Abstract
Sarcopenia is a multifactorial disease that limits autonomy for the growing elderly population. An optimal amount of dietary protein has shown to be important to maintain muscle mass during aging. Yet, the optimal distribution of that dietary protein has not been fully clarified. The aim of the present study was to examine whether an even, compared to a skewed, distribution of daily dietary protein leads to higher muscle protein synthesis and amino acid utilization. Twelve healthy males and twelve healthy females aged between 65 and 80 years were block randomized to either an even (EVEN, n = 12) or skewed (SKEWED, n = 12) dietary protein distribution for three daily main meals. Seven days of habituation were followed by three trial days, which were initiated by oral intake of deuterium oxide (D2O). The dietary protein throughout all trial meals was intrinsically labelled with 2H5-phenylalanine. Blood samples were drawn daily, and muscle biopsies were taken before and at the end of the trial to measure muscle protein synthesis (FSR) and muscle protein incorporation of the dietary-protein-derived tracer. Muscle protein FSR was no different between the two groups (EVEN 2.16 ± 0.13%/day and SKEWED 2.23 ± 0.09%/day, p = 0.647), and the muscle protein incorporation of the intrinsically labeled 2H5-phenylalanine tracer was not different between the two groups (EVEN 0.0049 ± 0.0004 MPE% and SKEWED 0.0054 ± 0.0003 MPE%, p = 0.306). In conclusion, the daily distribution pattern of the dietary protein did not affect muscle protein synthesis or the utilization of dietary protein.
1. Introduction
The degenerative loss of skeletal muscle mass and function, also known as sarcopenia, is a factor with great negative influence on the physical health and autonomy of older adults [1]. Besides being important for the ability to move around and carry out everyday tasks, skeletal muscle is also important for our metabolism [2]. In men >60 years of age, muscle strength is a predictor of all-cause mortality [3]; muscle mass is a predictor of all-cause mortality in women >60 years [4], and low muscle strength with low physical performance has been shown to be a predictor of mortality independent of other mortality risk factors such as ischemic heart disease, activities of daily living, age, or gender [5]. Hence, delaying or minimizing the development of sarcopenia is a target of efforts to maintain the health and autonomy of the aging population.
The etiology behind the development of sarcopenia is multifactorial, and is the result of both age-dependent cellular changes as well as age-related changes in lifestyle, such as diet and physical activity [6,7,8]. Regarding diet, the recommended daily protein intake for elderly people is debated; however, consensus is arising that people >65 years of age should have a daily protein intake of 1.2 g per kg body weight to maintain skeletal muscle mass [9].
The net protein balance of skeletal muscle is determined by protein synthesis and protein breakdown, and the balance between these two turnover rates changes throughout the day as a response to anabolic stimuli such as protein-feeding and resistance exercise [10]. Whereas studies have tried to clarify how much protein is needed on daily basis, there has been less focus on ways to optimize daily distribution of the protein to improve utilization of the ingested protein and in order to ensure an optimal net balance in skeletal muscle protein turnover in the elderly. Acute metabolic studies with the application of infused amino acid tracers have shown a graded increase in muscle protein synthesis (MPS) in the elderly with increasing doses of protein or essential amino acid (EAA) intake [8,11,12]. A protein content above 0.40 g per kg body weight (BW) has shown to maximally stimulate postprandial MPS in older men [13]. This suggests that to optimize MPS at each meal during the day and positively affecting the skeletal muscle net protein balance, elderly individuals could benefit from ingesting 0.40 g per kg BW of protein in all main meals during the day, i.e., an even protein distribution.
Nevertheless, it seems that most elderly people have a skewed protein intake during the day, where most of the daily protein is ingested at dinner while less protein is ingested at breakfast and lunch [14,15,16]. With a skewed protein distribution, breakfast and lunch meals could have a protein content below the 0.40 g per kg BW needed to maximally stimulate MPS [16]. In addition, it is possible that more protein than needed could be consumed at dinner, with the excess amino acids being oxidized and wasted. Consequently, the skeletal muscle’s net protein balance could be stimulated less optimally during a day with a skewed protein distribution when compared to evening out the protein distribution and, thereby, having multiple meals with a protein intake reaching the 0.4 g per kg BW.
It has been indicated that evenness of dietary protein distribution is associated with a higher muscle mass [17]. In addition, the importance of daily protein distribution has been shown in a cross-sectional study where men and women aged 75–96 with a skewed protein distribution had increased risk of frailty [18]. In contrast, in elderly malnourished at-risk patients in an inpatient rehabilitation unit, a skewed protein distribution had a positive effect on lean body mass compared to an even distribution [19]. When looking at acute metabolic studies, the effect of the protein distribution is unclear. Two studies by Kim and colleagues found no effect of an even protein distribution compared to a skewed distribution [20,21]. Yet, in adults aged 25–52, Mamerow and colleagues showed a greater mixed-muscle fractional synthesis rate with an even protein distribution compared to a skewed protein distribution [22].
Hence, the current literature is sparse and ambiguous. Therefore, the aim of the present study was to assess the importance of daily protein distribution through an integrated measurement of muscle protein synthesis and amino acid utilization over consecutive days. Importantly, the study was designed as a randomized controlled trial that mimics everyday life, looks at a demographic relevant population, and controls sufficiently for total protein intake, protein distribution, and total calorie intake. It was hypothesized that an even vs. a skewed daily protein distribution would result in a greater utilization of dietary amino acids for de novo muscle protein synthesis.
2. Materials and Methods
2.1. Subjects
Before inclusion in the study, each subject had the study design, purpose, and possible risks explained to them. Subsequently, all subjects gave their written consent to participate in the protocol, which adhered to the Declaration of Helsinki and was approved by the Ethics Committee of Copenhagen and Frederiksberg (H-18026529). The study is registered at ClinicalTrials.net (NCT03870425).
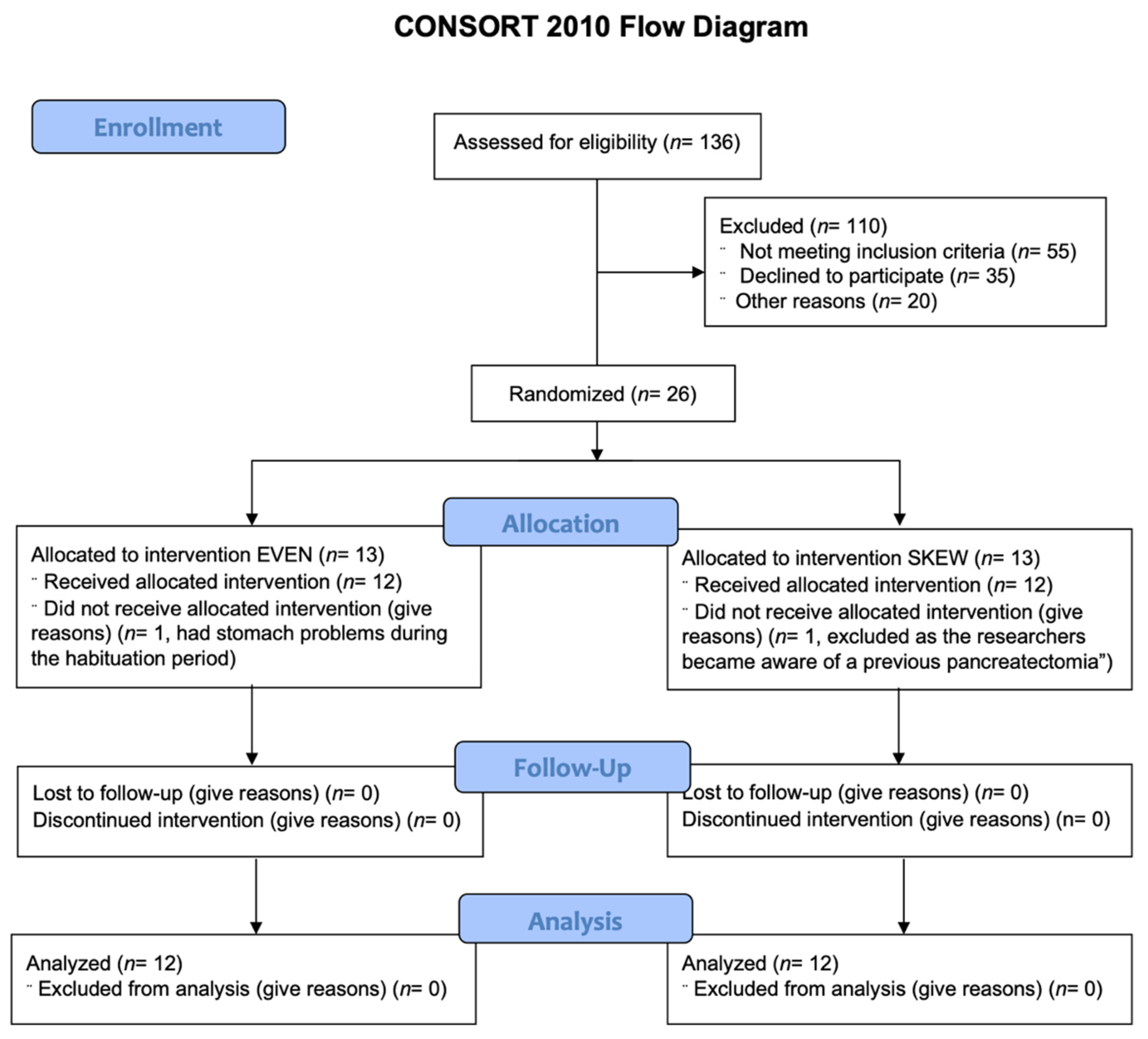
Inclusion protocol. Twelve healthy elderly males and twelve healthy elderly females were block randomized to either the EVEN (n = 12) or the SKEWED (n = 12) protein distribution throughout all 11 days (Table 1). See Figure 1 for CONSORT flow diagram.

Table 1.
Subject characteristics at inclusion.
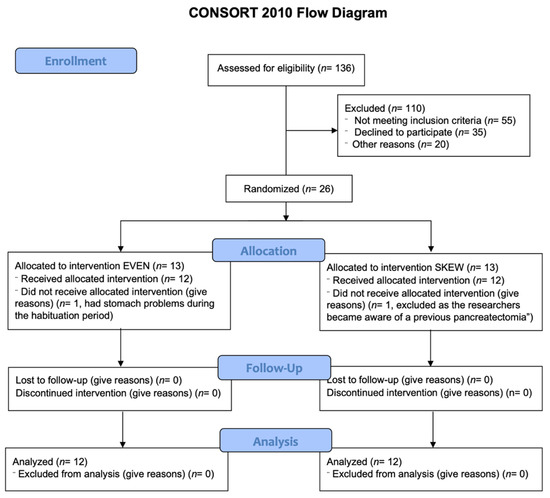
Figure 1.
CONSORT diagram. 136 potential subjects were assessed for eligibility. 110 were excluded either due to not meeting inclusion criteria (55), declining to participate (35) or other reasons (20), leaving 26 subjects fit for inclusion. The 26 remaining subjects were randomized to either the EVEN or SKEWED intervention group. 1 subject from each intervention group were lost during the habituation period, leaving 12 subjects in each intervention group that all completed the entire study and who were all included in the later analysis.
Exclusion criteria for this study were an age below 65 or above 80 years of age, body mass index (BMI) below 18.5 or above 30 kg/m2, smoking, vegetarianism, diabetes or any other metabolic diseases, gastrointestinal diseases, impaired kidney or liver function, inflammatory diseases, hypertension, signs of arteriosclerosis, or more than 5 h of weekly systematic training, except for activity associated with transportation as well as dancing and stretching.
Written informed consent was obtained from all subjects before enrollment to the study. Subject’s height, weight and blood pressure were measured, and blood samples were taken for health screening. In addition, DEXA scans were carried out to determine lean body mass (LBM).
2.2. Diet
Diet allocation. Before each trial, subjects were randomly allocated to either an even or a skewed protein distribution for the entire trial, which consisted of a 7-day habituation period followed by a 3-day trial period at the hospital (Figure 2). Subjects were block randomized in three blocks with 8 subjects in each block in order to ensure an equal number of subjects as well as an equal gender distribution in the EVEN and SKEWED groups.
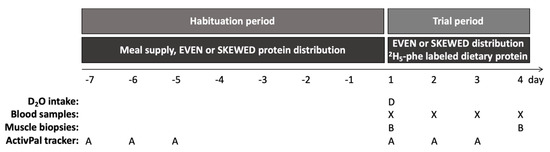
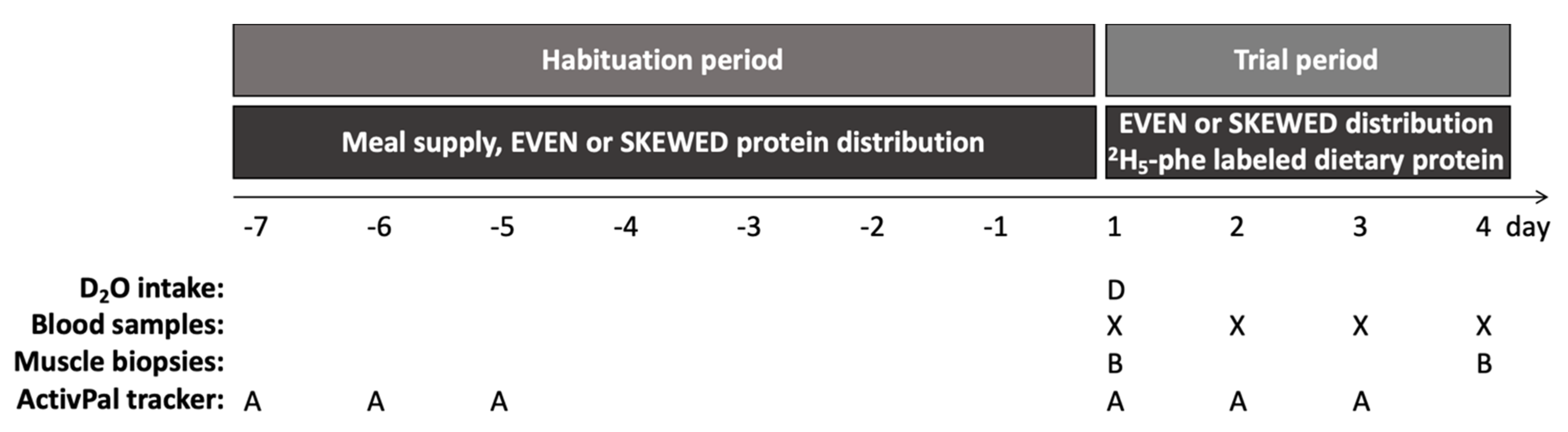
Figure 2.
Study design. Indicating the initial 7-day dietary habituation period from day -7 to 1, with habituation to the skewed or even protein distribution by home delivered meals, followed by the three-day trial period started on day 1. Subjects showed up at the hospital from 8.00 a.m. to 18.00 p.m. on all trial days. After background muscle biopsy (B) and blood sample (X) were taken on day 1, the subjects ingested 5.25 mL D2O/kg LBM and the trial meals were commenced. All trial meals were prepared by the researchers and contained intrinsically labeled 2H5-phenylalanine protein, and the meal intake was monitored to ensure full compliance. On day 2 and 3, subjects had a blood sample taken and followed the schedule of the trial meals. On day 4 subjects had breakfast, and two hours later muscle biopsy and blood sample were collected. During the habituation period, the subjects followed their habitual lifestyle except from adhering to the meals, whereas during the trial days a pre-determined activity schedule (walks and cycling) was followed. The activity level was monitored (A) during the initial 3 days during the habituation and trial period, respectively.
The food ingested by the subjects during the entire trial was made at the Research Kitchen at the Department of Human Nutrition, University of Copenhagen.
Habituation period. Prior to the 3 trial days at the hospital, subjects went through 7 days of dietary habituation (Figure 2) where they maintained their everyday lifestyle but were served a standardized diet for all 7 days, with either an even or skewed protein distribution. The standardized meals (breakfast, lunch, and dinner) were prepacked for all seven days. Daily calorie intake was determined by the Harris–Benedict equation, calculated on the basis of total LBM and with an activity factor of 1.53 [23]. The calorie intake during habituation was divided into four levels. Subjects with LBM between 30–38 kg received 7.7 mega joule (MJ) daily, subjects with LBM between 39–46 kg received 8.8 MJ daily, subjects with LBM between 47–54 kg received 9.9 MJ daily, and subjects with LBM between 55–63 kg received 11 MJ daily. The calories of the main meals were equal, with 33% of the total daily calorie intake at breakfast, 33% at lunch, and 33% at dinner. In addition, subjects were allowed to choose a snack low in protein twice daily. The subjects were instructed on which type of snacks were allowed (low in protein) and not allowed (high in protein). Additionally, the subjects filled out a questionnaire each day answering whether they had eaten a snack that day, and if so, what snack had been eaten. The reporting of the snacks through the questionnaire was continuously monitored by the researchers.
The daily protein intake during habituation was between 1.3–1.6 g/kg LBM in both groups, within all four levels of calorie intake: 7.7, 8.8, 9.9, and 11 MJ, respectively. Importantly, the distribution of the daily protein intake was 33% at breakfast, 33% at lunch, and 33% at dinner for the EVEN group, whereas in the SKEWED group, the distribution was 17% at breakfast, 17% at lunch, and 66% at dinner.
Subjects were thoroughly instructed to remain compliant with their diets and, except for the snacks, not to ingest anything containing energy at the meals. Tap water, sparkling water without energy, and coffee/tea without milk or sugar were allowed. Subjects had to eat breakfast between 7.00–9.00 a.m., lunch between 11.00 a.m.–1.00 p.m., and dinner between 6.00–8.00 p.m. Importantly, the subjects were instructed not to skip any meals, not to save food for later, to finish their meals, and to try an extra time if the meal was difficult to finish. However, if they were unable to finish a meal, they were instructed to freeze the leftovers and bring them to the researchers during the following trial days at the hospital. Here, the leftovers would be measured and registered. Subjects were allowed to add salt, pepper, or other seasonings to the meals.
Trial days. During the trial days, the majority of the dietary protein in all meals and snacks consisted of minced beef intrinsically labeled with 2H5-phenylalanine in order to measure the utilization of the dietary protein. To limit the dietary dilution of the 2H5-phenylalanine tracer from the minced meat with phenylalanine from other protein sources, whole foods very low in protein were chosen for the remainder of the meals and snacks. Information on the production of intrinsically labeled minced meat has previously been published [24]. The only meal not containing intrinsically 2H5-phenylalanine-labeled minced meat was the initial breakfast on the first trial day, as this meal was consumed before the background blood sample was collected.
During the three trial days at the hospital, subjects continued eating according to their respective protein distribution with a total protein intake of 1.5 g/kg LBM/day, equivalent to approximately 1.0–1.2 g/kg BW, in both the EVEN and SKEWED group (Table 2). The protein intake of the EVEN group was divided into 0.450 g/kg LBM at breakfast, 0.075 g/kg LBM as a noon snack, 0.450 g/kg LBM at lunch, 0.075 g/kg LBM as an afternoon snack, and 0.450 g/kg LBM at dinner (30%-5%-30%-5%-30% distribution). The protein intake of the SKEWED group was divided into 0.225 g/kg LBM at breakfast, 0.075 g/kg LBM as a noon snack, 0.225 g/kg LBM at lunch, 0.075 g/kg LBM as an afternoon snack, and 0.900 g/kg LBM at dinner (15%-5%-15%-5%-60% distribution). With this protein distribution design, the present study is comparable to previous studies by Mamerow and colleagues and Kim and colleagues [20,22]. The total daily intake of calories was calculated on the basis of the individual LBM of each subject with the Harris–Benedict equation, with a correction factor of 1.53 for light activity. All meals were prepared and weighted by the researchers. The subjects were instructed to finish all meals, and this was controlled by the researchers. Tap water, sparkling water without energy, and coffee/tea without milk or sugar were allowed. Breakfast was served at 8.00 a.m., noon snack at 10.15 a.m., lunch at 12.30 p.m., afternoon snack at 3.00 p.m., and dinner at 5.45 p.m. For both groups, the total daily calories were divided as follows: 30% in the morning, 5% as a noon snack, 30% at lunch, 5% as an afternoon snack and 30% at dinner. Each day at 6.00 p.m., subjects were allowed to return home. They would then return to the hospital each day at 7.45 a.m. Subjects were instructed not to ingest anything containing energy or protein while at home. Only tap water, sparkling water without energy, and coffee/tea without milk or sugar were allowed while subjects were not at the hospital.

Table 2.
Meal distribution during the trial period.
2.3. Activity and Activity Monitoring
Activity monitoring. During both the habituation period and the trial days at the hospital, the subjects were fitted with an ActivPal activity tracker (PAL Technologies Ltd., Scotland, UK). Activity was tracked for 3 out of 7 days during the habituation period and during the 3-day trial period. The ActivPal would collect data from the first day at 6.00 a.m. until the fourth day at 6.00 a.m. During the trial days at the hospital, the ActivPal would collect data from the first day at 11.00 a.m. until the fourth day at 6.00 a.m. The ActivPal was taped to the middle of the anterior side of the subject’s thigh. The ActivPal measured time spent lying, sitting, and standing during the day, while also registering daily step count. Two ActivPals failed to collect data during the habituation period, one in the SKEWED group and one in the EVEN group.
Activity. Throughout the habituation days, the participants were asked to maintain their usual activity levels. During the trial days at the hospital, the activity level was controlled by the researchers.
During the three initial trial days, subjects had three planned daily activities. All activities were supervised by a researcher. At 10.30 a.m., a walk on a predetermined 3 km route in the vicinity of the hospital was completed, lasting for approximately 45 min. At 2.00 p.m., 15 min of cycling was performed on a cycle ergometer with a cadence of 60–80 rpm and fitted with a heart rate monitor to ensure exercise at 60–80% of their estimated maximal heart rate.
Finally, at 4.30 p.m., an approximately 30 min walk was completed along a predetermined 1.5 km route around the hospital that included climbing of 357 stairs at an easy walking pace. On the first trial day, the 3 km walk was skipped in order to collect the initial background muscle biopsy and blood sample.
2.4. Measurements
Blood sampling. On the first trial day, a background blood sample was collected at approximately 9.00 a.m. before consumption of intrinsically labeled 2H5-phenylalanine beef and oral D2O intake. For the remainder of the trial days, blood samples were taken at 9.00 a.m. Blood samples were collected in EDTA tubes. After 10–30 min on ice, the tubes were centrifuged at 3200× g for 10 min at 4 °C, after which the plasma were aliquoted and stored at −80 °C until further analysis.
Muscle biopsies. At approximately 10.00 a.m. on the first trial day a background muscle biopsy was taken, and a second biopsy was taken on the fourth trial day at 10.00 a.m.
Initially, by randomization, either the dominant or the non-dominant leg would be chosen as the site for all biopsies for each subject. Biopsies were taken with 3 cm between each site, and the order of the biopsies within the leg was randomized as well.
The muscle biopsies were taken from the lateral portion of the vastus lateralis muscle in the leg under local anesthetic treatment (lidocaine, 1%). The biopsies were taken with 4 mm Bergström biopsy needles (Stille, Stockholm, Sweden) with manual suction. From the muscle specimen, blood, visible fat, and connective tissue were quickly removed, and the muscle specimen was rinsed with saline water before being snap frozen in liquid nitrogen and stored at –80 °C until further analysis.
D2O administration. Administration of D2O (DLM-2259-PK, Cambridge Isotope Laboratories Inc., Tewksbury, MA, USA) was used to measure the muscle protein synthesis during the trial days. The 99.8% D2O, diluted 1:1 in tap water, was administered orally in six hourly boluses during the first trial day starting at 11.00 a.m. to minimize the adverse effects of D2O [25]. In total, the subjects received 5.25 mL 99.8% D2O/kg LBM, and all subjects consumed the entire dose.
2.5. Mass Spectrometry Analysis
For the mass spectrometry analysis of 2H-alanine and 2H5-phenylalanine muscle enrichment, 20 mg of muscle tissue was used. The muscle tissue was homogenized in homogenization buffer (0.02 M Tris [pH 7.4], 0.15 M NaCl, 2 mM EDTA, 2 mM EGTA, 0.5% TritonX-100 and 0.25 M sucrose). The proteins were hydrolyzed by addition of 1 mL 1 M HCl and 1 mL resin slurry, and left overnight at 110 °C.
For mass spectrometry analysis of 2H-alanine precursor enrichment from the plasma samples, 200 μL of plasma per sample was used. Isotopically labeled internal standards (uniformly labeled 13C/15N) of each amino acid were added to the plasma in a 50% acetic acid solution.
Hereafter, the solution from either the muscle or plasma samples was poured over cation exchange columns with resin (AG 50 W-X8 resin, Bio-Rad Laboratories, Hercules, CA, USA) which had been prepared by adding 3 × 2 mL 1 M HCl. The resin columns were washed 5 times with 3 mL deionized water before the amino acids were eluted into collection vials by adding 2 × 2 mL 4 M NH4OH. The solution of each of the muscle samples was divided into two portions for liquid chromatography–tandem mass spectrometry (LC–MS/MS) and gas chromatography–combustion–isotope ratio mass spectrometer (GC–C–IRMS) analysis, respectively.
To measure the plasma 2H-alanine precursor enrichment and muscle incorporation of the dietary-protein-derived 2H5-phenylalanine, the solvents eluted from the resin columns were evaporated under a stream of N2 flow at 70 °C and samples were derivatized into their PITC derivative with phenylisothiocyanate (PITC). Ten microliters of the derivatized samples were loaded and analyzed by LC–MS/MS (TSQ Quantiva; Thermo Fisher Scientific, San Jose, CA, USA) as described elsewhere [26].
For determining the 2H-alanine abundance in the muscle tissue samples, amino acids in the solvent eluted from the resin column were converted to the N-acetyl-propyl (NAP) derivatives and analyzed by GC–C–IRMS as previously described by Bornø et al. [27]
2.6. Fractional Synthesis Rate Calculation
The fractional synthesis rate (FSR) of muscle proteins was calculated form the direct incorporation rate,
The FSR is expressed in % × day−1 and was calculated from the change in muscle protein tracer enrichment (ΔEprotein) from the background muscle tissue sample taken on the first trial day to the muscle tissue sample obtained on the last trial day, divided by the weighted average of the precursor enrichment measured from the plasma of the daily blood sampling (Êprecursor) multiplied by the incorporation time (Δt).
2.7. Statistics
All data were analyzed with the Shapiro–Wilk normality test before parametric statistics were applied. Subject characteristics, total calorie intake, muscle protein synthesis, and 2H5-phenylalanine incorporation were analyzed by two-tailed unpaired t-test. Data on protein and calorie intake per meal during the 7 habituation days were analyzed by two-way ANOVA with repeated measures for meal (breakfast, lunch, dinner). Activity data was analyzed by two-way ANOVA with repeated measures for time (habituation vs. trial period). Subject characteristics and protein and calorie intake are presented as mean ± SD. The remaining data are presented as mean ± SEM with individual data indicated. All data was analyzed by Sigma Plot version 13.0 (Systat Software Inc., San Jose, CA, USA).
3. Result
3.1. Subject Characteristics
Before the trial, no significant differences existed between groups in regard to age (p = 0.784), height (p = 0.648), weight (p = 0.132), BMI (p = 0.163), LBM (p = 0.502), fat percentage (p = 0.363), systolic blood pressure (BP) (p = 0.439), diastolic BP (p = 0.480), HbA1c (p = 0.702), thyroid-stimulating hormone (TSH) (p = 0.605), total cholesterol (p = 0.557), high-density lipoprotein (HDL) (p = 0.081), or low-density lipoprotein (LDL) (p = 0.940) (Table 1).
3.2. Diet
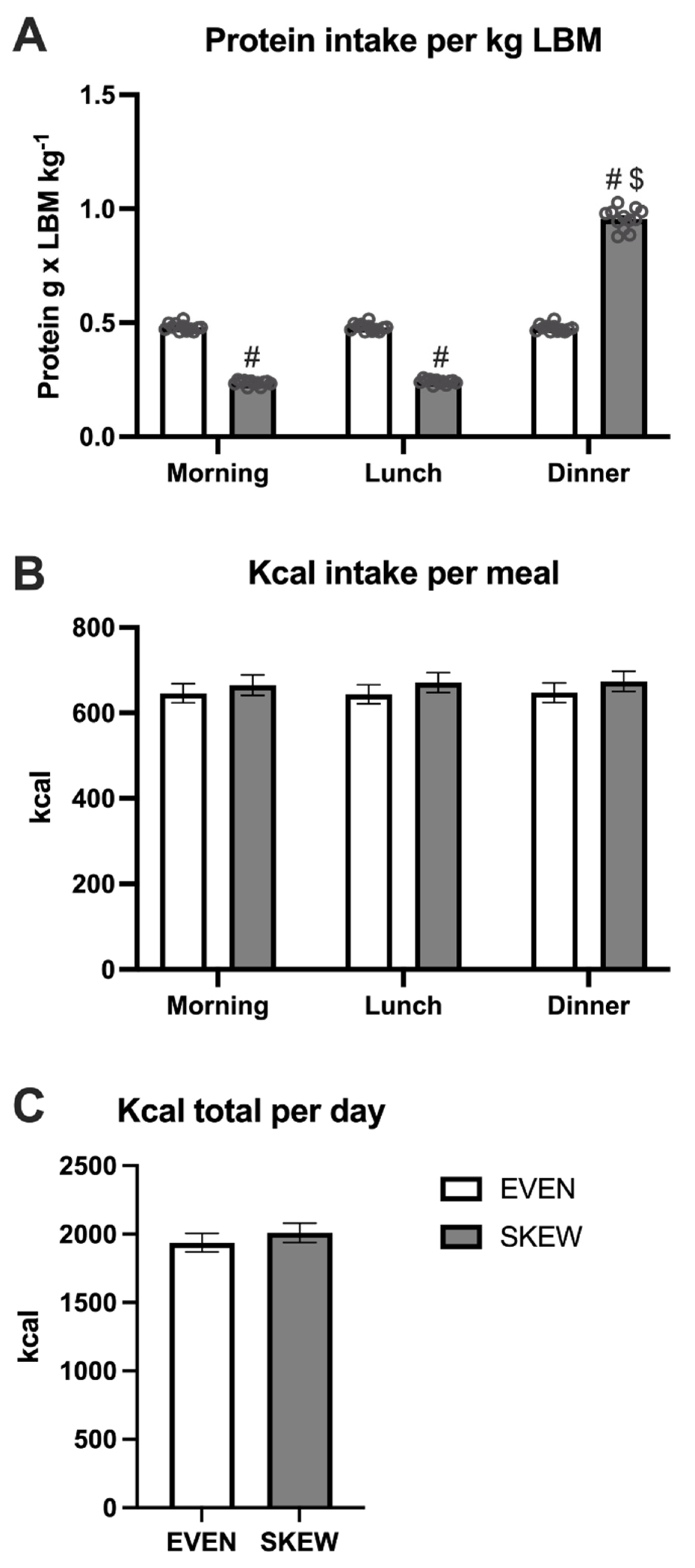
During the habituation period, no differences in protein intake per kg LBM were seen between meals in the EVEN group, whereas the intake was greater at dinner compared to breakfast (p < 0.001) and lunch (p < 0.001) in the SKEWED group (Figure 3A). The protein intake per kg LBM was greater at breakfast (p < 0.001) and lunch (p < 0.001) in EVEN vs. SKEWED, whereas the intake at dinner was smaller in EVEN vs. SKEWED (p < 0.001). No significant differences between the two groups or between meals were found for total kcal per day (Figure 3B). The total daily calorie intake was the same for the EVEN and SKEWED groups (Figure 3C).
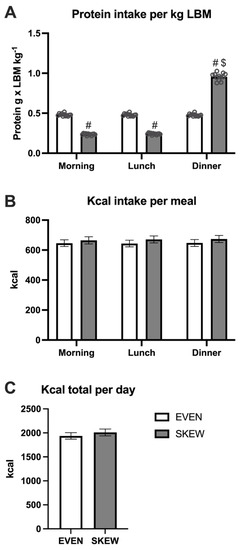
Figure 3.
The daily intake of (A) protein per meal, (B) kcal per meal, and (C) total ingested kcal during the habituation period. 3A: No differences in protein intake per kg LBM were seen between meals in EVEN, whereas the intake was greater at dinner compared to breakfast (p < 0.001) and lunch (p < 0.001) in SKEWED. The protein intake per kg LBM was greater at breakfast (p < 0.001) and lunch (p < 0.001) in EVEN vs. SKEWED, whereas the intake at dinner were smaller at EVEN vs. SKEWED (p < 0.001). 3B: No significant differences between the two groups or between meals were found for total kcal per day. 3C: The total daily calorie intake was the same for the EVEN and SKEWED group. Data shown as mean ± SEM. # denote different from EVEN, $ denote different from breakfast and lunch.
3.3. Activity and Activity Monitoring
No significant main effect of intervention was found for any of the five activity parameters (Table 3). A significant effect of time was found, with a greater number of steps (p < 0.001) and step time (p = 0.005) during the trial period compared to habituation and a shorter stand time (p < 0.001) during the trial period compared to habituation. The energy expenditure (MET) was slightly lower during the trial period compared to habituation (p = 0.006). No significant effect of time was found for sit-lie time (p = 0.084)

Table 3.
Activity monitoring during habituation and trial days:.
3.4. Muscle Protein Synthesis and Intrinsically Labeled Tracer Incorporation
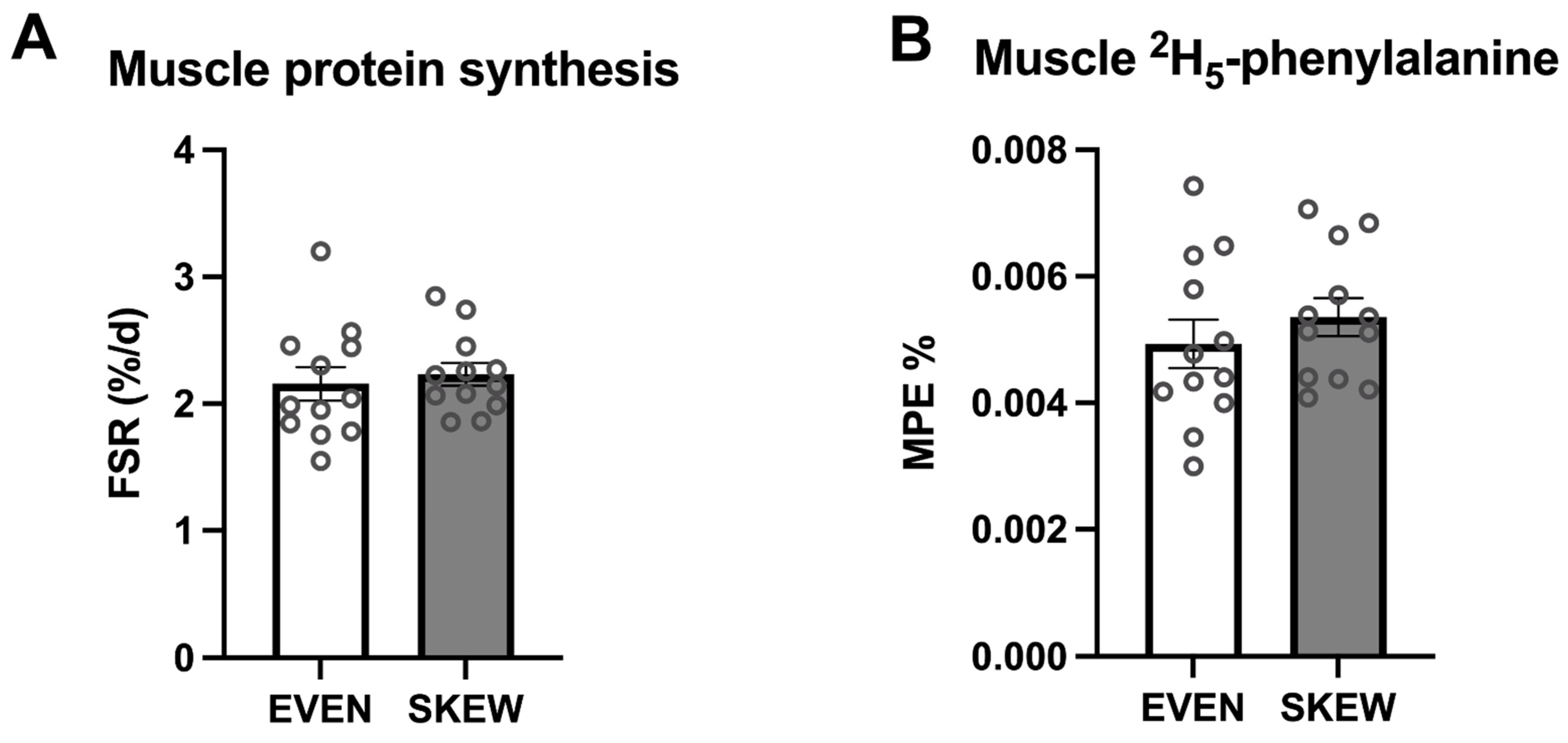
During the trial period, no significant difference between the two groups was found for mixed-muscle protein FSR (p = 0.647) (Figure 4A). No significant difference between the two groups was found for incorporation of dietary-protein-derived 2H5-phenylalanine (p = 0.387) (Figure 4B).
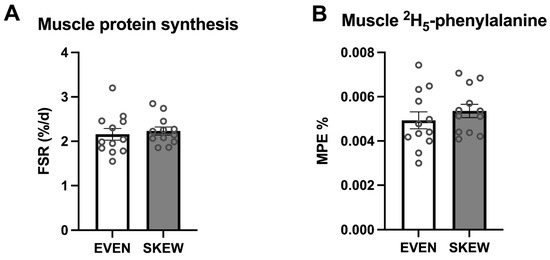
Figure 4.
(A) Muscle protein FSR and (B) muscle 2H5-phenylalanine incorporation. 4A: No significant difference between the two groups was found for mixed muscle protein FSR (p = 0.647). 4B: No significant difference between the two groups was found for incorporation of the dietary protein derived 2H5-phenylalanine (p = 0.387). Data shown as mean ± SEM, with individual data indicated as circles.
4. Discussion
The present study explored the importance of how the daily protein intake is distributed with regards to muscle protein synthesis and utilization of dietary protein. To the knowledge of the authors, this is the first study on healthy older adults to explore the effect of dietary protein distribution in an energy-balanced diet by applying an integrated measurement of muscle protein synthesis over consecutive days and assessing the utilization of dietary-protein-derived amino acids using intrinsically labeled protein. It was found that a sufficient daily protein intake at 1.5 g protein per kg LBM with either an even or skewed distribution resulted in the same rates of muscle protein synthesis, as well as similar utilization of the dietary-protein-derived amino acids for synthesis of muscle proteins. The study was performed with a randomized controlled trial design that mimicked everyday life, included a demographically relevant population, employed strict dietary control that ensured a distinct protein distribution in the two intervention groups—EVEN and SKEWED, respectively—and, at the same time, ensured identical total protein intake and total calorie intake for both groups.
The integrated measurement of muscle protein synthesis over multiple days is, to a large degree, affected by physical activity level and diet habits [28]. Therefore, the translatability of the muscle protein synthesis outcome in a parallel group design, as in the present study, is affected by the ability to control for physical activity level, energy expenditure, and dietary intake. To ensure that the prior diet habits would not influence the change to an EVEN or SKEWED distribution, a run-in habituation period of 7 days was performed before any outcome measurements were commenced. Furthermore, as previously described, the diet was designed based on the subject’s LBM, and the protein and calorie intake were controlled through daily reporting during the habituation period as well as by food preparations and observations by the researchers during the trial period. The activity levels of the subjects were monitored both during the habituation days and during the trial days in the two groups. Notably, the physical activity for the subjects during the trial was not designed to be physical training, but merely to ensure an activity level and energy expenditure comparable to the habitual activity as observed during the habituation period. Despite differences being seen in stand time, step time, and total steps per day from habituation to the trial period, only a slight difference in the energy expenditure was seen. Importantly, no differences in the monitored physical activity were observed between the EVEN and SKEWED groups, which otherwise could have affected the integrated measurement of muscle protein synthesis. Therefore, by strict dietary and activity design and control, it is possible to translate the outcome of the study to be based on dietary protein distribution—EVEN and SKEWED, respectively—as the major diverging parameter.
The results from previous studies exploring the muscle protein synthesis response from an even or skewed dietary protein distribution are inconsistent. Studies from Kim and colleagues showed no difference in mixed-muscle FSR with an even or skewed dietary protein intake [20,21], which is in line with the findings of the present study. In contrast, the findings of Mamerow et al. [22] contradict the results of these studies, showing a greater mixed-muscle FSR with an even compared to a skewed protein distribution. The protein dose provided in the study by Kim et al. was given at a low dose of 0.8 g per kg BW per day or high dose of 1.5 g per kg BW per day, and in the study by Mamerow et al., protein dose was 1.2 g per kg BW; in both studies, the dietary protein was given in mixed meals of macronutrients. Therefore, the protein dose and source of the meals was comparable to the present study. It should be noted that the study by Mamerow and colleagues is on a younger population compared to the present study and the studies by Kim and colleagues. Whether an age difference exists in the effect of an even dietary protein distribution can only be speculated. There could be several mechanisms for such a distinct effect, e.g., different protein metabolism, or higher habitual activity level and, thereby, greater amino acid demand in younger vs. older adults. In the above-mentioned studies by Kim et al. and Mamerow et al., muscle protein synthesis was measured over 24 h with a stable-isotope amino acid tracer infusion. Except for a 15–30 min treadmill walk during these 24 h, the tracer infusion necessitated that the subjects be primarily bed-ridden during the 24 h tracer infusion. In such a setting, the stimulatory response of habitual daily activity on muscle protein synthesis becomes negligible. In contrast, the subjects in the present study were not restricted to bed rest, as the tracer was given orally on day 1 which warranted a subsequent synthesis measurement under free-living conditions. Furthermore, the integrated measurement over multiple days decreased the risk of interference by day-to-day variations in protein synthesis.
In addition to the measurement of muscle protein synthesis, the present study assessed the muscle utilization of the ingested dietary protein by consuming intrinsically amino-acid-labeled meat at all meals during the three-day trial period. As mentioned in the introduction, it has been shown that a protein intake of 0.4 g per kg body weight per meal is needed to maximally stimulate muscle protein synthesis in older adults [13], which formed the basis of the meal-size design in the present study. Therefore, it was hypothesized that the greater protein intake at the evening meal of the SKEWED distribution (0.9 g per kg LBM) would not be fully utilized, and an excess appearance of amino acids into circulation would be oxidized instead of being used for de novo synthesis of proteins. It should be noted that the study indicating a maximum stimulation of muscle protein synthesis at 0.4 g per kg body weight was performed with a bolus intake of pure protein, and not together with a mixed meal. The amino acid absorption after a bolus of protein is fast and transient, whereas a more prolonged release of amino acids into circulation is seen after intake of protein in a mixed meal [24,29]. Such a prolonged release could potentiate full utilization of a greater protein intake over the post-prandial period, which could be why a difference was not seen between the EVEN and SKEWED distribution groups in muscle-tissue incorporation of the intrinsically labeled 2H5-phenylalanine tracer in the current study. It should be noted that, when exploring the incorporation of a dietary derived amino acid tracer over multiple days, a potential recirculation of the tracer could occur due to an efflux of the tracer from body proteins because of protein breakdown. However, by limiting the study period to three days, the recirculation is minor and, at the same time, the study duration makes it possible still to measure the muscle incorporation of the dietary derived amino acid tracer.
The study was conducted with a total daily protein intake that was sufficient. The daily protein intake during the trial period, 1.5 g of protein per kg LBM, is equivalent to approximately 1.0–1.2 g of protein per kg body weight. Furthermore, the activity level of the subjects was low to moderate. Thus, the conclusion that no difference between even and skewed protein intake exists should not be extrapolated to either a situation where the total protein intake is substantially lower or a situation with highly physically active older adults. In both such situations, the metabolic amino acid demand would be greater and a potential effect of distributing the dietary protein throughout the day could be significant. Previously, we have indicated that an even protein intake is associated with a higher muscle mass [17], and it has been shown that a skewed protein intake was associated with a higher risk of frailty [18]. Therefore, the dietary protein distribution could be clinically relevant, e.g., in patients at risk of sarcopenia that could also experience malnourishment due to a reduced appetite. Thus, as previously discussed, in a practical setting, a dietary protein intake that is evenly distributed throughout the daily meals could increase the total daily protein intake and, thereby, ensure a sufficient protein intake to maintain the protein pool of the body [17].
Although the diet of the subjects was controlled to the best of our abilities, the subjects were at home during the entire seven-day habituation period, and during the three trial days, the subjects were at home between 6:00 p.m. and 8:00 a.m. each day. Therefore, the dietary control is limited by the inability to closely observe the subject during the habituation period and at nighttime during the trial period. Nevertheless, with clear instructions to the subjects throughout the entire experiment on the importance of complying with the dietary intervention and with registration of all meals and snacks, it is believed that compliance with the diet was high and deviations were negligible.
5. Conclusions
Through a randomized controlled trial on healthy older adults with a sufficient total daily dietary protein intake distributed evenly or skewed throughout daily meals, no difference in the level of muscle protein synthesis was found. Furthermore, no difference in the utilization of the dietary protein in the skeletal muscle was seen between the EVEN and a SKEWED protein distributions.
Author Contributions
Conceptualization, L.H. and J.A.; Methodology, G.v.H. and J.A.; Formal Analysis, T.E.H.J. and J.A.; Investigation, T.E.H.J., S.E.J., T.T.T. and J.A.; Resources, J.A.; Data Curation, T.E.H.J. and J.A.; Writing—Original Draft Preparation, T.E.H.J.; Writing—Review & Editing, T.E.H.J., S.E.J., T.T.T., L.H., G.v.H. and J.A.; Visualization, T.E.H.J. and J.A.; Supervision, G.v.H. and J.A.; Project Administration, J.A.; Funding Acquisition, J.A. All authors have read and agreed to the published version of the manuscript.
Funding
The Danish Innovation Fund and Danish Crown Ingredients A/S have funded the project.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Copenhagen and Frederiksberg (H-18026529).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Acknowledgments
Thanks to the subjects volunteering for the study, and thanks to Charlotte Kostecki from the Department of Human Nutrition, University of Copenhagen for designing and producing all meals.
Conflicts of Interest
None of the authors have any conflicts of interest.
References
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal Muscle Mass and Distribution in 468 Men and Women Aged 18–88 Yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Distefano, G.; Goodpaster, B.H. Effects of Exercise and Aging on Skeletal Muscle. Cold Spring Harb. Perspect. Med. 2018, 8, a029785. [Google Scholar] [CrossRef] [PubMed]
- Metter, E.J.; Talbot, L.A.; Schrager, M.; Conwit, R. Skeletal Muscle Strength as a Predictor of All-Cause Mortality in Healthy Men. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B359–B365. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Mackenzie, T.A.; Barre, L.K.; Lopez-Jimenez, F.; Bartels, S.J. Sarcopenia, Sarcopenic Obesity and Mortality in Older Adults: Results from the National Health and Nutrition Examination Survey III. Eur. J. Clin. Nutr. 2014, 68, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Arango-Lopera, V.E.; Arroyo, P.; Gutiérrez-Robledo, L.M.; Perez-Zepeda, M.U.; Cesari, M. Mortality as an Adverse Outcome of Sarcopenia. J. Nutr. Health Aging 2013, 17, 259–262. [Google Scholar] [CrossRef]
- Morley, J.E.; Baumgartner, R.N.; Roubenoff, R.; Mayer, J.; Nair, K.S. Sarcopenia. J. Lab. Clin. Med. 2001, 137, 231–243. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European Consensus on Definition and Diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic Signaling Deficits Underlie Amino Acid Resistance of Wasting, Aging Muscle. FASEB J. 2005, 19, 422–424. [Google Scholar] [CrossRef]
- Nordic Council of Ministers, N.C. of M. Nordic Nutrition Recommendations 2012. Nordic. Nutr. Recomm. 2008, 5, 1–3. [Google Scholar] [CrossRef]
- Breen, L.; Phillips, S.M. Skeletal Muscle Protein Metabolism in the Elderly: Interventions to Counteract the “anabolic Resistance” of Ageing. Nutr. Metab. 2011, 8, 68. [Google Scholar] [CrossRef]
- Yang, Y.; Breen, L.; Burd, N.A.; Hector, A.J.; Churchward-Venne, T.A.; Josse, A.R.; Tarnopolsky, M.A.; Phillips, S.M. Resistance Exercise Enhances Myofibrillar Protein Synthesis with Graded Intakes of Whey Protein in Older Men. Br. J. Nutr. 2012, 108, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Witard, O.C.; Jackman, S.R.; Breen, L.; Smith, K.; Selby, A.; Tipton, K.D. Myofibrillar Muscle Protein Synthesis Rates Subsequent to a Meal in Response to Increasing Doses of Whey Protein at Rest and after Resistance Exercise. Am. J. Clin. Nutr. 2014, 99, 86–95. [Google Scholar] [CrossRef]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein Ingestion to Stimulate Myofibrillar Protein Synthesis Requires Greater Relative Protein Intakes in Healthy Older versus Younger Men. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Borgonjen-Van den Berg, K.J.; Van Loon, L.J.C.; de Groot, L.C.P.G.M. Dietary Protein Intake in Dutch Elderly People: A Focus on Protein Sources. Nutrients 2015, 7, 9697–9706. [Google Scholar] [CrossRef] [PubMed]
- Farsijani, S.; Morais, J.A.; Payette, H.; Gaudreau, P.; Shatenstein, B.; Gray-Donald, K.; Chevalier, S. Relation between Mealtime Distribution of Protein Intake and Lean Mass Loss in Free-Living Older Adults of the NuAge Study. Am. J. Clin. Nutr. 2016, 104, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Nygård, L.K.; Dahl, L.; Mundal, I.; Šaltytė Benth, J.; Rokstad, A.M.M. Protein Intake, Protein Mealtime Distribution and Seafood Consumption in Elderly Norwegians: Associations with Physical Function and Strength. Geriatrics 2020, 5, 100. [Google Scholar] [CrossRef]
- Jespersen, S.E.; Agergaard, J. Evenness of Dietary Protein Distribution Is Associated with Higher Muscle Mass but Not Muscle Strength or Protein Turnover in Healthy Adults: A Systematic Review. Eur. J. Nutr. 2021, 60, 3185–3202. [Google Scholar] [CrossRef] [PubMed]
- Bollwein, J.; Diekmann, R.; Kaiser, M.J.; Bauer, J.M.; Uter, W.; Sieber, C.C.; Volkert, D. Distribution but Not Amount of Protein Intake Is Associated with Frailty: A Cross-Sectional Investigation in the Region of Nürnberg. Nutr. J. 2013, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Curis, E.; Hamon-Vilcot, B.; Nicolis, I.; Chrétien, P.; Schauer, N.; Vincent, J.-P.; Cynober, L.; Aussel, C. Impact of Protein Pulse Feeding on Lean Mass in Malnourished and At-Risk Hospitalized Elderly Patients: A Randomized Controlled Trial. Clin. Nutr. 2013, 32, 186–192. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Schutzler, S.; Schrader, A.; Spencer, H.; Kortebein, P.; Deutz, N.E.P.; Wolfe, R.R.; Ferrando, A.A. Quantity of Dietary Protein Intake, but Not Pattern of Intake, Affects Net Protein Balance Primarily through Differences in Protein Synthesis in Older Adults. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E21–E28. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Schutzler, S.; Schrader, A.M.; Spencer, H.J.; Azhar, G.; Wolfe, R.R.; Ferrando, A.A. Protein Intake Distribution Pattern Does Not Affect Anabolic Response, Lean Body Mass, Muscle Strength or Function over 8 Weeks in Older Adults: A Randomized-Controlled Trial. Clin. Nutr. 2018, 37, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Mamerow, M.M.; Mettler, J.A.; English, K.L.; Casperson, S.L.; Arentson-Lantz, E.; Sheffield-Moore, M.; Layman, D.K.; Paddon-Jones, D. Dietary Protein Distribution Positively Influences 24-h Muscle Protein Synthesis in Healthy Adults. J. Nutr. 2014, 144, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Heshka, S.; Gallagher, D.; Boozer, C.N.; Kotler, D.P.; Heymsfield, S.B. Resting Energy Expenditure-Fat-Free Mass Relationship: New Insights Provided by Body Composition Modeling. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E539–E545. [Google Scholar] [CrossRef]
- Reitelseder, S.; Tranberg, B.; Agergaard, J.; Dideriksen, K.; Højfeldt, G.; Merry, M.E.; Storm, A.C.; Poulsen, K.R.; Hansen, E.T.; van Hall, G.; et al. Phenylalanine Stable Isotope Tracer Labeling of Cow Milk and Meat and Human Experimental Applications to Study Dietary Protein-Derived Amino Acid Availability. Clin. Nutr. 2020, 39, 3652–3662. [Google Scholar] [CrossRef] [PubMed]
- Holm, L.; O’Rourke, B.; Ebenstein, D.; Toth, M.J.; Bechshoeft, R.; Holstein-Rathlou, N.-H.; Kjaer, M.; Matthews, D.E. Determination of Steady-State Protein Breakdown Rate in Vivo by the Disappearance of Protein-Bound Tracer-Labeled Amino Acids: A Method Applicable in Humans. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E895–E907. [Google Scholar] [CrossRef]
- Bornø, A.; van Hall, G. Quantitative Amino Acid Profiling and Stable Isotopically Labeled Amino Acid Tracer Enrichment Used for in Vivo Human Systemic and Tissue Kinetics Measurements. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 951–952, 69–77. [Google Scholar] [CrossRef]
- Bornø, A.; Hulston, C.J.; van Hall, G. Determination of Human Muscle Protein Fractional Synthesis Rate: An Evaluation of Different Mass Spectrometry Techniques and Considerations for Tracer Choice. J. Mass Spectrom. 2014, 49, 674–680. [Google Scholar] [CrossRef]
- Miller, B.F.; Reid, J.J.; Price, J.C.; Lin, H.J.L.; Atherton, P.J.; Smith, K. CORP: The Use of Deuterated Water for the Measurement of Protein Synthesis. J. Appl. Physiol. 2020, 128, 1163–1176. [Google Scholar] [CrossRef]
- Agergaard, J.; Hansen, E.T.; van Hall, G.; Holm, L. Postprandial Amino Acid Availability after Intake of Intact or Hydrolyzed Meat Protein in a Mixed Meal in Healthy Elderly Subjects: A Randomized, Single Blind Crossover Trial. Amino Acids 2021, 53, 951–959. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).