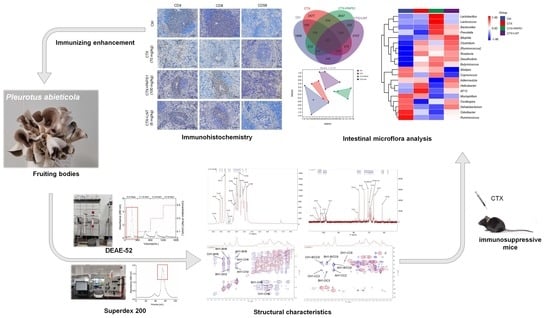
The Structural Characterization and Immunomodulatory Activity of Polysaccharides from Pleurotus abieticola Fruiting Bodies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Purification of Polysaccharides from P. abieticola
2.1.1. Extraction of Polysaccharides
2.1.2. Purification of Polysaccharides
2.2. Composition and Structural Analysis of PAPS1
2.2.1. Composition Analysis of PAPS1
2.2.2. Ultraviolet-Visible (UV-Vis) Analysis of PAPS1
2.2.3. Molecular Weight (Mw) and Homogeneity Analysis of PAPS1
2.2.4. Monosaccharide Composition Analysis of PAPS1
2.2.5. Methylation Analysis of PAPS1
2.2.6. Nuclear Magnetic Resonance (NMR) Analysis of PAPS1
2.3. Animal Immunosuppression Model and Agent Administration
2.4. Histopathological Analysis
2.4.1. Hematoxylin and Eosin (H&E) Staining
2.4.2. Immunohistochemistry Staining
2.5. Assessment of Biochemical Indices
2.6. Intestinal Microflora Analysis
2.7. Statistical Analysis
3. Results
3.1. Purification and Composition Analysis of PAPS1
3.2. Structural Characterization of PAPS1
3.3. Immunoregulatory Effects of PAPS1 in Immunosuppressed Mice
3.4. PAPS1 Regulated Intestinal Microflora in Immunosuppressed Mice
3.5. PAPS1 Regulated Cytokines in Immunosuppressed Mice
3.6. PAPS1 Suppressed Oxidative Stress in Immunosuppressed Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, F.; Zhou, L.-W.; Yang, Z.-L.; Bau, T.; Li, T.-H.; Dai, Y.-C. Resource diversity of Chinese macrofungi: Edible, medicinal and poisonous species. Fungal Divers. 2019, 98, 1–76. [Google Scholar] [CrossRef]
- Motta, F.; Gershwin, M.E.; Selmi, C. Mushrooms and immunity. J. Autoimmun. 2021, 117, 102576. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Q.; Yue, G.L.; Yue, R.Q.; Dong, C.X.; Chan, C.L.; Ko, C.H.; Cheung, W.S.; Luo, K.W.; Dai, H.; Wong, C.K.; et al. Structure elucidation and immunomodulatory activity of a beta glucan from the fruiting bodies of Ganoderma sinense. PLoS ONE 2014, 9, e100380. [Google Scholar] [CrossRef]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Wan, Z.; Zhang, X.; Li, J.; Li, H.; Wang, C. Dietary Chlorella vulgaris Ameliorates Altered Immunomodulatory Functions in Cyclophosphamide-Induced Immunosuppressive Mice. Nutrients 2017, 9, 708. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef] [Green Version]
- Carasi, P.; Racedo, S.M.; Jacquot, C.; Romanin, D.E.; Serradell, M.A.; Urdaci, M.C. Impact of kefir derived Lactobacillus kefiri on the mucosal immune response and gut microbiota. J. Immunol. Res. 2015, 2015, 361604. [Google Scholar] [CrossRef] [Green Version]
- Llorente, C.; Schnabl, B. The gut microbiota and liver disease. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Nejati, M.; Dehghan, P.; Hashempour-Baltork, F.; Alizadeh, A.M.; Farshi, P.; Khosravi-Darani, K. Potential Dietary Interventions for COVID-19 Infection Based on the Gut-Immune Axis: An Update Review on Bioactive Component of Macronutrients. Int. J. Prev. Med. 2021, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zheng, Z.; Guo, T.; Wang, K.; Zhang, Y. Molecular dynamics simulation of lentinan and its interaction with the innate receptor dectin-1. Int. J. Biol. Macromol. 2021, 171, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Cerletti, C.; Esposito, S.; Iacoviello, L. Edible Mushrooms and Beta-Glucans: Impact on Human Health. Nutrients 2021, 13, 2195. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Qi, J.; Ho, C.-T.; Li, B.; Mu, J.; Zhang, Y.; Hu, H.; Mo, W.; Chen, Z.; Xie, Y. Structural characterization and immunomodulatory activity of a water-soluble polysaccharide from Ganoderma leucocontextum fruiting bodies. Carbohydr. Polym. 2020, 249, 116874. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.S.P.; Behera, B.; Mishra, D.; Maiti, T.K. Immune augmentation and Dalton’s Lymphoma tumor inhibition by glucans/glycans isolated from the mycelia and fruit body of Pleurotus ostreatus. Int. Immunopharmacol. 2015, 25, 207–217. [Google Scholar] [CrossRef]
- Ellefsen, C.F.; Wold, C.W.; Wilkins, A.L.; Rise, F.; Samuelsen, A.B.C. Water-soluble polysaccharides from Pleurotus eryngii fruiting bodies, their activity and affinity for Toll-like receptor 2 and dectin-1. Carbohydr. Polym. 2021, 264, 117991. [Google Scholar] [CrossRef]
- Zhi-Hui, L.; Xin, N.; Sheng-Long, W.; Hai-Ping, Y.; Bo, Z.; Yu, L. Biological characteristics and cultivation conditions of Pleurotus abieticola from Qilian Mountains, northwestern China. Mycosystema 2020, 39, 1741–1749. [Google Scholar] [CrossRef]
- Xiao-Bin, L.; Jian-Wei, L.; Zhu-Liang, Y. A new edible mushroom resource, Pleurotus abieticola, in southwestern China. Mycosystema 2015, 34, 581–588. [Google Scholar] [CrossRef]
- Guo, X.; Sun, L.; Li, C.; Fu, Y.; Song, B.; Li, Y. The yield and quality of Pleurotus abieticola grown on nematode-infected Pinus massoniana chips. RSC Adv. 2021, 11, 883–890. [Google Scholar] [CrossRef]
- Hu, W.; Song, M.; Wang, C.; Guo, Z.; Li, Y.; Wang, D. Structural characterization of polysaccharide purified from Hericium erinaceus fermented mycelium and its pharmacological basis for application in Alzheimer’s disease: Oxidative stress related calcium homeostasis. Int. J. Biol. Macromol. 2021, 193, 358–369. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhäusser, S.M. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, J.; Wang, D.; Zhang, N.; Lu, J.; Meng, Q.; Zhou, Y.; Wang, N.; Liu, Y.; Wang, D.; et al. The anti-membranous glomerulonephritic activity of purified polysaccharides from Irpex lacteus Fr. Int. J. Biol. Macromol. 2016, 84, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hao, J.; Liu, X.; Li, C.; Yuan, X.; Lee, R.J.; Bai, T.; Wang, D. Isoforsythiaside Attenuates Alzheimer’s Disease via Regulating Mitochondrial Function Through the PI3K/AKT Pathway. Int. J. Mol. Sci. 2020, 21, 5687. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cai, X.; Wang, R.; Zhai, S.; Zhang, Y.; Hu, W.; Zhang, Y.; Wang, D. Neuroprotective effects of verbascoside against Alzheimer’s disease via the relief of endoplasmic reticulum stress in Aβ-exposed U251 cells and APP/PS1 mice. J. Neuroinflamm. 2020, 17, 309. [Google Scholar] [CrossRef]
- Jiang, X.; Hao, J.; Liu, Z.; Ma, X.; Feng, Y.; Teng, L.; Li, Y.; Wang, D. Anti-obesity effects of Grifola frondosa through the modulation of lipid metabolism via ceramide in mice fed a high-fat diet. Food Funct. 2021, 12, 6725–6739. [Google Scholar] [CrossRef]
- Cheng, Y.; Xie, Y.; Ge, J.-C.; Wang, L.; Peng, D.-Y.; Yu, N.-J.; Zhang, Y.; Jiang, Y.-H.; Luo, J.-P.; Chen, W.-D. Structural characterization and hepatoprotective activity of a galactoglucan from Poria cocos. Carbohydr. Polym. 2021, 263, 117979. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Sarker, M.M.R.; Yan, X.; Yang, C.; Zhao, L.; Lv, X.; Liu, B.; Zhao, C. Structural characterization and antidiabetic potential of a novel heteropolysaccharide from Grifola frondosa via IRS1/PI3K-JNK signaling pathways. Carbohydr. Polym. 2018, 198, 452–461. [Google Scholar] [CrossRef]
- Shi, H.; Bi, S.; Li, H.; Li, J.; Li, C.; Yu, R.; Song, L.; Zhu, J. Purification and characterization of a novel mixed-linkage α,β-d-glucan from Arca subcrenata and its immunoregulatory activity. Int. J. Biol. Macromol. 2021, 182, 207–216. [Google Scholar] [CrossRef]
- Xue, W.; Gao, Y.; Li, Q.; Lu, Q.; Bian, Z.; Tang, L.; Zeng, Y.; Chen, C.; Guo, W. Immunomodulatory activity-guided isolation and characterization of a novel polysaccharide from Atractylodis macrocephalae Koidz. Int. J. Biol. Macromol. 2020, 161, 514–524. [Google Scholar] [CrossRef]
- Borchani, C.; Fonteyn, F.; Jamin, G.; Destain, J.; Willems, L.; Paquot, M.; Blecker, C.; Thonart, P. Structural Characterization, Technological Functionality, and Physiological Aspects of Fungal β-D-glucans: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1746–1752. [Google Scholar] [CrossRef]
- Bao, L.; Hao, C.; Wang, J.; Wang, D.; Zhao, Y.; Li, Y.; Yao, W. High-Dose Cyclophosphamide Administration Orchestrates Phenotypic and Functional Alterations of Immature Dendritic Cells and Regulates Th Cell Polarization. Front. Pharmacol. 2020, 11, 775. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, W.; He, Y.; Sun, M.; Yu, J.; Jiao, X.; Han, Q.; Tang, H.; Zhang, B.; Xian, Y.; et al. HLA-A2.1-restricted ECM1-derived epitope LA through DC cross-activation priming CD8(+) T and NK cells: A novel therapeutic tumour vaccine. J. Hematol. Oncol. 2021, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qu, Y.; Wang, Y.; Wang, X.; Xu, J.; Zhao, H.; Zheng, D.; Sun, L.; Tai, G.; Zhou, Y.; et al. β-1,6-Glucan From Pleurotus eryngii Modulates the Immunity and Gut Microbiota. Front. Immunol. 2022, 13, 859923. [Google Scholar] [CrossRef] [PubMed]
- Keyt, B.A.; Baliga, R.; Sinclair, A.M.; Carroll, S.F.; Peterson, M.S. Structure, Function, and Therapeutic Use of IgM Antibodies. Antibodies 2020, 9, 53. [Google Scholar] [CrossRef]
- Breedveld, A.; van Egmond, M. IgA and FcαRI: Pathological Roles and Therapeutic Opportunities. Front. Immunol. 2019, 10, 553. [Google Scholar] [CrossRef]
- Habijanic, J.; Berovic, M.; Boh, B.; Plankl, M.; Wraber, B. Submerged cultivation of Ganoderma lucidum and the effects of its polysaccharides on the production of human cytokines TNF-α, IL-12, IFN-γ, IL-2, IL-4, IL-10 and IL-17. New Biotechnol. 2015, 32, 85–95. [Google Scholar] [CrossRef]
- Lusty, E.; Poznanski, S.M.; Kwofie, K.; Mandur, T.S.; Lee, D.A.; Richards, C.D.; Ashkar, A.A. IL-18/IL-15/IL-12 synergy induces elevated and prolonged IFN-γ production by ex vivo expanded NK cells which is not due to enhanced STAT4 activation. Mol. Immunol. 2017, 88, 138–147. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Alvarez, C.A.; Cobb, B.A. Integration of IL-2 and IL-4 signals coordinates divergent regulatory T cell responses and drives therapeutic efficacy. Elife 2021, 10, e57417. [Google Scholar] [CrossRef]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2019, 195, 74–85. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Fang, Q.; Dong, N.; Fang, Q.; Cui, S.W.; Nie, S. A polysaccharide from natural Cordyceps sinensis regulates the intestinal immunity and gut microbiota in mice with cyclophosphamide-induced intestinal injury. Food Funct. 2021, 12, 6271–6282. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, M.; Jin, H.; Yang, J.; Kang, S.; Liu, Y.; Yang, S.; Ma, S.; Ni, J. Effects of Lycium barbarum Polysaccharides on Immunity and the Gut Microbiota in Cyclophosphamide-Induced Immunosuppressed Mice. Front. Microbiol. 2021, 12, 701566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Arnold, I.C.; Müller, A. Mechanisms of persistence, innate immune activation and immunomodulation by the gastric pathogen Helicobacter pylori. Curr. Opin. Microbiol. 2020, 54, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rizzuti, D.; Ang, M.; Sokollik, C.; Wu, T.; Abdullah, M.; Greenfield, L.; Fattouh, R.; Reardon, C.; Tang, M.; Diao, J.; et al. Helicobacter pylori Inhibits Dendritic Cell Maturation via Interleukin-10-Mediated Activation of the Signal Transducer and Activator of Transcription 3 Pathway. J. Innate Immun. 2015, 7, 199–211. [Google Scholar] [CrossRef] [Green Version]
- Hao, Z.; Wang, X.; Yang, H.; Tu, T.; Zhang, J.; Luo, H.; Huang, H.; Su, X. PUL-Mediated Plant Cell Wall Polysaccharide Utilization in the Gut Bacteroidetes. Int. J. Mol. Sci. 2021, 22, 3077. [Google Scholar] [CrossRef]
- Yang, C.; Mogno, I.; Contijoch, E.J.; Borgerding, J.N.; Aggarwala, V.; Li, Z.; Siu, S.; Grasset, E.K.; Helmus, D.S.; Dubinsky, M.C.; et al. Fecal IgA Levels Are Determined by Strain-Level Differences in Bacteroides ovatus and Are Modifiable by Gut Microbiota Manipulation. Cell Host Microbe 2020, 27, 467–475.e6. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-H.; Sun, Y.; Wang, M.; Zhang, S.-J.; Gao, Y.-S.; Chen, L.; Wu, M.-Y.; Zhang, X.-L.; Zhou, L.; Yuan, H.-M.; et al. Effects of dachaihu decoction and its “prescription elements” on intestinal flora of nonalcoholic fatty liver disease model rats. World J. Tradit. Chin. Med. 2020, 6, 97–105. [Google Scholar] [CrossRef]
- Hu, C.; Yan, Y.; Ji, F.; Zhou, H. Maternal Obesity Increases Oxidative Stress in Placenta and It Is Associated With Intestinal Microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 671347. [Google Scholar] [CrossRef]
- Ko, S.H.; Jeon, J.I.; Woo, H.A.; Kim, J.M. Bacteroides fragilis enterotoxin upregulates heme oxygenase-1 in dendritic cells via reactive oxygen species-, mitogen-activated protein kinase-, and Nrf2-dependent pathway. World J. Gastroenterol. 2020, 26, 291–306. [Google Scholar] [CrossRef]
- Mimura, K.; Kua, L.-F.; Shimasaki, N.; Shiraishi, K.; Nakajima, S.; Siang, L.K.; Shabbir, A.; So, J.; Yong, W.-P.; Kono, K. Upregulation of thioredoxin-1 in activated human NK cells confers increased tolerance to oxidative stress. Cancer Immunol. Immunother. 2017, 66, 605–613. [Google Scholar] [CrossRef]







| Retention Time (min) | Linkage Pattern | Methylated Sugar | Characteristic Ions (m/z) | Relative Mole Percentage * (%) |
|---|---|---|---|---|
| 6.773 | t-Fuc(p) | 1,5-di-O-acetyl-6-deoxy-2,3,4-tri-O-methyl fucitol | 59, 72, 89, 102, 115, 118, 131, 162, 175 | 1.81 |
| 8.624 | t-Man(p) | 1,5-di-O-acetyl-2,3,4,6-tetra-O-methyl mannitol | 59, 71, 87, 102, 113, 118, 129, 145, 161, 162, 205, 246 | 32.19 |
| 9.683 | t-Gal(p) | 1,5-di-O-acetyl-2,3,4,6-tetra-O-methyl galactitol | 59, 71, 87, 102, 113, 118, 129, 145, 161, 162, 205 | 1.67 |
| 11.908 | 3-Glc(p) | 1,3,5-tri-O-acetyl-2,4,6-tri-O-methyl glucitol | 59, 71, 74, 87, 101, 118, 129, 143, 161, 174, 203, 217, 234, 277 | 2.82 |
| 15.228 | 6-Gal(p) | 1,5,6-tri-O-acetyl-2,3,4-tri-O-methyl galactitol | 59, 71, 87, 99, 102, 118, 129, 143, 159, 162, 189, 204, 233 | 31.59 |
| 19.398 | 2,6-Gal(p) | 1,2,5,6-tetra-O-acetyl-3,4-di-O-methyl galactitol | 60, 74, 87,100, 114, 130, 143, 160, 174, 190, 204, 234 | 29.93 |
| Residue | Glycosyl Residues | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6a /C6 | H6b | Reference |
|---|---|---|---|---|---|---|---|---|---|
| A | β-D-Manp-(1→ | 4.72 | 4.08 | 3.57 | 3.75 | 3.48 | 3.85 | 3.66 | [16] |
| 101.69 | 72.93 | 76.25 | 70.43 | 75.67 | 61.07 | ||||
| B | →6)-α-D-Galp-(1→ | 4.90 | 3.78 | 4.01 | 4.08 | 4.21 | 3.85 | 3.50 | [26] |
| 98.30 | 69.19 | 69.50 | 69.63 | 68.54 | 66.82 | ||||
| C | →2,6)-α-D-Galp-(1→ | 5.04 | 3.82 | 3.91 | 4.00 | 4.10 | 3.85 | 3.58 | [27] |
| 98.15 | 76.97 | 69.20 | 70.43 | 68.81 | 66.78 | ||||
| D | → 3)-β-D-Glcp-(1→ | 4.47 | 3.64 | 4.25 | 3.51 | 3.48 | 3.29 | 3.22 | [28,29] |
| 102.08 | 73.17 | 78.80 | 70.50 | 75.65 | 60.26 |
| Group | Taxa | Abundance (log10) | LDA Score | p |
|---|---|---|---|---|
| Ctrl | Bacteria.Firmicutes.Erysipelotrichi.Erysipelotrichales | 4.185 | 3.362 | 0.022 |
| Bacteria.Firmicutes.Clostridia.Clostridiales.Ruminococcaceae.Ruminococcus | 4.185 | 3.697 | 0.019 | |
| Bacteria.Firmicutes.Bacilli.Lactobacillales.Streptococcaceae.Streptococcus | 4.158 | 2.534 | 0.032 | |
| Bacteria.Firmicutes.Erysipelotrichi.Erysipelotrichales.Erysipelotrichaceae | 3.714 | 3.379 | 0.022 | |
| Bacteria.Bacteroidetes.Bacteroidia.Bacteroidales._Odoribacteraceae_ | 3.714 | 3.653 | 0.02 | |
| Bacteria.Proteobacteria.Gammaproteobacteria.Pseudomonadales.Moraxellaceae.Perlucidibaca | 3.714 | 2.41 | 0.017 | |
| Bacteria.Firmicutes.Erysipelotrichi | 2.91 | 3.365 | 0.022 | |
| Bacteria.Bacteroidetes.Bacteroidia.Bacteroidales._Odoribacteraceae_.Odoribacter | 1.952 | 3.623 | 0.02 | |
| Model | Bacteria.Proteobacteria.Epsilonproteobacteria | 4.19 | 3.835 | 0.029 |
| Bacteria.Proteobacteria.Epsilonproteobacteria.Campylobacterales.Helicobacteraceae.Helicobacter | 4.19 | 3.838 | 0.029 | |
| Bacteria.Proteobacteria.Epsilonproteobacteria.Campylobacterales.Helicobacteraceae | 4.19 | 3.787 | 0.029 | |
| Bacteria.Chloroflexi.Anaerolineae.SBR1031 | 4.19 | 2.738 | 0.009 | |
| Bacteria.Chloroflexi.Anaerolineae.SBR1031.A4b | 2.078 | 3.3 | 0.017 | |
| Bacteria.Proteobacteria.Epsilonproteobacteria.Campylobacterales | 2.03 | 3.836 | 0.029 | |
| Bacteria.Chloroflexi.Anaerolineae.SBR1031.SHA_31 | 1.911 | 2.44 | 0.007 | |
| Bacteria.Chloroflexi.Anaerolineae | 1.409 | 2.538 | 0.03 | |
| PAPS1 | Bacteria.Bacteroidetes.Bacteroidia.Bacteroidales._Paraprevotellaceae_.Paraprevotella | 2.711 | 2.461 | 0.045 |
| Bacteria.Bacteroidetes.Bacteroidia.Bacteroidales._Paraprevotellaceae_ | 2.711 | 2.44 | 0.039 | |
| Bacteria.Proteobacteria.Gammaproteobacteria.Alteromonadales.Idiomarinaceae | 1.72 | 2.774 | 0.017 | |
| LEP | Bacteria.Firmicutes.Clostridia.Clostridiales.Lachnospiraceae.Clostridium | 3.201 | 2.704 | 0.014 |
| Bacteria.Firmicutes.Clostridia.Clostridiales.Ruminococcaceae.Ruminococcus.Ruminococcus_albus | 1.545 | 2.95 | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, M.; Kong, F.; Xing, L.; Yao, L.; Li, Y.; Liu, Y.; Li, C.; Li, L. The Structural Characterization and Immunomodulatory Activity of Polysaccharides from Pleurotus abieticola Fruiting Bodies. Nutrients 2022, 14, 4410. https://doi.org/10.3390/nu14204410
Pan M, Kong F, Xing L, Yao L, Li Y, Liu Y, Li C, Li L. The Structural Characterization and Immunomodulatory Activity of Polysaccharides from Pleurotus abieticola Fruiting Bodies. Nutrients. 2022; 14(20):4410. https://doi.org/10.3390/nu14204410
Chicago/Turabian StylePan, Meichen, Fange Kong, Lei Xing, Lan Yao, Yu Li, Yang Liu, Changtian Li, and Lanzhou Li. 2022. "The Structural Characterization and Immunomodulatory Activity of Polysaccharides from Pleurotus abieticola Fruiting Bodies" Nutrients 14, no. 20: 4410. https://doi.org/10.3390/nu14204410