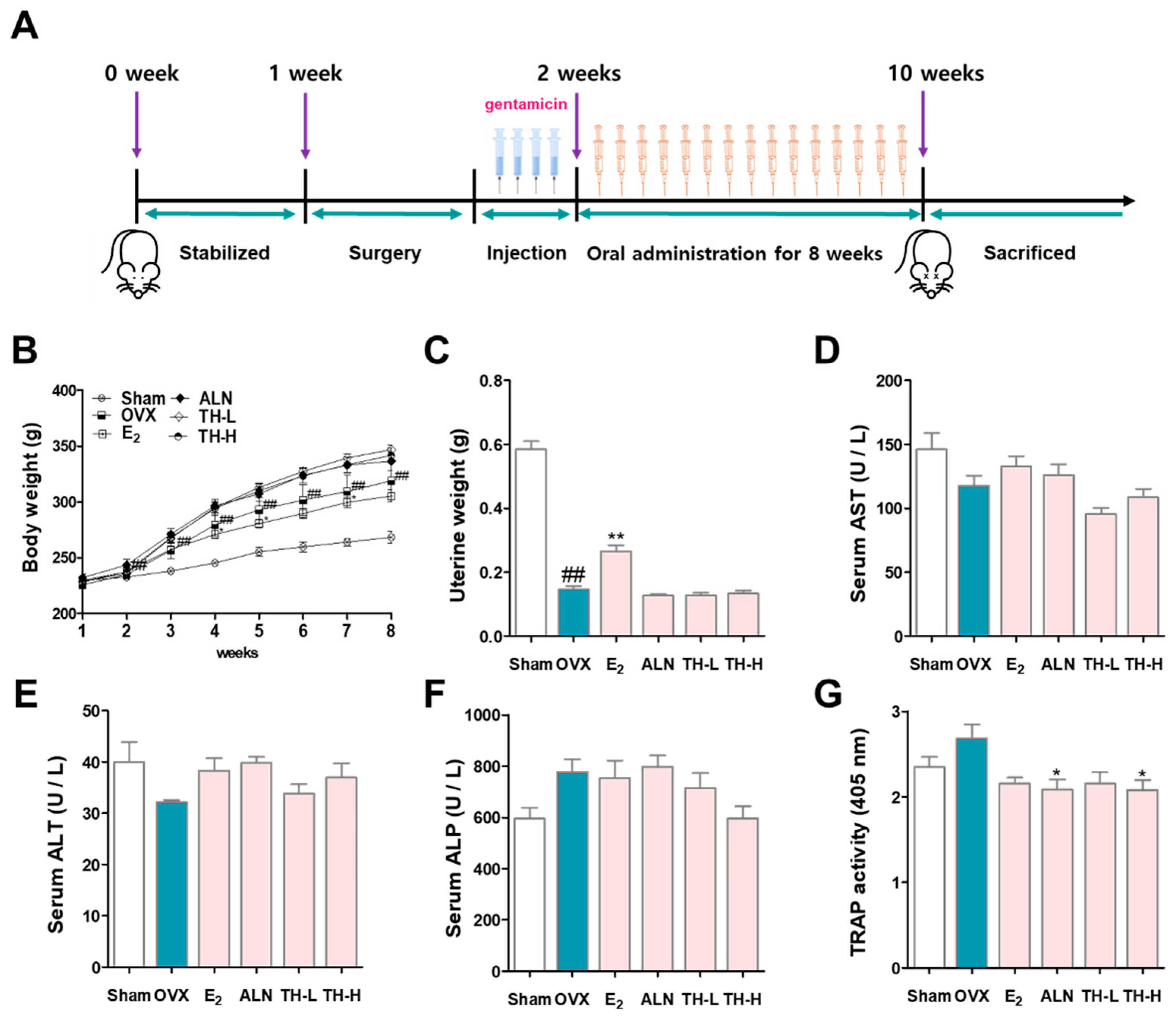
2.9. Experimental Protocols
2.10. Serum Analysis
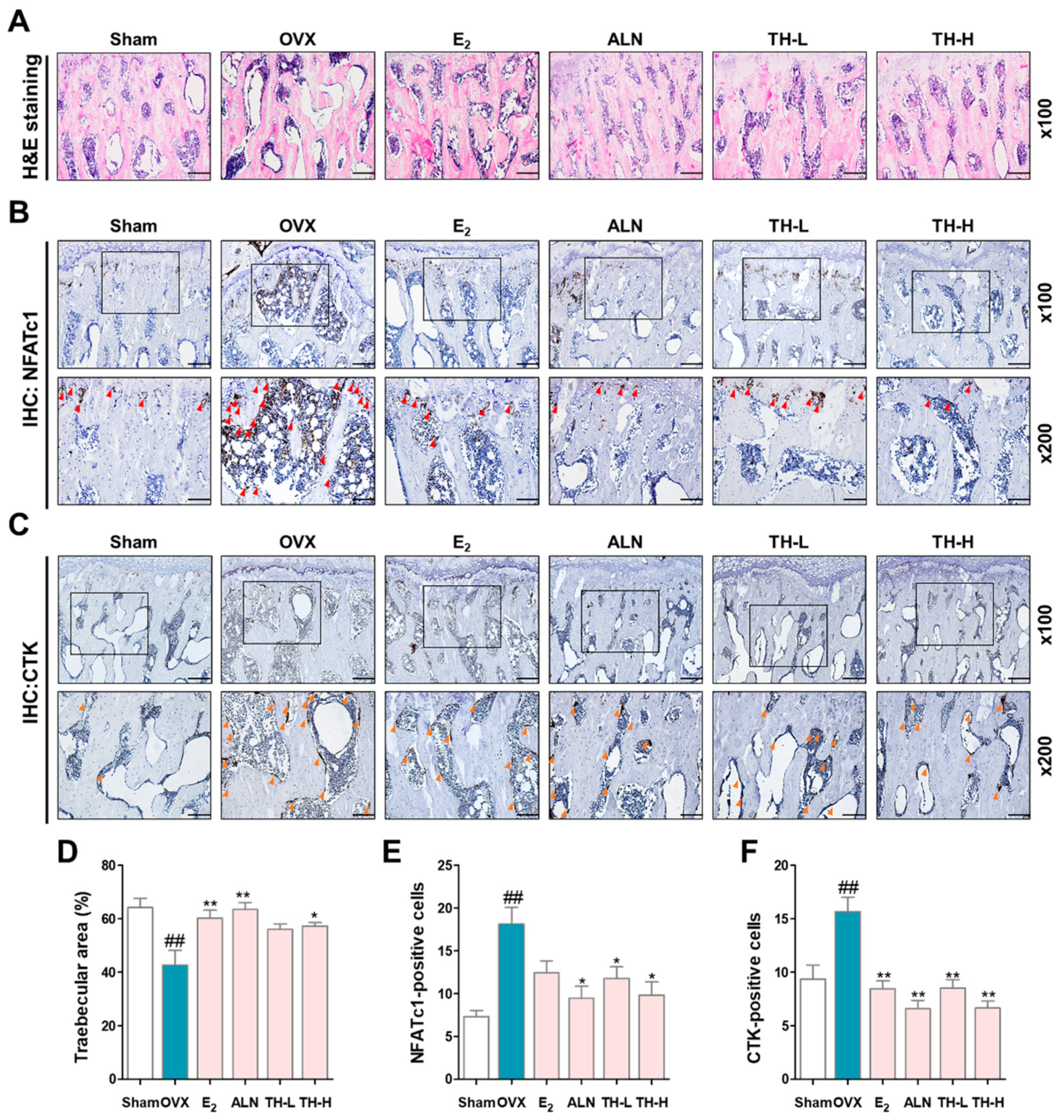
2.11. Micro-Computed Tomography (Micro-CT) Analysis
2.12. Histological Examination and Immunohistochemistry
2.13. Data Statistics and Analysis
3. Results
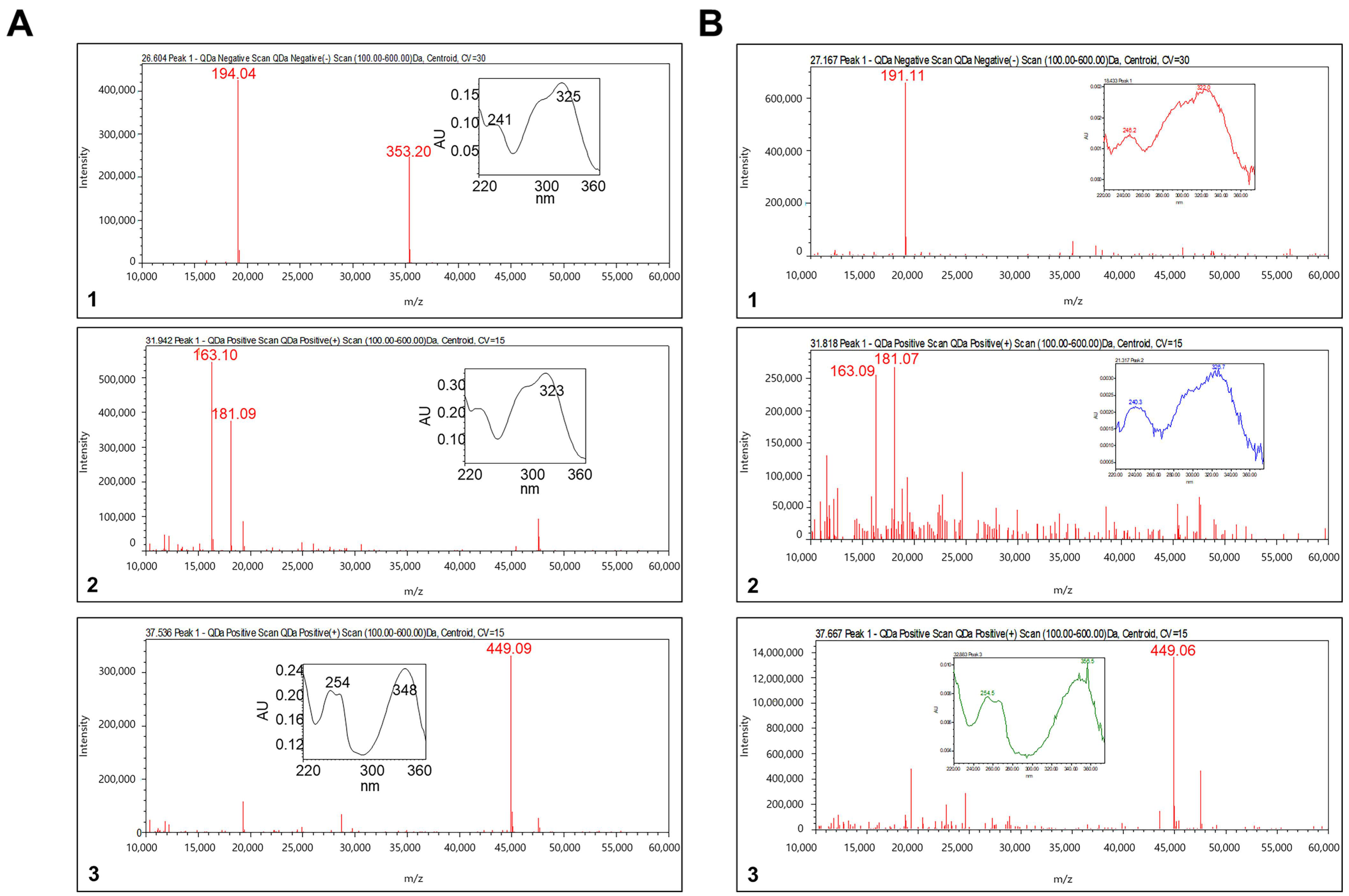
3.1. LC-MS Analysis for TH Extract
3.2. TH Restrained RANKL-Induced Osteoclast Differentiation in RAW 264.7 Cells
3.3. TH Suppresses Osteoclast Function and Bone Resorption
3.4. TH Lower the Expression of Osteoclast Differentiation Transcription Factor
3.5. TH Lowers the Expression of Osteoclast-Related Genes by Inhibiting the Expression of Transcription Factors Such as c-Fos and NFATc1
3.6. Changes in Body Weight and Effects of TH on Serum Levels
3.7. TH Improved Bone Loss in the Postmenopausal Osteoporosis Rat Model
3.8. TH Increased the Trabecular Area and Reduced the Number of NFATc1 and c-Fos in Femur Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoon, H.K.; Lee, Y.K.; Ha, Y.C. Characteristics of Patients Diagnosed with Osteoporosis in South Korea: Results from the National Claim Registry. J. Bone Metab. 2017, 24, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reginster, J.Y.; Burlet, N. Osteoporosis: A still increasing prevalence. Bone 2006, 38, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. WHO Study Group. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, H.; Cheng, Y.; Yu, W.; Luo, G.; Duan, C.; Yao, F.; Xiao, B.; Feng, C.; Xia, X.; et al. Reduction in activating transcription factor 4 promotes carbon tetrachloride and lipopolysaccharide/Dgalactosaminemediated liver injury in mice. Mol. Med. Rep. 2018, 18, 1718–1725. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, E.; Kim, S.; Lee, T.J. Economic Burden of Osteoporotic Fracture of the Elderly in South Korea: A National Survey. Value Health Reg. Issues 2016, 9, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Painter, S.E.; Kleerekoper, M.; Camacho, P.M. Secondary osteoporosis: A review of the recent evidence. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2006, 12, 436–445. [Google Scholar] [CrossRef]
- Ji, M.X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Dobbs, M.B.; Buckwalter, J.; Saltzman, C. Osteoporosis: The increasing role of the orthopaedist. Iowa Orthop. J. 1999, 19, 43–52. [Google Scholar]
- Kim, B.; Cho, Y.J.; Lim, W. Osteoporosis therapies and their mechanisms of action (Review). Exp. Ther. Med. 2021, 22, 1379. [Google Scholar] [CrossRef]
- Chen, L.R.; Ko, N.Y.; Chen, K.H. Medical Treatment for Osteoporosis: From Molecular to Clinical Opinions. Int. J. Mol. Sci. 2019, 20, 2213. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, D.R.; Nedwin, G.E.; Bringman, T.S.; Smith, D.D.; Mundy, G.R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature 1986, 319, 516–518. [Google Scholar] [CrossRef] [PubMed]
- Caplan, L.; Pittman, C.B.; Zeringue, A.L.; Scherrer, J.F.; Wehmeier, K.R.; Cunningham, F.E.; Eisen, S.A.; McDonald, J.R. An observational study of musculoskeletal pain among patients receiving bisphosphonate therapy. Mayo Clin. Proc. 2010, 85, 341–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, D.M.; Eastell, R.; Adams, A.L. Atypical Femur Fracture Risk versus Fragility Fracture Prevention with Bisphosphonates. Reply. New Engl. J. Med. 2020, 383, 2189–2190. [Google Scholar] [CrossRef] [PubMed]
- Thurston, R.C.; Chang, Y.; Barinas-Mitchell, E.; Jennings, J.R.; Landsittel, D.P.; Santoro, N.; von Kanel, R.; Matthews, K.A. Menopausal Hot Flashes and Carotid Intima Media Thickness Among Midlife Women. Stroke 2016, 47, 2910–2915. [Google Scholar] [CrossRef]
- Lewiecki, E.M. Bisphosphonates for the treatment of osteoporosis: Insights for clinicians. Ther. Adv. Chronic Dis. 2010, 1, 115–128. [Google Scholar] [CrossRef]
- Boyce, B.F. Advances in the regulation of osteoclasts and osteoclast functions. J. Dent. Res. 2013, 92, 860–867. [Google Scholar] [CrossRef] [Green Version]
- Collin-Osdoby, P.; Osdoby, P. RANKL-mediated osteoclast formation from murine RAW 264.7 cells. Methods Mol. Biol. 2012, 816, 187–202. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X. Receptor activator of nuclear factor-kappaB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues (review). Mol. Med. Rep. 2015, 11, 3212–3218. [Google Scholar] [CrossRef] [Green Version]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Martinez, M.; Poirrier, P.; Chamy, R.; Prufer, D.; Schulze-Gronover, C.; Jorquera, L.; Ruiz, G. Taraxacum officinale and related species-An ethnopharmacological review and its potential as a commercial medicinal plant. J. Ethnopharmacol. 2015, 169, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Schutz, K.; Carle, R.; Schieber, A. Taraxacum—A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.; Kwon, M.; Park, M.; Oh, J.H.; Sung, K.K.; Lee, B.H. Combined treatment of Taraxaci Herba and R7050 alleviates the symptoms of herpes simplex virus-induced Behcet’s disease in rats. Integr. Med. Res. 2021, 10, 100720. [Google Scholar] [CrossRef]
- Seo, S.W.; Koo, H.N.; An, H.J.; Kwon, K.B.; Lim, B.C.; Seo, E.A.; Ryu, D.G.; Moon, G.; Kim, H.Y.; Kim, H.M.; et al. Taraxacum officinale protects against cholecystokinin-induced acute pancreatitis in rats. World J. Gastroenterol. 2005, 11, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Sun-Waterhouse, D. The potential of dandelion in the fight against gastrointestinal diseases: A review. J. Ethnopharmacol. 2022, 293, 115272. [Google Scholar] [CrossRef] [PubMed]
- Ginaldi, L.; Di Benedetto, M.C.; De Martinis, M. Osteoporosis, inflammation and ageing. Immun. Ageing I A 2005, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Koh, Y.J.; Cha, D.S.; Ko, J.S.; Park, H.J.; Choi, H.D. Anti-inflammatory effect of Taraxacum officinale leaves on lipopolysaccharide-induced inflammatory responses in RAW 264.7 cells. J. Med. Food 2010, 13, 870–878. [Google Scholar] [CrossRef]
- Williams, C.A.; Goldstone, F.; Greenham, J. Flavonoids, cinnamic acids and coumarins from the different tissues and medicinal preparations of Taraxacum officinale. Phytochemistry 1996, 42, 121–127. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, J.; Wang, L.; Li, B.; Guo, J.; Guan, X.; Han, Q.; Zhang, H. Caffeic acid reduces cutaneous tumor necrosis factor alpha (TNF-alpha), IL-6 and IL-1beta levels and ameliorates skin edema in acute and chronic model of cutaneous inflammation in mice. Biol. Pharm. Bull. 2014, 37, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Kim, Y.W.; Park, Y.; Lee, H.J.; Kim, K.W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2014, 63, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Song, Y.S. Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-kappaB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells. Nutr. Res. Pract. 2013, 7, 423–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, G.; Li, F.; Li, X.; Wang, Z.G.; Zhang, B. TNFalpha and RANKL promote osteoclastogenesis by upregulating RANK via the NFkappaB pathway. Mol. Med. Rep. 2018, 17, 6605–6611. [Google Scholar] [CrossRef] [Green Version]
- Weitzmann, M.N. The Role of Inflammatory Cytokines, the RANKL/OPG Axis, and the Immunoskeletal Interface in Physiological Bone Turnover and Osteoporosis. Scientifica 2013, 2013, 125705. [Google Scholar] [CrossRef] [Green Version]
- Ekeuku, S.O.; Pang, K.L.; Chin, K.Y. Effects of Caffeic Acid and Its Derivatives on Bone: A Systematic Review. Drug Des. Dev. Ther. 2021, 15, 259–275. [Google Scholar] [CrossRef]
- Kwak, S.C.; Lee, C.; Kim, J.Y.; Oh, H.M.; So, H.S.; Lee, M.S.; Rho, M.C.; Oh, J. Chlorogenic acid inhibits osteoclast differentiation and bone resorption by down-regulation of receptor activator of nuclear factor kappa-B ligand-induced nuclear factor of activated T cells c1 expression. Biol. Pharm. Bull. 2013, 36, 1779–1786. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Jung, J.W.; Ha, B.G.; Hong, J.M.; Park, E.K.; Kim, H.J.; Kim, S.Y. The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J. Nutr. Biochem. 2011, 22, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Tschop, M.H.; Speakman, J.R.; Arch, J.R.; Auwerx, J.; Bruning, J.C.; Chan, L.; Eckel, R.H.; Farese, R.V., Jr.; Galgani, J.E.; Hambly, C.; et al. A guide to analysis of mouse energy metabolism. Nat. Methods 2011, 9, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.Y.; Kim, J.H.; Kim, M.; Park, J.H.; Sohn, Y.; Jung, H.S. Abeliophyllum distichum Nakai alleviates postmenopausal osteoporosis in ovariectomized rats and prevents RANKL-induced osteoclastogenesis in vitro. J. Ethnopharmacol. 2020, 257, 112828. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Ther. 2007, 9 (Suppl. S1), S1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omi, M.; Mishina, Y. Role of osteoclasts in oral homeostasis and jawbone diseases. Oral Sci. Int. 2020, 18, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Vincent, C.; Kogawa, M.; Findlay, D.M.; Atkins, G.J. The generation of osteoclasts from RAW 264.7 precursors in defined, serum-free conditions. J. Bone Miner. Metab. 2009, 27, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Camassa, J.A.; Diogo, C.C.; Bordelo, J.P.A.; Bonelli, M.A.; Viegas, C.A.; Azevedo, J.T.; Dourado, N.; Dias, I.R. Tartrate-resistant acid phosphate as biomarker of bone turnover over the lifespan and different physiologic stages in sheep. BMC Vet. Res. 2017, 13, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komarova, S.V.; Pereverzev, A.; Shum, J.W.; Sims, S.M.; Dixon, S.J. Convergent signaling by acidosis and receptor activator of NF-kappaB ligand (RANKL) on the calcium/calcineurin/NFAT pathway in osteoclasts. Proc. Natl. Acad. Sci. USA 2005, 102, 2643–2648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vu, T.H.; Shipley, J.M.; Bergers, G.; Berger, J.E.; Helms, J.A.; Hanahan, D.; Shapiro, S.D.; Senior, R.M.; Werb, Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998, 93, 411–422. [Google Scholar] [CrossRef] [Green Version]
- Takito, J.; Inoue, S.; Nakamura, M. The Sealing Zone in Osteoclasts: A Self-Organized Structure on the Bone. Int. J. Mol. Sci. 2018, 19, 984. [Google Scholar] [CrossRef] [Green Version]
- Saftig, P.; Hunziker, E.; Wehmeyer, O.; Jones, S.; Boyde, A.; Rommerskirch, W.; Moritz, J.D.; Schu, P.; von Figura, K. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc. Natl. Acad. Sci. USA 1998, 95, 13453–13458. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Rho, J.; Jeong, D.; Sul, J.Y.; Kim, T.; Kim, N.; Kang, J.S.; Miyamoto, T.; Suda, T.; Lee, S.K.; et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat. Med. 2006, 12, 1403–1409. [Google Scholar] [CrossRef]
- Nedeva, I.R.; Vitale, M.; Elson, A.; Hoyland, J.A.; Bella, J. Role of OSCAR Signaling in Osteoclastogenesis and Bone Disease. Front. Cell Dev. Biol. 2021, 9, 641162. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.S. Animal models of osteoporosis—Necessity and limitations. Eur. Cells Mater. 2001, 1, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Kalu, D.N. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991, 15, 175–191. [Google Scholar] [CrossRef]
- Yousefzadeh, N.; Kashfi, K.; Jeddi, S.; Ghasemi, A. Ovariectomized rat model of osteoporosis: A practical guide. EXCLI J. 2020, 19, 89–107. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, J.; Genant, H.K.; Dequeker, J.; Geusens, P. Long-term changes in bone mineral and biomechanical properties of vertebrae and femur in aging, dietary calcium restricted, and/or estrogen-deprived/-replaced rats. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1997, 12, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Stournaras, E.; Tziomalos, K. Herbal medicine-related hepatotoxicity. World J. Hepatol. 2015, 7, 2189–2193. [Google Scholar] [CrossRef]
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, X.; Karsdal, M.A.; Leeming, D.J.; Genovese, F. Molecular serum markers of liver fibrosis. Biomark. Insights 2012, 7, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Pfingstgraf, I.O.; Taulescu, M.; Pop, R.M.; Orasan, R.; Vlase, L.; Uifalean, A.; Todea, D.; Alexescu, T.; Toma, C.; Parvu, A.E. Protective Effects of Taraxacum officinale L. (Dandelion) Root Extract in Experimental Acute on Chronic Liver Failure. Antioxidants 2021, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.R.; Chen, C.H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Kim, J.H.; Hong, S.; Kwon, B.; Kim, E.Y.; Jung, H.S.; Sohn, Y. Effects of Melandrium firmum Rohrbach on RANKLinduced osteoclast differentiation and OVX rats. Mol. Med. Rep. 2021, 24, 610. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.M.; Sophocleous, A. Quantitative analysis of bone and soft tissue by micro-computed tomography: Applications to ex vivo and in vivo studies. BoneKEy Rep. 2014, 3, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Tang, C.; Serrano-Sosa, M.; Hu, J.; Zhu, J.; Tang, G.; Huang, C.; Huang, M. Bone microarchitectural parameters can detect oxytocin induced changes prior to bone density on mitigating bone deterioration in rabbit osteoporosis model using micro-CT. BMC Musculoskelet. Disord. 2019, 20, 560. [Google Scholar] [CrossRef] [Green Version]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. CJASN 2008, 3 (Suppl. S3), S131–S139. [Google Scholar] [CrossRef] [Green Version]
- Dalle Carbonare, L.; Valenti, M.T.; Bertoldo, F.; Zanatta, M.; Zenari, S.; Realdi, G.; Lo Cascio, V.; Giannini, S. Bone microarchitecture evaluated by histomorphometry. Micron 2005, 36, 609–616. [Google Scholar] [CrossRef]
- Magaki, S.; Hojat, S.A.; Wei, B.; So, A.; Yong, W.H. An Introduction to the Performance of Immunohistochemistry. Methods Mol. Biol. 2019, 1897, 289–298. [Google Scholar] [CrossRef]





| Gene Name | Primer Sequence (5′-3′) | Base Pair | Cycle | Temperature | Accession Number |
|---|---|---|---|---|---|
| Mmp9 (MMP-9) | F: CGA CTT TTG TGG TCT TCC CC | 258 | 30 | 58 | NM_013599.4 |
| R: TGA AGG TTT GGA ATC GAC CC | |||||
| Acp5 (TRAP) | F: ACT TCC CCA GCC CTT ACT ACC G | 381 | 30 | 58 | NM_007388.3 |
| R: TCA GCA CAT AGC CCA CAC CG | |||||
| Ctks (CTK) | F: AGG CGG CTA TAT GAC CAC TG | 403 | 26 | 58 | NM_007802.4 |
| R: CGA CAG CGT CAA ACA AAG GCT TGT A | |||||
| Ca2 (CA2) | F: CTC TCA GGA CAA TGC AGT GCT GA | 411 | 32 | 58 | NM_001357334.1 |
| R: ATC CAG GTC ACA CAT TCC AGC A | |||||
| Atp6v0d2 (ATP6v0d2) | F: ATG GGG CCT TGC AAA AGA AAT CTG | 504 | 30 | 58 | NM_175406.3 |
| R: CGA CAG CGT CAA ACA AAG GCT TGT A | |||||
| Oscar (OSCAR) | F: CTG CTG GTA ACG GAT CAG CTC CCC AGA | 310 | 35 | 53 | NM_001290377.1 |
| R: CCA AGG AGC CAG AAC CTT CGA AAC T | |||||
| Actb (β-actin) | F: TTC TAC AAT GAG CTG CGT GT | 267 | 30 | 58 | NM_008084.3 |
| R: CTC ATA GCT CTT CTC CAG GG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, J.; Kim, M.; Kim, J.-H.; Shin, H.; Lim, S.-E.; Jung, H.-S.; Sohn, Y.; Ku, J. Effect of Taraxaci Herba on Bone Loss in an OVX-Induced Model through the Regulation of Osteoclast Differentiation. Nutrients 2022, 14, 4354. https://doi.org/10.3390/nu14204354
Heo J, Kim M, Kim J-H, Shin H, Lim S-E, Jung H-S, Sohn Y, Ku J. Effect of Taraxaci Herba on Bone Loss in an OVX-Induced Model through the Regulation of Osteoclast Differentiation. Nutrients. 2022; 14(20):4354. https://doi.org/10.3390/nu14204354
Chicago/Turabian StyleHeo, Jun, Minsun Kim, Jae-Hyun Kim, Hwajeong Shin, Seo-Eun Lim, Hyuk-Sang Jung, Youngjoo Sohn, and Jaseung Ku. 2022. "Effect of Taraxaci Herba on Bone Loss in an OVX-Induced Model through the Regulation of Osteoclast Differentiation" Nutrients 14, no. 20: 4354. https://doi.org/10.3390/nu14204354
APA StyleHeo, J., Kim, M., Kim, J.-H., Shin, H., Lim, S.-E., Jung, H.-S., Sohn, Y., & Ku, J. (2022). Effect of Taraxaci Herba on Bone Loss in an OVX-Induced Model through the Regulation of Osteoclast Differentiation. Nutrients, 14(20), 4354. https://doi.org/10.3390/nu14204354







