Lactic Acid Bacteria: A Promising Tool for Menopausal Health Management in Women
Abstract
:1. Introduction
2. Menopausal Symptoms
2.1. Anxiety and Depression
2.2. Urogenital Atrophy
2.3. Osteoporosis
2.4. Cognitive Disorders
2.5. Cardiovascular Risk
2.6. Metabolic Disorders
3. The Mainstream Method of Managing Menopause
3.1. HRT
3.2. Non-Hormonal Therapy
3.3. Non-Pharmaceutical Treatments
3.3.1. Phytoestrogens
3.3.2. Vitamin and Mineral Supplements
4. Health-Promoting Benefits and Clinical Implications of LAB in Menopausal Women
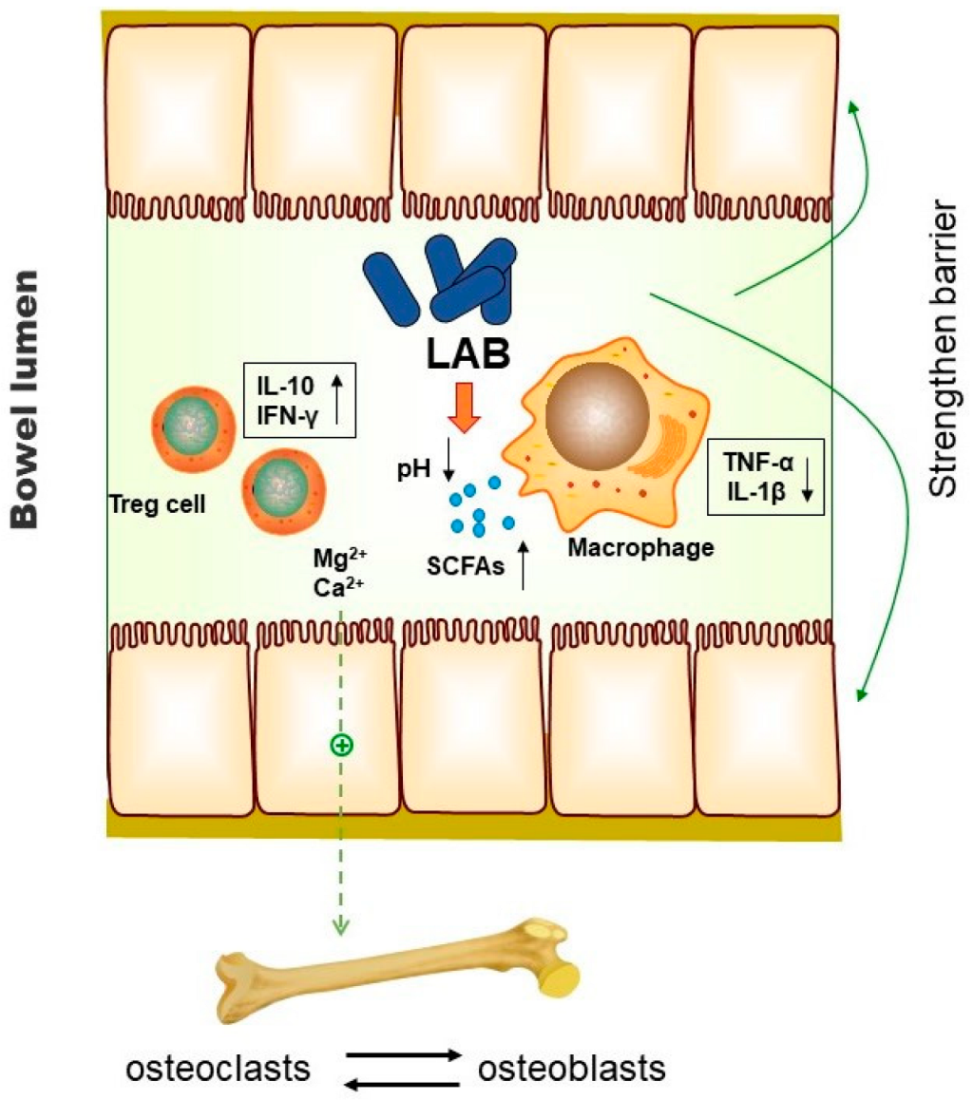
4.1. LAB for Healthy Ageing
4.2. Promoting Oestrogen Receptor Response
4.3. Role of LAB on Menopausal Osteoporosis
4.4. Prevention of Dyslipidaemia and Obesity by LAB
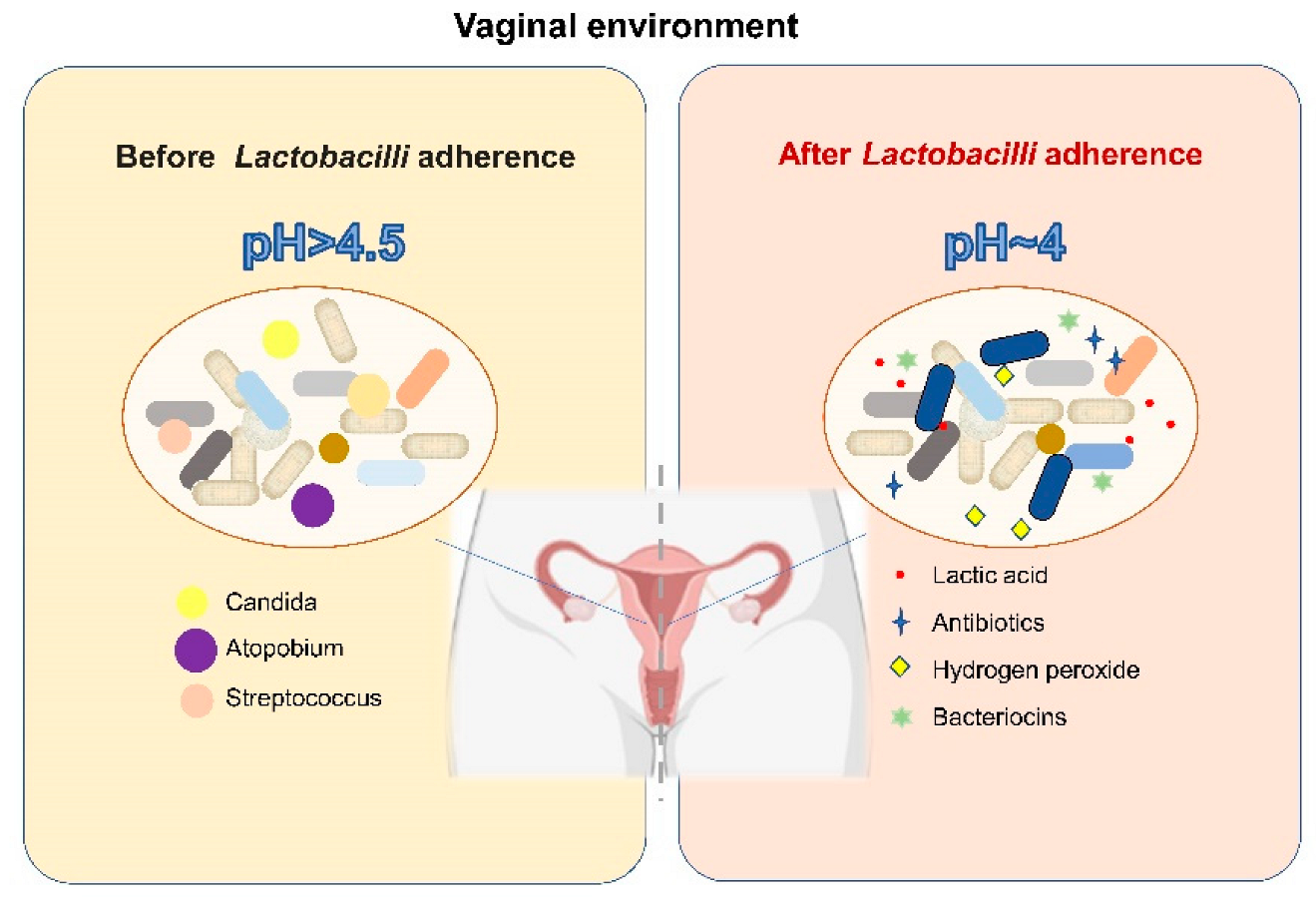
4.5. LAB and Their Influence on the Vaginal Microbiome
4.6. LAB and CNS-Related Symptoms
5. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Honour, J.W. Biochemistry of the menopause. Ann. Clin. Biochem. 2018, 55, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.A.; Gompel, A. Management of menopause: A view towards prevention. Lancet Diabetes Endocrinol. 2022, 10, 457–470. [Google Scholar] [CrossRef]
- Lee, P.-S.; Lee, C.-L. Prevalence of symptoms and associated factors across menopause status in Taiwanese women. Menopause 2020, 28, 182–188. [Google Scholar] [CrossRef]
- Monteleone, P.; Mascagni, G.; Giannini, A.; Genazzani, A.R.; Simoncini, T. Symptoms of menopause—Global prevalence, physiology and implications. Nat. Rev. Endocrinol. 2018, 14, 199–215. [Google Scholar] [CrossRef]
- Greendale, G.A.; Lee, N.P.; Arriola, E.R. The menopause. Lancet 1999, 353, 571–580. [Google Scholar] [CrossRef]
- Jaspers, L.; Daan, N.M.; van Dijk, G.M.; Gazibara, T.; Muka, T.; Wen, K.-X.; Meun, C.; Zillikens, M.C.; van Lennep, J.E.R.; Roos-Hesselink, J.W.; et al. Health in middle-aged and elderly women: A conceptual framework for healthy menopause. Maturitas 2015, 81, 93–98. [Google Scholar] [CrossRef]
- Mehta, J.; Kling, J.M.; Manson, J.E. Risks, benefits, and treatment modalities of menopausal hormone therapy: Current concepts. Front. Endocrinol. 2021, 12, 564781. [Google Scholar] [CrossRef]
- E Nappi, R.; Chedraui, P.; Lambrinoudaki, I.; Simoncini, T. Menopause: A cardiometabolic transition. Lancet Diabetes Endocrinol. 2022, 10, 442–456. [Google Scholar] [CrossRef]
- Stiles, M.E.; Holzapfel, W.H. Holzapfel, Lactic acid bacteria of foods and their current taxonomy. Int. J. Food Microbiol. 1997, 36, 1–29. [Google Scholar] [CrossRef]
- Garbacz, K. Anticancer activity of lactic acid bacteria. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Naumova, I.; Castelo-Branco, C. Current treatment options for postmenopausal vaginal atrophy. Int. J. Women’s Health 2018, 10, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosset, A.; Pouillès, J.-M.; Trémollieres, F. Menopausal hormone therapy for the management of osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101551. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.; Ashraf, A.; Zeynali, N.; Ebrahimi, B.; A Jehu, D. Balance and functional mobility predict low bone mineral density among postmenopausal women undergoing recent menopause with osteoporosis, osteopenia, and normal bone mineral density: A cross-sectional study. Geriatr. Nurs. 2021, 42, 33–36. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Greendale, G.A.; Karlamangla, A.S.; Maki, P.M. The menopause transition and cognition. JAMA 2020, 323, 1495–1496. [Google Scholar] [CrossRef]
- Andrew, M.K.; Tierney, M.C. The puzzle of sex, gender and Alzheimer’s disease: Why are women more often affected than men? Women’s Health 2018, 14, 1745506518817995. [Google Scholar] [CrossRef] [Green Version]
- El Khoudary, S.R. Age at menopause onset and risk of cardiovascular disease around the world. Maturitas 2020, 141, 33–38. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, D.; Wang, M.; Sun, J.-Y.; Liu, J.; Qi, Y.; Hao, Y.-C.; Deng, Q.-J.; Liu, J.; Liu, J.; et al. Combined effect of menopause and cardiovascular risk factors on death and cardiovascular disease: A cohort study. BMC Cardiovasc. Disord. 2021, 21, 109. [Google Scholar] [CrossRef]
- Razmjou, S.; Abdulnour, J.; Bastard, J.-P.; Fellahi, S.; Doucet, É.; Brochu, M.; Lavoie, J.-M.; Rabasa-Lhoret, R.; Prud’Homme, D. Body composition, cardiometabolic risk factors, physical activity, and inflammatory markers in premenopausal women after a 10-year follow-up: A MONET study. Menopause 2018, 25, 89–97. [Google Scholar] [CrossRef]
- Woodward, M. Cardiovascular disease and the female disadvantage. Int. J. Environ. Res. Public Health 2019, 16, 1165. [Google Scholar] [CrossRef]
- Slopien, R.; Wender-Ozegowska, E.; Rogowicz-Frontczak, A.; Meczekalski, B.; Zozulinska-Ziolkiewicz, D.; Jaremek, J.D.; Cano, A.; Chedraui, P.; Goulis, D.G.; Lopes, P.; et al. Menopause and diabetes: EMAS clinical guide. Maturitas 2018, 117, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meneses, M.J.; Silvestre, R.; Sousa-Lima, I.; Macedo, M.P. Paraoxonase-1 as a regulator of glucose and lipid homeostasis: Impact on the onset and progression of metabolic disorders. Int. J. Mol. Sci. 2019, 20, 4049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozakowski, J.; Gietka-Czernel, M.; Leszczyńska, D.; Majos, A. Obesity in menopause–our negligence or an unfortunate inevitability? Prz. Menopauzalny = Menopause Rev. 2017, 16, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Fait, T. Menopause hormone therapy: Latest developments and clinical practice. Drugs Context 2019, 8, 1–9. [Google Scholar] [CrossRef]
- Vigneswaran, K.; Hamoda, H. Hormone replacement therapy-current recommendations. Best Pract. Res. Clin. Obstet. Gynaecol. 2021. [Google Scholar] [CrossRef]
- Pan, M.; Pan, X.; Zhou, J.; Wang, J.; Qi, Q.; Wang, L. Update on hormone therapy for the management of postmenopausal women. BioScience. Trends 2022, 16, 46–57. [Google Scholar] [CrossRef]
- Chester, R.C.; Kling, J.M.; Manson, J.E. What the Women’s Health Initiative has taught us about menopausal hormone therapy. Clin. Cardiol. 2018, 41, 247–252. [Google Scholar]
- Papadakis, G.; Hans, D.; Gonzalez-Rodriguez, E.; Vollenweider, P.; Waeber, G.; Marques-Vidal, P.M.; Lamy, O. The benefit of menopausal hormone therapy on bone density and microarchitecture persists after its withdrawal. J. Clin. Endocrinol. Metab. 2016, 101, 5004–5011. [Google Scholar] [CrossRef] [Green Version]
- Del Río, J.P.; Molina, S.; Hidalgo-Lanussa, O.; Garcia-Segura, L.M.; Barreto, G.E. Tibolone as hormonal therapy and neuroprotective agent. Trends Endocrinol. Metab. 2020, 31, 742–759. [Google Scholar] [CrossRef]
- Pinkerton, J.V.; Conner, E.A. Beyond estrogen: Advances in tissue selective estrogen complexes and selective estrogen receptor modulators. Climacteric 2019, 22, 140–147. [Google Scholar] [CrossRef]
- Gómez-Coronado, D.; Lasunción, M.A.; Martínez-Botas, J.; Fernández-Suárez, M.E. Role of cholesterol metabolism in the anticancer pharmacology of selective estrogen receptor modulators. Semin. Cancer Biol. 2020, 73, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I. Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives. Eur. J. Med. Chem. 2018, 143, 515–531. [Google Scholar]
- Stubbs, C.; Mattingly, L.; A Crawford, S.; A Wickersham, E.; Brockhaus, J.L.; McCarthy, L.H. Do SSRIs and SNRIs reduce the frequency and/or severity of hot flashes in menopausal women. J. Okla. State Med Assoc. 2017, 110, 272–274. [Google Scholar] [PubMed]
- Gorzkiewicz, J.; Bartosz, G.; Sadowska-Bartosz, I. The Potential Effects of Phytoestrogens: The Role in Neuroprotection. Molecules 2021, 26, 2954. [Google Scholar] [CrossRef]
- Chen, L.-R.; Ko, N.-Y.; Chen, K.-H. Isoflavone Supplements for Menopausal Women: A Systematic Review. Nutrients 2019, 11, 2649. [Google Scholar] [CrossRef] [Green Version]
- Saghafi, N.; Ghazanfarpour, M.; Sadeghi, R.; Najarkolaei, A.H.; Omid, M.G.; Azad, A.; Najarkolaei, E.H. Effects of Phytoestrogens in Alleviating the Menopausal Symptoms: A Systematic Review and Meta-Analysis. IJPR 2017, 16, 99–111. [Google Scholar] [CrossRef]
- Vitale, S.G.; Caruso, S.; Rapisarda, A.M.C.; Cianci, S.; Cianci, A. Isoflavones, calcium, vitamin D and inulin improve quality of life, sexual function, body composition and metabolic parameters in menopausal women: Result from a prospective, randomized, placebo-controlled, parallel-group study. Menopausal Rev. 2018, 17, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Lobo, R.A. Menopause and aging. In Yen and Jaffe’s Reproductive Endocrinology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 322–356.e9. [Google Scholar]
- Wise, P.M.; Krajnak, K.M.; Kashon, M.L. Menopause: The aging of multiple pacemakers. Science 1996, 273, 67–70. [Google Scholar] [CrossRef]
- Fischer, V.; Haffner-Luntzer, M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Han, A.; Kim, J.Y.; Kwak-Kim, J.; Lee, S.K. Menopause is an inflection point of age-related immune changes in women. J. Reprod. Immunol. 2021, 146, 103346. [Google Scholar] [CrossRef]
- Levit, R.; De Giori, G.S.; Leblanc, A.D.M.D.; Leblanc, J.G. Folate-producing lactic acid bacteria reduce inflammation in mice with induced intestinal mucositis. J. Appl. Microbiol. 2018, 125, 1494–1501. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Shi, J.; Zhu, J.; Shao, D.; Huang, Q.; Yang, H.; Jin, M. Capacity of lactic acid bacteria in immunity enhancement and cancer prevention. Appl. Microbiol. Biotechnol. 2016, 101, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, Z.; Tian, P.; Wang, G. Lactic acid bacteria and host immunity. In Lactic Acid Bacteria; Springer: Berlin/Heidelberg, Germany, 2019; pp. 261–296. [Google Scholar]
- LeBlanc, J.G.; Levit, R.; de Giori, G.S.; LeBlanc, A.D.M.D. Application of vitamin-producing lactic acid bacteria to treat intestinal inflammatory diseases. Appl. Microbiol. Biotechnol. 2020, 104, 3331–3337. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, M.A.; Zacarías-Flores, M.; Arronte-Rosales, A.; Correa-Muñoz, E.; Mendoza-Núñez, V.M. Menopause as risk factor for oxidative stress. Menopause 2012, 19, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-C.; Kwong, H.K.; Chen, H.-Y.; Hsu, H.-Y.; Yu, S.-H.; Hsieh, C.-W.; Lin, H.-W.; Chu, Y.-L.; Cheng, K.-C. Enhanced antioxidant activity of Chenopodium formosanum Koidz. by lactic acid bacteria: Optimization of fermentation conditions. PLoS ONE 2021, 16, e0249250. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hwang, K.-T.; Chung, M.-Y.; Cho, D.-H.; Park, C.-S. Resistance of Lactobacillus casei KCTC 3260 to Reactive Oxygen Species (ROS): Role for a Metal Ion Chelating Effect. J. Food Sci. 2005, 70, m388–m391. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12. [Google Scholar] [CrossRef]
- Ni, Y.; Yang, X.; Zheng, L.; Wang, Z.; Wu, L.; Jiang, J.; Yang, T.; Ma, L.; Fu, Z. Lactobacillus and Bifidobacterium Improves Physiological Function and Cognitive Ability in Aged Mice by the Regulation of Gut Microbiota. Mol. Nutr. Food Res. 2019, 63, e1900603. [Google Scholar] [CrossRef]
- Sharma, R.; Kapila, R.; Kapasiya, M.; Saliganti, V.; Dass, G.; Kapila, S. Dietary supplementation of milk fermented with probiotic Lactobacillus fermentum enhances systemic immune response and antioxidant capacity in aging mice. Nutr. Res. 2014, 34, 968–981. [Google Scholar] [CrossRef]
- Pennell, L.M.; Galligan, C.L.; Fish, E.N. Sex affects immunity. J. Autoimmun. 2012, 38, J282–J291. [Google Scholar] [CrossRef]
- Moxley, G.; Posthuma, D.; Carlson, P.; Estrada, E.; Han, J.; Benson, L.L.; Neale, M.C. Sexual dimorphism in innate immunity. Arthritis Care Res. 2002, 46, 250–258. [Google Scholar] [CrossRef]
- Abbès, S.; Ben Salah-Abbès, J.; Jebali, R.; Ben Younes, R.; Oueslati, R. Interaction of aflatoxin B1 and fumonisin B1 in mice causes immunotoxicity and oxidative stress: Possible protective role using lactic acid bacteria. J. Immunotoxicol. 2015, 13, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, H.U. Effects of potential synbiotic interaction between Lactobacillus rhamnosus GG and salicylic acid on human colon and prostate cancer cells. Arch. Microbiol. 2021, 203, 1221–1229. [Google Scholar] [PubMed]
- Chen, Q.; Kong, Q.; Tian, P.; He, Y.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Lactic acid bacteria alleviate di-(2-ethylhexyl) phthalate-induced liver and testis toxicity via their bio-binding capacity, antioxidant capacity and regulation of the gut microbiota. Environ. Pollut. 2022, 305. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Zhang, Q.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. The synergistic effect of Lactobacillus plantarum CCFM242 and zinc on ulcerative colitis through modulating intestinal homeostasis. Food Funct. 2019, 10, 6147–6156. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, S.; Mei, C.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Capabilities of bio-binding, antioxidant and intestinal environmental repair jointly determine the ability of lactic acid bacteria to mitigate perfluorooctane sulfonate toxicity. Environ. Int. 2022, 166, 107388. [Google Scholar] [PubMed]
- Vaghef-Mehrabany, E.; Rad, A.H.; Alipour, B.; Sharif, S.; Vaghef-Mehrabany, L.; Alipour-Ajiry, S. Effects of Probiotic Supplementation on Oxidative Stress Indices in Women with Rheumatoid Arthritis: A Randomized Double-Blind Clinical Trial. J. Am. Coll. Nutr. 2015, 35, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, L.; Alipour, B.; Jafarabadi, M.A.; Behrooz, M.; Gargari, B.P. Daily consumption effects of probiotic yogurt containing Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 on oxidative stress in metabolic syndrome patients. Clin. Nutr. ESPEN 2020, 41, 136–142. [Google Scholar] [CrossRef]
- Mazloom, Z.; Yousefinejad, A.; Dabbaghmanesh, M.H. Effect of Probiotics on Lipid Profile, Glycemic Control, Insulin Action, Oxidative Stress, and Inflammatory Markers in Patients with Type 2 Diabetes: A Clinical Trial. Iran. J. Med Sci. 2013, 38, 38–43. [Google Scholar]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar]
- Hameed, A.S.S.; Rawat, P.S.; Meng, X.; Liu, W. Biotransformation of dietary phytoestrogens by gut microbes: A review on bidirectional interaction between phytoestrogen metabolism and gut microbiota. Biotechnol. Adv. 2020, 43, 107576. [Google Scholar] [CrossRef]
- Stojanov, S.; Kreft, S. Gut Microbiota and the Metabolism of Phytoestrogens. Rev. Bras. Farm. 2020, 30, 145–154. [Google Scholar] [CrossRef]
- Kwon, J.E.; Lim, J.; Kim, I.; Kim, D.; Kang, S.C. Isolation and identification of new bacterial stains producing equol from Pueraria lobata extract fermentation. PLoS ONE 2018, 13, e0192490. [Google Scholar] [CrossRef] [PubMed]
- Izaguirre, J.; Barañano, L.; Castañón, S.; Alkorta, I.; Quirós, L.; Garbisu, C. Optimization of the Bioactivation of Isoflavones in Soymilk by Lactic Acid Bacteria. Processes 2021, 9, 963. [Google Scholar] [CrossRef]
- Ribeiro, A.E.; Monteiro, N.E.S.; De Moraes, A.V.G.; Costa-Paiva, L.H.; Pedro, A.O. Can the use of probiotics in association with isoflavone improve the symptoms of genitourinary syndrome of menopause? Results from a randomized controlled trial. Menopause 2018, 26, 643–652. [Google Scholar] [CrossRef]
- Monteiro, N.E.S.; Queirós, L.D.; Lopes, D.B.; Pedro, A.O.; Macedo, G.A. Impact of microbiota on the use and effects of isoflavones in the relief of climacteric symptoms in menopausal women—A review. J. Funct. Foods 2018, 41, 100–111. [Google Scholar] [CrossRef]
- De Franciscis, P.; Grauso, F.; Luisi, A.; Schettino, M.T.; Torella, M.; Colacurci, N. Adding Agnus Castus and Magnolia to Soy Isoflavones Relieves Sleep Disturbances Besides Postmenopausal Vasomotor Symptoms-Long Term Safety and Effectiveness. Nutrients 2017, 9, 129. [Google Scholar] [CrossRef] [Green Version]
- Desfita, S.; Sari, W.; Yusmarini, Y.; Pato, U.; Zakłos-Szyda, M.; Budryn, G. Effect of Fermented Soymilk-Honey from Different Probiotics on Osteocalcin Level in Menopausal Women. Nutrients 2021, 13, 3581. [Google Scholar] [CrossRef]
- Łaniewski, P.; Herbst-Kralovetz, M.M. Connecting microbiome and menopause for healthy ageing. Nat. Microbiol. 2022, 7, 354–358. [Google Scholar] [CrossRef]
- He, Y.; Wang, Q.; Li, X.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Lactic acid bacteria alleviate polycystic ovarian syndrome by regulating sex hormone related gut microbiota. Food Funct. 2020, 11, 5192–5204. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Z.; Jiang, S.; Bai, X.; Ma, C.; Peng, Q.; Chen, K.; Chang, H.; Fang, T.; Zhang, H. Probiotic Bifidobacterium lactis V9 Regulates the Secretion of Sex Hormones in Polycystic Ovary Syndrome Patients through the Gut-Brain Axis. mSystems 2019, 4, e00017-19. [Google Scholar] [CrossRef] [Green Version]
- Poutahidis, T.; Springer, A.D.; Levkovich, T.; Qi, P.; Varian, B.J.; Lakritz, J.; Ibrahim, Y.M.; Chatzigiagkos, A.; Alm, E.J.; Erdman, S.E. Probiotic Microbes Sustain Youthful Serum Testosterone Levels and Testicular Size in Aging Mice. PLoS ONE 2014, 9, e84877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdman, S.; Poutahidis, T. Probiotic ‘glow of health’: It’s more than skin deep. Benef. Microbes 2014, 5, 109. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, B.; Wang, S.; Qian, X.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Modulation of the Gut Microbiota Structure with Probiotics and Isoflavone Alleviates Metabolic Disorder in Ovariectomized Mice. Nutrients 2021, 13, 1793. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.-H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z. Ovarian aging and osteoporosis. Aging Aging-Relat. Dis. 2018, 199–215. [Google Scholar]
- Binda, S.; Ouwehand, A.C. Chapter 12: Lactic Acid Bacteria for Fermented Dairy Products. In Lactic Acid Bacteria: Microbiological and Functional Aspects; CRC Press: Boca Raton, FL, USA, 2019; pp. 175–198. [Google Scholar]
- Campbell, J.M.; Fahey, J.G.C.; Wolf, B.W. Selected Indigestible Oligosaccharides Affect Large Bowel Mass, Cecal and Fecal Short-Chain Fatty Acids, pH and Microflora in Rats. J. Nutr. 1997, 127, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Matar, C.; Amiot, J.; Savoie, L.; Goulet, J. The Effect of Milk Fermentation by Lactobacillus helveticus on the Release of Peptides During In Vitro Digestion. J. Dairy Sci. 1996, 79, 971–979. [Google Scholar] [CrossRef]
- Jafarnejad, S.; Djafarian, K.; Fazeli, M.R.; Yekaninejad, M.S.; Rostamian, A.; Keshavarz, S.A. Effects of a Multispecies Probiotic Supplement on Bone Health in Osteopenic Postmenopausal Women: A Randomized, Double-blind, Controlled Trial. J. Am. Coll. Nutr. 2017, 36, 497–506. [Google Scholar] [CrossRef]
- Ohlsson, C.; Engdahl, C.; Fåk, F.; Andersson, A.; Windahl, S.H.; Farman, H.H.; Movérare-Skrtic, S.; Islander, U.; Sjögren, K. Probiotics Protect Mice from Ovariectomy-Induced Cortical Bone Loss. PLoS ONE 2014, 9, e92368. [Google Scholar] [CrossRef]
- Dar, H.Y.; Shukla, P.; Mishra, P.K.; Anupam, R.; Mondal, R.; Tomar, G.B.; Sharma, V.; Srivastava, R.K. Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating Treg-Th17 cell balance. Bone Rep. 2018, 8, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.L.; Rios-Arce, N.D.; Atkinson, S.; Bierhalter, H.; Schoenherr, D.; Bazil, J.N.; McCabe, L.R.; Parameswaran, N. Temporal and regional intestinal changes in permeability, tight junction, and cytokine gene expression following ovariectomy-induced estrogen deficiency. Physiol. Rep. 2017, 5, e13263. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhang, Q.; de Haan, B.J.; Faas, M.M.; Zhang, H.; de Vos, P. Protective effects of lactic acid bacteria on gut epithelial barrier dysfunction are Toll like receptor 2 and protein kinase C dependent. Food Funct. 2020, 11, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency–associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansson, P.-A.; Curiac, D.; Ahrén, I.L.; Hansson, F.; Niskanen, T.M.; Sjögren, K.; Ohlsson, C. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019, 1, e154–e162. [Google Scholar] [CrossRef]
- Chen, C.; Dong, B.; Wang, Y.; Zhang, Q.; Wang, B.; Feng, S.; Zhu, Y. The role of Bacillus acidophilus in osteoporosis and its roles in osteocyte proliferation and differentiation. J. Clin. Lab. Anal. 2019, 34, e23471. [Google Scholar]
- Kim, D.E.; Kim, J.K.; Han, S.K.; Jang, S.E.; Han, M.J.; Kim, D.H. Lactobacillus plantarum NK3 and Bifidobacterium longum NK49 alleviate bacterial vaginosis and osteoporosis in mice by suppressing NF-κ B-Linked TNF-α expression. J. Med. Food 2019, 22, 1022–1031. [Google Scholar] [CrossRef]
- Nilsson, A.G.; Sundh, D.; Bäckhed, F.; Lorentzon, M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: A randomized, placebo-controlled, double-blind, clinical trial. J. Intern. Med. 2018, 284, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Belguesmia, Y.; Domenger, D.; Caron, J.; Dhulster, P.; Ravallec, R.; Drider, D.; Cudennec, B. Novel probiotic evidence of lactobacilli on immunomodulation and regulation of satiety hormones release in intestinal cells. J. Funct. Foods 2016, 24, 276–286. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Yi, S.; Li, X.; Guo, Z.; Zhou, X.; Mu, J.; Yi, R. Lactobacillus plantarum CQPC02 prevents obesity in mice through the PPAR-α signaling pathway. Biomolecules 2019, 9, 407. [Google Scholar]
- Long, X.; Zeng, X.; Tan, F.; Yi, R.; Pan, Y.; Zhou, X.; Mu, J.; Zhao, X. Lactobacillus plantarum KFY04 prevents obesity in mice through the PPAR pathway and alleviates oxidative damage and inflammation. Food Funct. 2020, 11, 5460–5472. [Google Scholar] [CrossRef] [PubMed]
- Kallus, S.J.; Brandt, L.J. The Intestinal Microbiota and Obesity. J. Clin. Gastroenterol. 2012, 46, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.-Y.; Zhao, C.-N.; Xu, X.-Y.; Tang, G.-Y.; Corke, H.; Gan, R.-Y.; Li, H.-B. Dietary plants, gut microbiota, and obesity: Effects and mechanisms. Trends Food Sci. Technol. 2019, 92, 194–204. [Google Scholar] [CrossRef]
- Mazloom, K.; Siddiqi, I.; Covasa, M. Probiotics: How effective are they in the fight against obesity? Nutrients 2019, 11, 258. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-H.; Kim, H.; Jeong, D.; Kang, I.-B.; Chon, J.-W.; Kim, H.-S.; Song, K.-Y.; Seo, K.-H. Kefir alleviates obesity and hepatic steatosis in high-fat diet-fed mice by modulation of gut microbiota and mycobiota: Targeted and untargeted community analysis with correlation of biomarkers. J. Nutr. Biochem. 2017, 44, 35–43. [Google Scholar] [CrossRef]
- Lim, E.; Song, E.-J.; Kim, J.; Jung, S.; Lee, S.-Y.; Shin, H.; Nam, Y.-D.; Kim, Y. Lactobacillus intestinalis YT2 restores the gut microbiota and improves menopausal symptoms in ovariectomized rats. Benef. Microbes 2021, 12, 503–516. [Google Scholar] [CrossRef]
- Szulińska, M.; Łoniewski, I.; van Hemert, S.; Sobieska, M.; Bogdański, P. Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. Nutrients 2018, 10, 773. [Google Scholar] [CrossRef] [Green Version]
- Szulińska, M.; Łoniewski, I.; Skrypnik, K.; Sobieska, M.; Korybalska, K.; Suliburska, J.; Bogdański, P. Multispecies Probiotic Supplementation Favorably Affects Vascular Function and Reduces Arterial Stiffness in Obese Postmenopausal Women—A 12-Week Placebo-Controlled and Randomized Clinical Study. Nutrients 2018, 10, 1672. [Google Scholar] [CrossRef]
- Zarezadeh, M.; Musazadeh, V.; Faghfouri, A.H.; Sarmadi, B.; Jamilian, P.; Jamilian, P.; Tutunchi, H.; Dehghan, P. Probiotic therapy, a novel and efficient adjuvant approach to improve glycemic status: An umbrella meta-analysis. Pharmacol. Res. 2022. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Srinivasan, S.; Zhan, X.; Wu, M.C.; Reed, S.; Guthrie, K.A.; LaCroix, A.Z.; Fiedler, T.; Munch, M.; Liu, C.; et al. Vaginal microbiota and genitourinary menopausal symptoms: A cross-sectional analysis. Menopause 2017, 24, 1160–1166. [Google Scholar] [CrossRef]
- Kumar, N.; Behera, B.; Sagiri, S.S.; Pal, K.; Ray, S.S.; Roy, S. Bacterial vaginosis: Etiology and modalities of treatment-A brief note. J. Pharm. Bioallied Sci. 2011, 3, 496. [Google Scholar] [PubMed]
- Selle, K.; Klaenhammer, T.R. Genomic and phenotypic evidence for probiotic influences of Lactobacillus gasseri on human health. FEMS Microbiol. Rev. 2013, 37, 915–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillier, S.L.; Krohn, M.A.; Rabe, L.K.; Klebanoff, S.J.; Eschenbach, D.A. The Normal Vaginal Flora, H2O2-Producing Lactobacilli, and Bacterial Vaginosis in Pregnant Women. Clin. Infect. Dis. 1993, 16 (Suppl. 4), S273–S281. [Google Scholar] [CrossRef] [PubMed]
- Vallor, A.C.; Antonio, M.A.D.; Hawes, S.E.; Hillier, S.L. Factors Associated with Acquisition of, or Persistent Colonization by, Vaginal Lactobacilli: Role of Hydrogen Peroxide Production. J. Infect. Dis. 2001, 184, 1431–1436. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.; Forney, L.J.; Ravel, J. Vaginal Microbiome: Rethinking Health and Disease. Annu. Rev. Microbiol. 2012, 66, 371–389. [Google Scholar] [CrossRef] [Green Version]
- McMillan, A.; Dell, M.; Zellar, M.P.; Cribby, S.; Martz, S.; Hong, E.; Fu, J.; Abbas, A.; Dang, T.; Miller, W.; et al. Disruption of urogenital biofilms by lactobacilli. Colloids Surf. B Biointerfaces 2011, 86, 58–64. [Google Scholar] [CrossRef]
- Bisanz, J.E.; Seney, S.; McMillan, A.; Vongsa, R.; Koenig, D.; Wong, L.; Dvoracek, B.; Gloor, G.; Sumarah, M.; Ford, B.; et al. A Systems Biology Approach Investigating the Effect of Probiotics on the Vaginal Microbiome and Host Responses in a Double Blind, Placebo-Controlled Clinical Trial of Post-Menopausal Women. PLoS ONE 2014, 9, e104511. [Google Scholar] [CrossRef] [Green Version]
- Özkinay, E.; Terek, M.C.; Yayci, M.; Kaiser, R.; Grob, P.; Tuncay, G. The effectiveness of live lactobacilli in combination with low dose oestriol (Gynoflor) to restore the vaginal flora after treatment of vaginal infections. BJOG Int. J. Obstet. Gynaecol. 2005, 112, 234–240. [Google Scholar] [CrossRef]
- Uehara, S.; Monden, K.; Nomoto, K.; Seno, Y.; Kariyama, R.; Kumon, H. A pilot study evaluating the safety and effectiveness of Lactobacillus vaginal suppositories in patients with recurrent urinary tract infection. Int. J. Antimicrob. Agents 2006, 28, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Capobianco, G.; Wenger, J.M.; Meloni, G.B.; Dessole, M.; Cherchi, P.; Dessole, S. Triple therapy with Lactobacilli acidophili, estriol plus pelvic floor rehabilitation for symptoms of urogenital aging in postmenopausal women. Arch. Gynecol. Obstet. 2013, 289, 601–608. [Google Scholar] [CrossRef]
- Visñuk, D.P.; de Giori, G.S.; LeBlanc, J.G.; de LeBlanc AD, M. Neuroprotective effects associated with immune modulation by selected lactic acid bacteria in a Parkinson’s disease model. Nutrition 2020, 79, 110995. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, P.; Dang, H. Lactic acid bacteria and γ-aminobutyric acid and diacetyl. In Lactic Acid Bacteria; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–19. [Google Scholar]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health Benefits of Lactobacillus gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.-I.; Wu, C.-C.; Tsai, P.-J.; Cheng, L.-H.; Hsu, C.-C.; Shan, I.-K.; Chan, P.-Y.; Lin, T.-W.; Ko, C.-J.; Chen, W.-L.; et al. Psychobiotic Supplementation of PS128TM Improves Stress, Anxiety, and Insomnia in Highly Stressed Information Technology Specialists: A Pilot Study. Front. Nutr. 2021, 8, 614105. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.S.; Chang, H.C.; Weng, Y.H.; Chen, C.C.; Kuo, Y.S.; Tsai, Y.C. The add-on effect of Lactobacillus plantarum PS128 in patients with Parkinson’s disease: A pilot study. Front. Nutr. 2021, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Chen, J.L.; Liao, J.F.; Chen, Y.H.; Chieu, M.W.; Ke, Y.Y.; Hsu, C.-C.; Tsai, Y.-J.; Hsieh-Li, H.M. Lactobacillus plantarum PS128 prevents cognitive dysfunction in Alzheimer’s disease mice by modulating propionic acid levels, glycogen synthase kinase 3 beta activity, and gliosis. BMC Complement. Med. Ther. 2021, 21, 259. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2021, 100, 233–241. [Google Scholar] [CrossRef]
- Zhu, G.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Administration of Bifidobacterium breve Improves the Brain Function of Aβ1-42-Treated Mice via the Modulation of the Gut Microbiome. Nutrients 2021, 13, 1602. [Google Scholar] [CrossRef]
| LAB Strain | Preclinical or Clinical Trials | Subjects | Sources of Oxidative Stress | Antioxidant Effects |
|---|---|---|---|---|
| L. paracasei BEJ01 | Preclinical trials | Mice | Aflatoxin B1 and fumonisin B1 | Reduced GSH concentration and inhibited expression of GPx and SOD [55] |
| L. rhamnosus GG | Preclinical trials | Mice | Colon and prostate cancer | Enhanced DPPH scavenging activity in colon and prostate cancer cells [56] |
| L. lactis subsp. lactis CCFM1018 | Preclinical trials | Rats | DEHP | Inhibited the level of MDA and enhanced the level of CAT in DEHP-exposed rats [57] |
| Lpb. plantarum CCFM242 | Preclinical trials | Mice | Ulcerative colitis | Decreased MDA content and increased the content of GSH, GPx, SOD, and CAT in the gut [58] |
| P. pentosaceus B19 | Preclinical trials | Mice | PFOS | Enhanced GSH activity in the liver [59] |
| L. casei 01 | Clinical trials | RA patients | RA | Decreased SOD and GPx activity [60] |
| L. acidophilus La5 and B. lactis Bb12 | Clinical trials | MetS patients | MetS | Significantly increased the level of TAC [61] |
| L. acidophilus, L. bulgaricus, L. bifidum, and L. casei. | Clinical trials | Type 2 diabetes patients | Type 2 diabetes | Reduced MDA levels [62] |
| LAB Strain | Preclinical or Clinical Trials | Subjects | Duration | Anti-Osteoporosis Results |
|---|---|---|---|---|
| L. acidophilus | Preclinical trials (in vitro) | MC3T3-E1 cells and RAW264.7 cells | - | Increased the number of cells in osteoblasts [90] |
| Lpb. plantarum NK3 | Preclinical trials (in vivo) | Ovariectomized mice | 4 weeks | Increased blood calcium, phosphorus, and osteocalcin levels [91] |
| L. acidophilus ATCC 4356 | Preclinical trials (in vivo) | Ovariectomized mice | 6 weeks | Strengthened both trabecular and cortical bone microstructure along with improved mineral density and heterogeneity of bones [85] |
| L. reuteri ATCC PTA 6475 | Clinical trials (in vivo) | Elderly women with osteopenia | 12 months | The loss in total VBMD of the distal tibia in women taking L. Reuteri 6475 was nearly half that of women taking placebo [92] |
| L. paracasei DSM 13434, Lpb. plantarum DSM 15312 and Lpb. plantarum DSM 15313 | Clinical trials (in vivo) | Early postmenopausal women (lumbar spine: T score > −2.5) | 12 months | Compared with placebo, LAB treatment reduced the LS-BMD loss [89] |
| Multispecies probiotic (Gerilact capsule) | Clinical trials (in vivo) | Postmenopausal women with mild bone loss | 6 months | Decreased BALP and CTX levels by multispecies probiotic supplementation in comparison with the control group [83] |
| Symptoms | Bacterial Strains | Subjects | Therapeutic Effects |
|---|---|---|---|
| Stress-related symptoms: anxiety, fatigue, sleep disturbance | L. gasseri CP2305 | Healthy young adults | Reduced anxiety and fatigue and improved sleep quality, including shortened sleep latency and increased sleep duration [117] |
| Stress-related symptoms: anxiety, depression, sleep disturbance; Parkinson’s disease (PD); Alzheimer’s disease (AD) | Lpb. plantarum PS128TM | IT specialists PD patients AD mice | 1. Apparently improved job and life satisfaction among IT specialists [118] 2. Lpb. plantarum PS128TM supplementation while continuously taking antiparkinsonian medicines could improve the motor ability of PD patients, inhibit the development of the disease, and improve the quality of life [119] 3. Effectively prevented damage in 3 × Tg-AD mice by ventricular injection of streptozotocin [120] |
| Depression disorder; Alzheimer’s disease | B. breve CCFM1025 | Major depressive disorder patients AD mice | 1. Significantly alleviated the psychiatric and gastrointestinal disorders of major depression disorder patients [121] 2. Significant improvement in cognitive impairment and neuroinflammation [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Wang, H.; Wang, G.; Zhao, J.; Chen, H.; Lu, X.; Chen, W. Lactic Acid Bacteria: A Promising Tool for Menopausal Health Management in Women. Nutrients 2022, 14, 4466. https://doi.org/10.3390/nu14214466
Chen Q, Wang H, Wang G, Zhao J, Chen H, Lu X, Chen W. Lactic Acid Bacteria: A Promising Tool for Menopausal Health Management in Women. Nutrients. 2022; 14(21):4466. https://doi.org/10.3390/nu14214466
Chicago/Turabian StyleChen, Qian, Haojue Wang, Gang Wang, Jianxin Zhao, Haiqin Chen, Xianyi Lu, and Wei Chen. 2022. "Lactic Acid Bacteria: A Promising Tool for Menopausal Health Management in Women" Nutrients 14, no. 21: 4466. https://doi.org/10.3390/nu14214466
APA StyleChen, Q., Wang, H., Wang, G., Zhao, J., Chen, H., Lu, X., & Chen, W. (2022). Lactic Acid Bacteria: A Promising Tool for Menopausal Health Management in Women. Nutrients, 14(21), 4466. https://doi.org/10.3390/nu14214466