Nutritional Programming: History, Hypotheses, and the Role of Prenatal Factors in the Prevention of Metabolic Diseases—A Narrative Review
Abstract
:1. Introduction
2. Methods
3. Nutritional Programming
3.1. History
3.2. Hypotheses
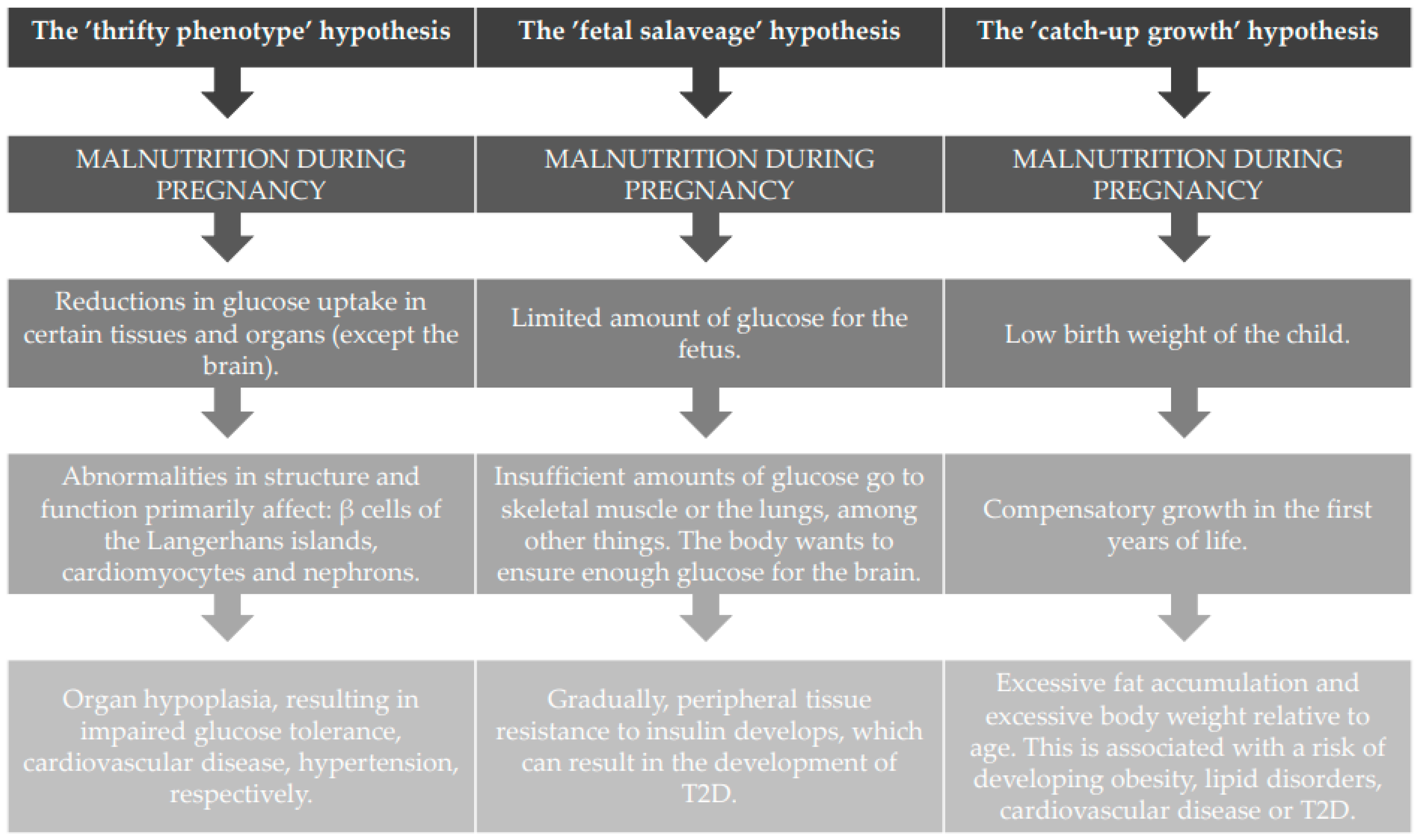
3.2.1. The Thrifty Phenotype Hypothesis
3.2.2. The ‘Fetal Salvage’ Hypothesis
3.2.3. The ‘Catch-Up Growth’ Hypothesis
3.3. Preconceptive and Prenatal Factors in Nutritional Programming
3.3.1. Epigenetics/DNA Methylation and Post-Translational Modification of Histones
3.3.2. Early Nutrition and Maternal Obesity
Animal Studies
Human Studies
3.3.3. Gestational Diabetes Mellitus
3.3.4. Gut Microbiota
4. Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pincock, S. David Barker. Lancet 2013, 382, 1170. [Google Scholar] [CrossRef]
- Selevan, S.G.; Kimmel, C.A.; Mendola, P. Identifying critical windows of exposure for children’s health. Environ. Health Perspect. 2000, 108 (Suppl. 3), 451–455. [Google Scholar] [CrossRef] [PubMed]
- Barr, M., Jr.; DeSesso, J.M.; Lau, C.S.; Osmond, C.; Ozanne, S.E.; Sadler, T.W.; Simmons, R.A.; Sonawane, B.R. Workshop to identify critical windows of exposure for children’s health: Cardiovascular and endocrine work group summary. Environ. Health Perspect. 2000, 108 (Suppl. 3), 569–571. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The Fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef] [Green Version]
- Barker, D.J. Fetal origins of coronary heart disease. BMJ 1995, 311, 171–174. [Google Scholar] [CrossRef]
- Meek, J.Y.; Noble, L. Section on Breastfeeding. Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics 2022, 150, e2022057988. [Google Scholar] [CrossRef]
- Marousez, L.; Lesage, J.; Eberlé, D. Epigenetics: Linking Early Postnatal Nutrition to Obesity Programming? Nutrients 2019, 11, 2966. [Google Scholar] [CrossRef] [Green Version]
- Guilloteau, P.; Zabielski, R.; Hammon, H.M.; Metges, C.C. Adverse effects of nutritional programming during prenatal and early postnatal life, some aspects of regulation and potential prevention and treatments. J. Physiol. Pharmacol. 2009, 60 (Suppl. 3), 17–35. [Google Scholar]
- Morandi, A.; Tommasi, M.; Soffiati, F.; Destro, F.; Fontana, L.; Grando, F.; Simonetti, G.; Bucolo, C.; Alberti, E.; Baraldi, L.; et al. Prevention of obesity in toddlers (PROBIT): A randomised clinical trial of responsive feeding promotion from birth to 24 months. Int. J. Obes. 2019, 43, 1961–1966. [Google Scholar] [CrossRef]
- Singhal, A.; Farooqi, I.S.; O’Rahilly, S.; Cole, T.J.; Fewtrell, M.; Lucas, A. Early nutrition and leptin concentrations in later life. Am. J. Clin. Nutr. 2002, 75, 993–999. [Google Scholar] [CrossRef] [Green Version]
- de Zegher, F.; Sebastiani, G.; Diaz, M.; Gómez-Roig, M.D.; López-Bermejo, A.; Ibáñez, L. Breast-feeding vs Formula-feeding for Infants Born Small-for-Gestational-Age: Divergent Effects on Fat Mass and on Circulating IGF-I and High-Molecular-Weight Adiponectin in Late Infancy. J. Clin. Endocrinol. Metab. 2013, 98, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Bartok, C.; Ventura, K.A. Mechanisms underlying the association between breastfeeding and obesity. Int. J. Pediatr. Obes. 2009, 4, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.; Lee, M. Breastfeeding during the first year promotes satiety responsiveness in children aged 18–24 months. International Association for the Study of Obesity. Pediatr. Obes. 2012, 7, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, L.; Zhu, Y.; Huang, G.; Wang, P.P. The association between breastfeeding and childhood obesity: A meta-analysis. BMC Public Heal. 2014, 14, 1267–1278. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Qiao, Y.; Zhao, P.; Li, W.; Katzmarzyk, P.T.; Chaput, J.; Fogelholm, M.; Kuriyan, R.; Lambert, E.; Maher, C.; et al. Breastfeeding and childhood obesity: A 12-country study. Matern. Child Nutr. 2020, 16, e12984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, J.; Dai, L.-J.; Zhang, Q.; Ouyang, Y.-Q. A Meta-Analysis of the Association Between Breastfeeding and Early Childhood Obesity. J. Pediatr. Nurs. 2020, 53, 57–66. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez; Visiedo-García, F.M.; Domínguez-Riscart, J.; González-Domínguez, R.; Mateos, R.M.; Lechuga-Sancho, A.M. Iron Metabolism in Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 5529. [Google Scholar] [CrossRef]
- Lind, M.V.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F. Dietary protein intake and quality in early life. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 71–76. [Google Scholar] [CrossRef]
- Arnesen, E.K.; Thorisdottir, B.; Lamberg-Allardt, C.; Bärebring, L.; Nwaru, B.; Dierkes, J.; Ramel, A.; Åkesson, A. Protein intake in children and growth and risk of overweight or obesity: A systematic review and meta-analysis. Food Nutr. Res. 2022, 66. [Google Scholar] [CrossRef]
- Rooij, W.H.; Yonker, J.E.; Painter, R.C.; Roseboom, T.J. Prenatal undernutrition and cognitive function in late adulthood. Proc. Natl. Acad. Sci. USA 2010, 107, 16881–16886. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, P.; Morriseau, T.S.; Kereliuk, S.M.; Doucette, C.A.; Wicklow, B.A.; Dolinsky, V.W. Maternal obesity, diabetes during pregnancy and epigenetic mechanisms that influence the developmental origins of cardiometabolic disease in the offspring. Crit. Rev. Clin. Lab. Sci. 2018, 55, 71–101. [Google Scholar] [CrossRef] [PubMed]
- Isganaitis, E.; Venditti, S.; Matthews, T.J.; Lerin, C.; Demerath, E.W.; A Fields, D. Maternal obesity and the human milk metabolome: Associations with infant body composition and postnatal weight gain. Am. J. Clin. Nutr. 2019, 110, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.W.; Longmate, J.A.; Frentzen, B. Excessive maternal weight and pregnancy outcome. Am. J. Obstet. Gynecol. 1992, 167, 353–372; discussion 370–372. [Google Scholar] [CrossRef]
- Jensen, D.M.; Damm, P.; Sørensen, B.; Mølsted-Pedersen, L.; Westergaard, J.G.; Ovesen, P.; Beck-Nielsen, H. Pregnancy outcome and prepregnancy body mass index in 2459 glucose-tolerant Danish women. Am. J. Obstet. Gynecol. 2003, 189, 239–244. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.M.; Louise, J.; Deussen, A.; Grivell, R.; Dodd, J.M. The effect of maternal obesity on fetal biometry, body composition, and growth velocity. J. Matern. Neonatal Med. 2018, 33, 2216–2226. [Google Scholar] [CrossRef]
- Dalrymple, K.V.; Tydeman, F.A.S.; Taylor, P.D.; Flynn, A.C.; O’Keeffe, M.; Briley, A.L.; Santosh, P.; Hayes, L.; Robson, S.C.; Nelson, S.M.; et al. Adiposity and cardiovascular outcomes in three-year-old children of participants in UPBEAT, an RCT of a complex intervention in pregnant women with obesity. Pediatr. Obes. 2020, 16, e12725. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.C.; Mahmood, T. Obesity in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 309–319. [Google Scholar] [CrossRef]
- Dai, R.X.; He, X.J.; Hu, C.L. Maternal pre-pregnancy obesity and the risk of macrosomia: A meta-analysis. Arch. Gynecol. Obstet. 2018, 297, 139–145. [Google Scholar] [CrossRef]
- Aceti, A.; Santhakumaran, S.; Logan, K.M.; Philipps, L.H.; Prior, E.; Gale, C.; Hyde, M.J.; Modi, N. The diabetic pregnancy and offspring blood pressure in childhood: A systematic review and meta-analysis. Diabetologia 2012, 55, 3114–3127. [Google Scholar] [CrossRef] [Green Version]
- Kashtanova, D.A.; Popenko, A.S.; Tkacheva, O.N.; Tyakht, A.; Alexeev, D.G.; Boytsov, S. Association between the gut microbiota and diet: Fetal life, early childhood, and further life. Nutrition 2016, 32, 620–627. [Google Scholar] [CrossRef]
- Kim, H.; Sitarik, A.R.; Woodcroft, K.; Johnson, C.C.; Zoratti, E. Birthmode, breastfeeding, petexposure, andantibioticuse: Associations with the gut microbiome and sensitization in children. Curr. Allergy Asthma Rep. 2019, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.Y.; Bloomfield, F.H.; O’Sullivan, J.M. Factors Affecting Gastrointestinal Microbiome Development in Neonates. Nutrients 2018, 10, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raspini, B.; Porri, D.; De Giuseppe, R.; Chieppa, M.; Liso, M.; Cerbo, R.M.; Civardi, E.; Garofoli, F.; Monti, M.C.; Vacca, M.; et al. Prenatal and postnatal determinants in shaping offspring’s microbiome in the first 1000 days: Study protocol and preliminary results at one month of life. Ital. J. Pediatr. 2020, 46, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blencowe, H.; Krasevec, J.; de Onis, M.; E Black, R.; An, X.; A Stevens, G.; Borghi, E.; Hayashi, C.; Estevez, D.; Cegolon, L.; et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: A systematic analysis. Lancet Glob. Health. 2019, 7, e849–e860. [Google Scholar] [CrossRef] [Green Version]
- Girard, A.W.; Olude, O. Nutrition education and counselling provided during pregnancy: Effects on maternal, neonatal and child health outcomes. Paediatr. Perinat. Epidemiol. 2012, 26 (Suppl. 1), 191–204. [Google Scholar] [CrossRef]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.J.M.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Caulfield, L.E.; Bennett, W.L.; Gross, S.M.; Hurley, K.M.; Ogunwole, S.M.; Venkataramani, M.; Lerman, J.L.; Zhang, A.; Sharma, R.; Bass, E.B. Maternal and Child Outcomes Associated with the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC); 22-EHC019; Agency for Healthcare Research and Quality: Rockville, MD, USA, April 2022. [Google Scholar]
- Godfrey, K.M.; NiPPeR Study Group; Cutfield, W.; Chan, S.-Y.; Baker, P.N.; Chong, Y.-S. Nutritional Intervention Preconception and During Pregnancy to Maintain Healthy Glucose Metabolism and Offspring Health (“NiPPeR”): Study protocol for a randomised controlled trial. Trials 2017, 18, 131. [Google Scholar] [CrossRef] [Green Version]
- Mellanby, E. Nutrition and child-bearing. Lancet 1933, 2, 1131–1137. [Google Scholar] [CrossRef]
- Williams, D.R.; Roberts, S.J.; Davies, T.W. Deaths from ischaemic heart disease and infant mortality in England and Wales. J. Epidemiol. Community Health 1979, 33, 199–202. [Google Scholar] [CrossRef] [Green Version]
- Barker, D.J.; Osmond, C. Infant mortality, childhood nutrition and ischaemic heart disease in England and Wales. Lancet 1986, 327, 1077–1081. [Google Scholar] [CrossRef]
- Marmot, M.G.; Shipley, M.J.; Rose, G. Inequalities in death—Specific explanations of general pattern? Lancet 1984, 323, 1003–1006. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C.; Winter, P.D.; Margetts, B.; Simmonds, S.J. Weight in infacy and death from ischaemic heart disease. Lancet 1989, 334, 577–580. [Google Scholar] [CrossRef]
- Bygren, L.O.; Tinghög, P.; Carstensen, J.; Edvinsson, S.; Kaati, G.; E Pembrey, M.; Sjöström, M. Change in paternal grandmothers’ early food supply influenced cardiovascular mortality of the female grandchildren. BMC Genet. 2014, 15, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaati, G.; Bygren, L.O.; Edvinsson, S. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur. J. Hum. Genet. 2002, 10, 682–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.L. Hunger in the U.S. Sei. Am. 1987, 256, 37–42. [Google Scholar] [CrossRef]
- Campbell, C.C. Food insecurity: A nutritional outcome or a predictor variable? J. Nutr. 1991, 121, 408–415. [Google Scholar] [CrossRef]
- Smil, V. China’s great famine: 40 years later. BMJ 1999, 319, 1619–1621. [Google Scholar] [CrossRef] [Green Version]
- Bruno, R.M.; Faconti, L.; Taddei, S.; Ghiadoni, L. Birth weight and arterial hypertension. Curr. Opin. Cardiol. 2015, 30, 398–402. [Google Scholar] [CrossRef]
- Vågerö, D.; Pinger, P.R.; Aronsson, V.; van den Berg, G.J. Paternal grandfather’s access to food predicts all-cause and cancer mortality in grandsons. Nat. Commun. 2018, 9, 5124. [Google Scholar] [CrossRef] [Green Version]
- Pembrey, M.E.; The ALSPAC Study Team; Bygren, L.O.; Kaati, G.; Edvinsson, S.; Northstone, K.; Sjöström, M.; Golding, J. Sex-specific, male-line transgenerational responses in humans. Eur. J. Hum. Genet. 2005, 14, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Paneth, N.; Susser, M. Early origin of coronary heart disease (the “Barker hypothesis”). BMJ 1995, 310, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Kuzawa, C.W. Modeling fetal adaptation to nutrient restriction: Testing the fetal origins hypothesis with a supply-demand model. J. Nutr. 2004, 134, 194–200. [Google Scholar] [CrossRef]
- Van Der Zee, H.A. The Hunger Winter: Occupied Holland 1944–1945; University of Nebraska Press: Lincoln, NE, USA, 1998. [Google Scholar]
- Lumey, L.H.; Ravelli, A.C.J.; Wiessing, L.G.; Koppe, J.G.; Treffers, P.E.; Stein, Z.A. The Dutch famine birth cohort study: Design, validation of exposure, and selected characteristics of subjects after 43 years follow-up. Paediatr. Périnat. Epidemiol. 1993, 7, 354–367. [Google Scholar] [CrossRef]
- Schultz, L.C. The Dutch Hunger Winter and the developmental origins of health and disease. Proc. Natl. Acad. Sci. USA 2010, 107, 16757–16758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenbosche, R.C.; Kirchner, J.T. Intrauterine growth retardation. Am. Fam. Physician 1998, 58, 1384–1390+1393–1394. [Google Scholar]
- Hales, C.N.; Barker, D.J. The thrifty phenotype hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Wadsworth, M.; Cripps, H.; Midwinter, R.; Colley, J. Blood pressure in a national birth cohort, at the age of 36 related to social and familial factors, smoking and body mass. Br. Med. J. 1985, 291, 1534–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Xiang, D.; Liu, Y.; Zhou, S.; Zhou, E.; Wang, Y. Protective Effects of Estrogen on Cardiovascular Disease Mediated by Oxidative Stress. Oxidative Med. Cell. Longev. 2021, 2021, 5523516. [Google Scholar] [CrossRef]
- Barker, D.; Godfrey, K.; Gluckman, P.; Harding, J.E.; Owens, J.; Robinson, J. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993, 341, 938–941. [Google Scholar] [CrossRef]
- Barker, D.J. The fetal origins of hypertension. J. Hypertens. Suppl. 1996, 14, S117–S120. [Google Scholar] [CrossRef]
- Edwards, M. The Barker Hypothesis. In Handbook of Famine, Starvation, and Nutrient Deprivation; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–21. [Google Scholar]
- Hofman, P.L.; Cutfield, W.S.; Robinson, E.M.; Bergman, R.N.; Menon, R.K.; Sperling, M.A.; Gluckman, P.D. Insulin Resistance in Short Children with Intrauterine Growth Retardation. J. Clin. Endocrinol. Metab. 1997, 82, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. Mothers, Babies and Diseases in Later Life; BMJ Publishing Group: London, UK, 1994; p. 80. [Google Scholar]
- Law, C.M.; Gordon, G.S.; Shiell, A.W.; Barker, D.J.; Hales, C.N. Thinners at birth and glucose tolerance in seven-year-old children. Diabet. Med. 1994, 12, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Law, C.M.; de Swiet, M.; Osmond, C.; Fayers, P.M.; Barker, D.J.; Cruddas, A.M.; Fall, C.H. Initiation of hypertension in utero and its amplification throughout life. BMJ 1993, 306, 24–27. [Google Scholar] [CrossRef] [Green Version]
- Barker, D.J.; Hales, C.N.; Fall, C.H.; Osmond, C.; Phipps, K.; Clark, P.M. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidemia (syndrome X): Relation to reduced fetal growth. Diabetologia 1993, 36, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Phillips, D.I.W.; Barker, D.J.P.; Hales, C.N.; Hirst, S.; Osmond, C. Thinness at birth and insulin resistance in adult life. Diabetologia 1994, 37, 150–154. [Google Scholar] [CrossRef] [Green Version]
- Fowden, A.L. The role of insulin prenatal growth. J. Dev. Physiol. 1989, 12, 173–182. [Google Scholar]
- Cota, B.; Allen, P. The developmental origins of health and disease hypothesis. Pediatr. Nurs. 2010, 36, 157–167. [Google Scholar]
- Martin, A.; Connelly, A.; Bland, R.M.; Reilly, J.J. Health impact of catch-up growth in low-birth weight infants: Systematic review, evidence appraisal, and meta-analysis. Matern. Child Nutr. 2017, 13. [Google Scholar] [CrossRef] [Green Version]
- Finkielstain, G.P.; Lui, J.C.; Baron, J. Catch-up growth: Cellular and molecular mechanisms. World Rev. Nutr. Diet. 2013, 106, 100–104. [Google Scholar]
- Galjaard, S.; Devlieger, R.; Van Assche, F.A. Fetal growth and developmental programming. J. Perinat. Med. 2013, 41, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.K.L.; Ahmed, M.L.; Emmett, P.M.; Preece, M.A.; Dunger, D. Association between postnatal catch-up growth and obesity in childhood: Prospective cohort study. BMJ 2000, 320, 967–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; Costa Louzada, M.D.; Pereira Machado, P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System; FAO: Rome, Italy, 2019. [Google Scholar]
- Juul, F.; Deierlein, A.L.; Vaidean, G.; Quatromoni, P.A.; Parekh, N. Ultra-processed Foods and Cardiometabolic Health Outcomes: From Evidence to Practice. Curr. Atheroscler. Rep. 2022, 24, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.S.; Rauber, F.; Leffa, P.S.; Sangalli, C.N.; Campagnolo, P.D.B.; Vitolo, M.R. Ultra-processed food consumption and its effects on anthropometric and glucose profile: A longitudinal study during childhood. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 177–184. [Google Scholar] [CrossRef]
- Eicher-Miller, H.A.; Fulgoni, V.L.; Keast, D.R. Processed Food Contributions to Energy and Nutrient Intake Differ among US Children by Race/Ethnicity. Nutrients 2015, 7, 10076–10088. [Google Scholar] [CrossRef]
- Rauber, F.; Campagnolo, P.; Hoffman, D.; Vitolo, M. Consumption of ultra-processed food products and its effects on children’s lipid profiles: A longitudinal study. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 116–122. [Google Scholar] [CrossRef]
- Smith, D.; Epigenetics, W.; Kreutzer, J. Encyclopedia of Clinical Neuropsychology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1113–1115. [Google Scholar]
- Popova, E.; Barnstable, C.J. Epigenetics Rules. J. Ocul. Biol. Dis. Inform. 2011, 4, 93–94. [Google Scholar] [CrossRef] [Green Version]
- Dolinoy, D.C.; Das, R.; Weidman, J.R.; Jirtle, R.L. Metastable Epialleles, Imprinting, and the Fetal Origins of Adult Diseases. Pediatr. Res. 2007, 61, 30–37. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Mitchell, M.D. Developmental origins of health and disease: Reducing the burden of chronic disease in the next generation. Genome Med. 2010, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Waterland, R.A.; Michels, K.B. Epigenetic epidemiology of the developmental origins hypothesis. Annu. Rev. Nutr. 2007, 27, 363–388. [Google Scholar] [CrossRef]
- Waterland, R.A.; Jirtle, R.L. Transposable elements: Targets for early nutritional effects on epigenetic gene regulation. Mol. Cell Biol. 2003, 23, 5293–5300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolinoy, D.C.; Weidman, J.R.; Waterland, R.A.; Jirtle, R.L. Maternal Genistein Alters Coat Color and Protects Avy Mouse Offspring from Obesity by Modifying the Fetal Epigenome. Environ. Health. Perspect. 2006, 114, 567–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, G.A.; Bale, T.L. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology 2009, 150, 4999–5009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuyama, H.; Hiramatsu, Y. Effects of a high-fat diet exposure in utero on the metabolic syndrome-like phenomenon in mouse offspring through epigenetic changes in adipocytokine gene expression. Endocrinology 2012, 153, 2823–2830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuyama, H.; Mitsui, T.; Nobumoto, E.; Hiramatsu, Y. The effects of high-fat diet exposure in utero on the obesogenic and diabetogenic traits through epigenetic changes in adiponectin and leptin gene expression for multiple generations in female mice. Endocrinology 2015, 156, 2482–2491. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Godfrey, K.M.; Pasupathy, D.; Levin, J.; Flynn, A.C.; Hayes, L.; Briley, A.L.; Bell, R.; A Lawlor, D.; Oteng-Ntim, E.; et al. Infant adiposity following a randomised controlled trial of a behavioural intervention in obese pregnancy. Int. J. Obes. 2017, 41, 1018–1026. [Google Scholar] [CrossRef] [Green Version]
- Poston, L.; Bell, R.; Croker, H.; Flynn, A.C.; Godfrey, K.M.; Goff, L.; Hayes, L.; Khazaezadeh, N.; Nelson, S.M.; Oteng-Ntim, E.; et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): A multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 767–777. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, Z.; Zhan, Y.; Wang, Y.; Ma, S.; Zhang, S.; Liu, J.; Wu, S.; Feng, Y.; Chen, Y.; et al. Effects of pre-pregnancy body mass index and gestational weight gain on maternal and infant complications. BMC Pregnancy Childbirth 2020, 20, 390. [Google Scholar] [CrossRef]
- Navarro, J.I.; Sigulem, D.M.; A Ferraro, A.; Polanco, J.J.; Barros, A.J. The double task of preventing malnutrition and overweight: A quasi-experimental community-based trial. BMC Public Health. 2013, 13, 212. [Google Scholar] [CrossRef]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef] [Green Version]
- Del Rosario, M.C.; Ossowski, V.; Knowler, W.C.; Bogardus, C.; Baier, L.J.; Hanson, R.L. Potential epigenetic dysregulation of genes associated with MODY and type 2 diabetes in humans exposed to a diabetic intrauterine environment: An analysis of genome-wide DNA methylation. Metabolism 2014, 63, 654–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gropper, S.S.; Smith, J.L.; Groff, J.L. Advanced Nutrition and Human Metabolism, 5th ed.; CENGAGE Learning Custom Publishing: Wadsworth, OH, USA, 2009; pp. 332–415. [Google Scholar]
- Le Doare, K.; Holder, B.; Bassett, A.; Pannaraj, P.S. Mother’s Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity. Front. Immunol. 2018, 9, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansaldo, E.; Farley, T.K.; Belkaid, Y. Control of Immunity by the Microbiota. Annu. Rev. Immunol. 2021, 39, 449–479. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Harrison, O.J. Homeostatic Immunity and the Microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, E.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Olivares, M.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Is meconium from healthy newborns sterile? Res. Microbiol. 2008, 159, 187–193. [Google Scholar] [CrossRef]
- Jimenez, E.; Fernández, L.; Marín, M.L. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr. Microbiol. 2005, 51, 270–274. [Google Scholar] [CrossRef]
- Chu, D.M.; Valentine, G.C.; Seferovic, M.D.; Aagaard, K.M. The Development of the Human Microbiome Why Moms Matter. Gastroenterol. Clin. N. Am. 2019, 48, 357–375. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Articles in English Nutritional programming and prenatal issues Nutritional programming and the preconception period Meta-analyses Systematic reviews Randomized clinical trials Observational studies Animal studies Historical data | Articles in a language other than English Nutritional programming and postnatal issues (breastfeeding, protein intake) In vitro studies Book Narrative reviews Review of reviews Research with pregnant adolescent (<16 years old) |
| Authors and Year of Publication | Factor under Examination | Size of the Study Group | Results and Conclusions |
|---|---|---|---|
| Waterland et al., 2003 [87] | Diet supplemented with methyl group donors | Mothers/offspring: n = 9/30, unsupplemented group; n = 10/39, supplemented group | Feeding pregnant mice a diet supplemented with methyl group donors resulted in a change in the color of the coat of the offspring from yellow to brown. The results of this study may suggest that dietary supplementation may have unintended deleterious effects on the establishment of epigenetic gene regulation in humans. |
| Dolinoy et al., 2006 [88] | Maternal genistein- supplemented diet of mice during gestation | Mothers/offspring: n = 15/52, unsupplemented group; n = 12/44, genistein-supplemented | The study reports that maternal supplementation of mice with genistein during gestation, at levels comparable to humans consuming a high-sugar diet, changed the coat color of heterozygous viable yellow agouti (A(vy/a) offspring toward pseudoagouti. The extent of this DNA methylation was similar in different tissues (endodermal, mesodermal, and ectodermal), indicating that genistein acts during early embryonic development. Furthermore, genistein-induced hypermethylation persisted into adulthood, reducing ectopic Agouti expression and protecting offspring from obesity. |
| Dunn et al., 2009 [89] | The 4.73-kcal/g high-fat diet consists of (by kcal): 7% corn starch, 10% maltodextrin, 17% sucrose, 39% lard, 20% casein, 0.3% l-cystine, 6% soybean oil, and essential vitamins and minerals. The 4.00-kcal/g house chow diet consisted of 28% protein, 12% fat, and 60% carbohydrate. | Mothers/first-generation offspring: n = 20/128, high-fat-fed (mHF); n = 12/65, chow-fed (mCH) | The study indicates that due to maternal exposure to a high-fat diet, the effect of a significant increase in body length persisted in the offspring for at least two generations. This phenotype is not attributed to altered intrauterine conditions or maternal dietary behaviour, as maternal and paternal lines were able to pass on the effect, supporting a true epigenetic mode of inheritance. A heritable trait of reduced insulin sensitivity was also detected between two generations. These studies suggest that the heritability of body length and glucose homeostasis is modulated by maternal diet over multiple generations, providing a mechanism in which length can increase rapidly in concert with caloric availability. |
| Masuyama et al., 2012 [90] | Maternal high-fat diet (HFD): energy content of 62% fat, 18% protein, and 20% carbohydrate. Maternal and offspring control diet (CD): 12% fat, 28% protein, and 60% carbohydrate. | Mothers/offspring: n = 6/24, offsprings from dams exposed to a high-fat diet during pregnancy (OH); n = 6/24, offsping from dams exposed to a conrtolled diet during pregnancy (OC) | OH mice were significantly heavier than OC mice. OH mice had higher blood pressure and worse glucose tolerance than OC mice. Total triglyceride and leptin levels were significantly higher, and adiponectin levels were significantly lower in OH mice compared to OC mice. This was due to changes in leptin and adiponectin expression in white adipose tissue. The study suggests that exposure to a high-fat diet in utero may cause a metabolic syndrome-like phenomenon through epigenetic modifications of adipocytokine, adiponectin and leptin gene expression. |
| Masuyama et al., 2015 [91] | High-fat diet 4 weeks before and during pregnancy and control diet (diet composition assumptions as Masuyama et al., 2012). | Mothers/offspring: differentiated according to the number of generations studied (group F0–2 or group F0–4). | The effect of maternal HFD on offspring over many generations in metabolic syndrome-like phenomena, such as weight gain and fat mass gain, glucose intolerance, hypertriglyceridemia, abnormal levels of adiponectin and leptin, and hypertension, was cumulative from expression and epigenetic changes in the genes of the aforementioned hormones. The controlled diet during pregnancy in subsequent generations after HFD in utero exposure decreased and the control diet during pregnancy for 3 generations completely abolished the effects of HFD in utero on body weight and fat mass gain, insulin resistance, serum triglyceride levels, adiponectin and leptin, with epigenetic changes in the adiponectin and leptin genes. |
| Authors and year of Publication | Type of Research | Factor under Examination | Characteristic of the Study Group | Results and Conclusions |
|---|---|---|---|---|
| Johnson et al., 1992 [23] | Longitudal restrospective study | The impact of increased maternal weight before pregnancy and increased weight gain during pregnancy affect pregnancy outcomes. | n = 3191 (BMI < 19.8, n = 755; BMI = 19.8–26, n = 1621; BMI = 27–29, n = 329; BMI > 29, n = 486) Mean age—data not available | Increased maternal weight before pregnancy (BMI) and increased weight gain during pregnancy were associated with increased risk of fetal macrosomia, birth abnormalities including unplanned cesarean section, postmaturity, though also with reduced incidence of low birth weight. Increased maternal weight gain during pregnancy results in a higher incidence of fetal macrosomia, which in turn leads to increased rates of cesarean section and other serious maternal and fetal complications. |
| Jensen et al., 2003 [24] | Cohort study | The relationship between pregnancy outcome and prepregnancy overweight or obesity in women with normoclycemia | n = 2459 (BMI = 18.5–24.9, n = 1094; BMI = 25.0–29.9, n = 728; BMI ≥ 30.0, n = 637) Age (mean ± SD) = 30.2 ± 5.1 years | Compared with normal-weight women (BMI 18.5–24.9 kg/m2), the risk of complications (preeclampsia, pregnancy-induced hypertension, cesarean section, induction of labor and macrosomia) was significantly increased in both overweight (BMI 25.0–29.9 kg/m2) and obese women (BMI ≥ 30.0 kg/m2). Pre-pregnancy overweight and obesity are associated with adverse pregnancy outcomes in normoglycemic women. |
| Poston et al. 2015 [92] | RCT | “The UK Pregnancies Better Eating and Activity Trial (UPBEAT study)” The relationship between an intervention including diet and physical activity and a reduction in the incidence of gestational diabetes and macrosomia in infants. | n = 1553 (Intervention group: n = 783, maternal age (mean ± SD) = 30.5 ± 5.5 years, BMI (mean ± SD) = 36.3 ± 5.0 kg/m2); Control group n = 772, maternal age (mean ± SD) = 30.4 ± 5.6 years, BMI (mean ± SD) = 36.3 ± 4.6 kg/m2) | Gestational diabetes was found in 160 (25%) women in the intervention group and in 172 (26%) women in the control group (p = 0.68). Macrosomia was found in 71 (9%) infants in the intervention group, compared to 61 (8%) infants in the control group (p = 0.40). Thus, there were no statistically significant differences in this regard. In contrast, there was a decrease in glycemia in the intervention group of women. Behavioral intervention including dietary change (promote a healthy pattern of eating but not to restrict energy intake) and physical activity in women with obesity during pregnancy is insufficient. There were no statistically significant differences in preventing gestational diabetes and reducing the incidence of infants with macrosomia between the intervention and control groups. |
| Patel et al., 2017 [93] | RCT | Postnatal follow up for a RCT ‘The UPBEAT study”. The impact of antenatal lifestyle intervention, including improvements in maternal diet and reduced gestational weight gain (GWG) in obese pregnant women affects reduction in infant adiposity | n = 698 (Intervention group: n = 342, maternal age (mean ± SD) = 31.3 ± 5.04 years, BMI (mean ± SD) = 36.17 ± 4.98 kg/m2); Control group n = 356, maternal age (mean ± SD) = 31.0 ± 5.58 years, BMI (mean ± SD) = 36.31 ± 4.69 kg/m2) | There were no differences in the z-score of triceps skinfold thickness between the intervention and standard care arms, though the z-score of subscapular skinfold thickness was lower in the intervention arm. Analysis of the data may indicate that the lower subscapular skinfold thickness in the infants was due to changes in the mother’s prenatal diet and weight gain during pregnancy, rather than postpartum diet. |
| O’Brien et al., 2020 [25] | RCT | The relationship of fetal growth velocity and fetal obesity under maternal obesity | n = 911 (BMI = 25.0–29.9, n = 376; BMI = 30.0–34.9, n = 271; BMI = 35.0–39.9, n = 153; BMI ≥ 40.0, n = 111) Age (mean) = 29.6 years | The fetus of women with giant obesity (BMI ≥ 40.0) showed the greatest increases in all z-scores of biometry, abdominal area (AA) and abdominal fat mass (AFM) compared to women classified as overweight (BMI 25.0–29.9). Maternal obesity was associated with an increase in all parameters, including: AFM and the velocity of estimated fetal weight (EFW) over time. Women with class 3 obesity (BMI ≥ 40.0) may be at increased risk of perpetuating intergenerational transmission of obesity to their offspring. |
| Sun et al., 2020 [94] | Clinical trial | The possible impact of pre-pregnancy BMI and gestational weight gain (GWG) on pregnancy outcomes and maternal and infant complications. | n = 3172 (BMI < 18.5, n = 420, BMI = 18.5–24.9, n = 2292; BMI = 25–29.9, n = 401, BMI ≥ 30.0, n = 59) | Overweight and inadequate GWG were risk factors for gestational diabetes mellitus (GDM) and large gestational age (LGA), and inadequate GWG was a risk factor for low birth weight. Overweight and obesity were risk factors for gestational hypertension, macrosomia, among others. Both overweight and obesity before pregnancy and excessive GWG are associated with a higher risk of developing GDM, GHp, macrosomia and LGA. |
| Dalrymple et al., 2021 [26] | RCT | The impact of behavioral intervention (“The UPBEAT study”) on cardiometabolic outcomes in children (and on sustained improvements in maternal lifestyle over 3 years postpartum) | Three-year-old children: n = 514: Intervention group: n = 250; Control group: n = 264. | There were no differences between the children of mothers in the intervention and control groups regarding the thickness of the subscapular skinfold. However, children of mothers in the intervention group had lower resting heart rates and a non-significantly lower likelihood of being overweight/obese. The study indicated that prenatal dietary and physical activity intervention in obese women is associated with lower offspring heart rates, as well as sustained improvements in maternal diet. However, the researchers point to the need to verify the study’s findings in future cohorts. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michońska, I.; Łuszczki, E.; Zielińska, M.; Oleksy, Ł.; Stolarczyk, A.; Dereń, K. Nutritional Programming: History, Hypotheses, and the Role of Prenatal Factors in the Prevention of Metabolic Diseases—A Narrative Review. Nutrients 2022, 14, 4422. https://doi.org/10.3390/nu14204422
Michońska I, Łuszczki E, Zielińska M, Oleksy Ł, Stolarczyk A, Dereń K. Nutritional Programming: History, Hypotheses, and the Role of Prenatal Factors in the Prevention of Metabolic Diseases—A Narrative Review. Nutrients. 2022; 14(20):4422. https://doi.org/10.3390/nu14204422
Chicago/Turabian StyleMichońska, Izabela, Edyta Łuszczki, Magdalena Zielińska, Łukasz Oleksy, Artur Stolarczyk, and Katarzyna Dereń. 2022. "Nutritional Programming: History, Hypotheses, and the Role of Prenatal Factors in the Prevention of Metabolic Diseases—A Narrative Review" Nutrients 14, no. 20: 4422. https://doi.org/10.3390/nu14204422
APA StyleMichońska, I., Łuszczki, E., Zielińska, M., Oleksy, Ł., Stolarczyk, A., & Dereń, K. (2022). Nutritional Programming: History, Hypotheses, and the Role of Prenatal Factors in the Prevention of Metabolic Diseases—A Narrative Review. Nutrients, 14(20), 4422. https://doi.org/10.3390/nu14204422







