The Effects of Nettle Extract Consumption on Liver PPARs, SIRT1, ACOX1 and Blood Lipid Levels in Male and Female C57Bl6 Mice
Abstract
1. Introduction
2. Methods and Materials
2.1. Experimental Animals and Treatment and Experimental Design
2.2. Blood and Liver Tissue Preparation
2.3. Liver PPAR-α, PPAR-γ, PGC1-α, ACOX1, SIRT1 Assays
2.4. Serum Lipids and Serum Biochemistry
2.5. Liver Oxidative Stress Analysis
2.6. Body Weight and Relative Mass of Liver (the Liver Organosomatic Index)
2.7. Serum Liver Markers
2.8. Statistical Analysis
3. Results
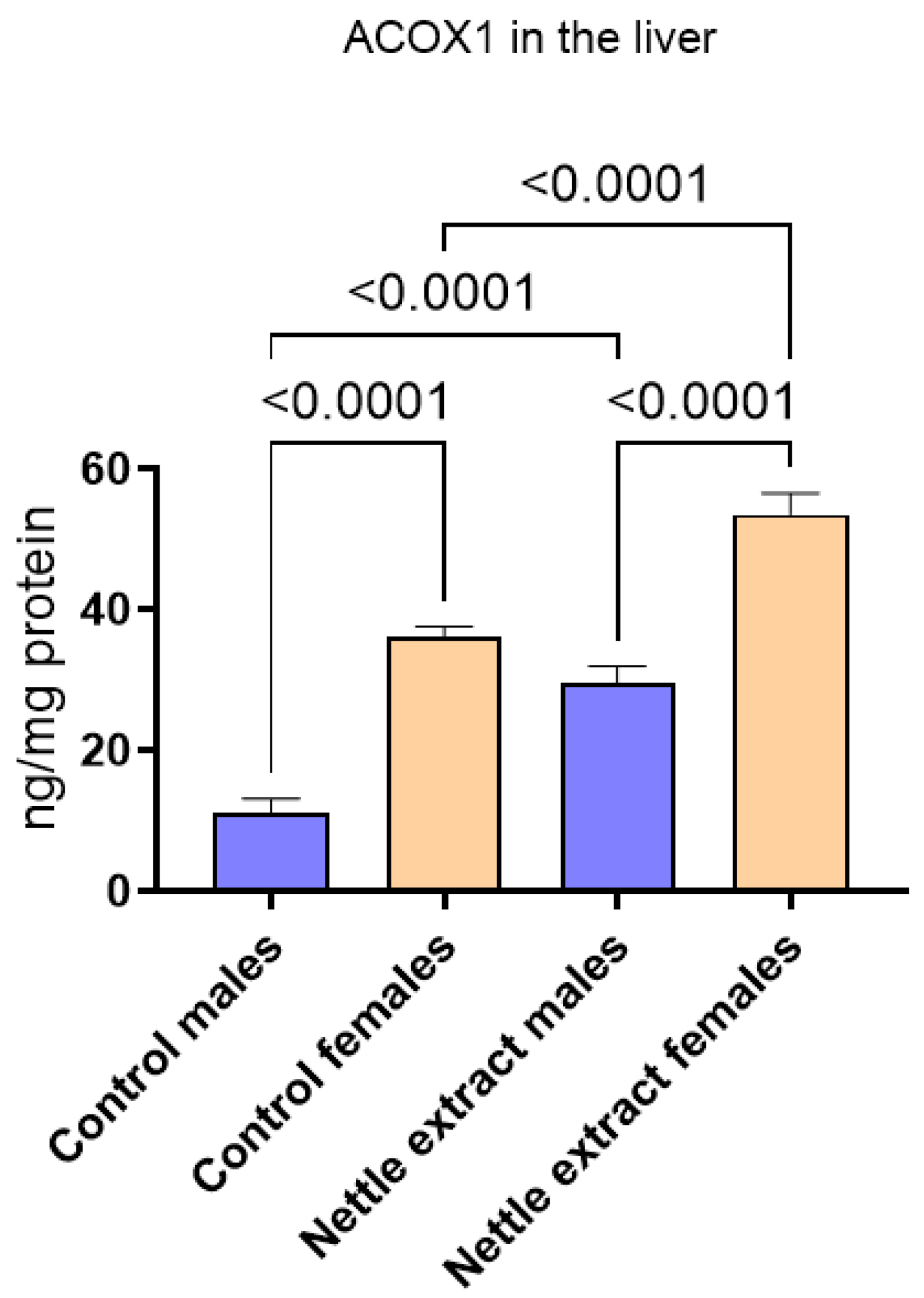
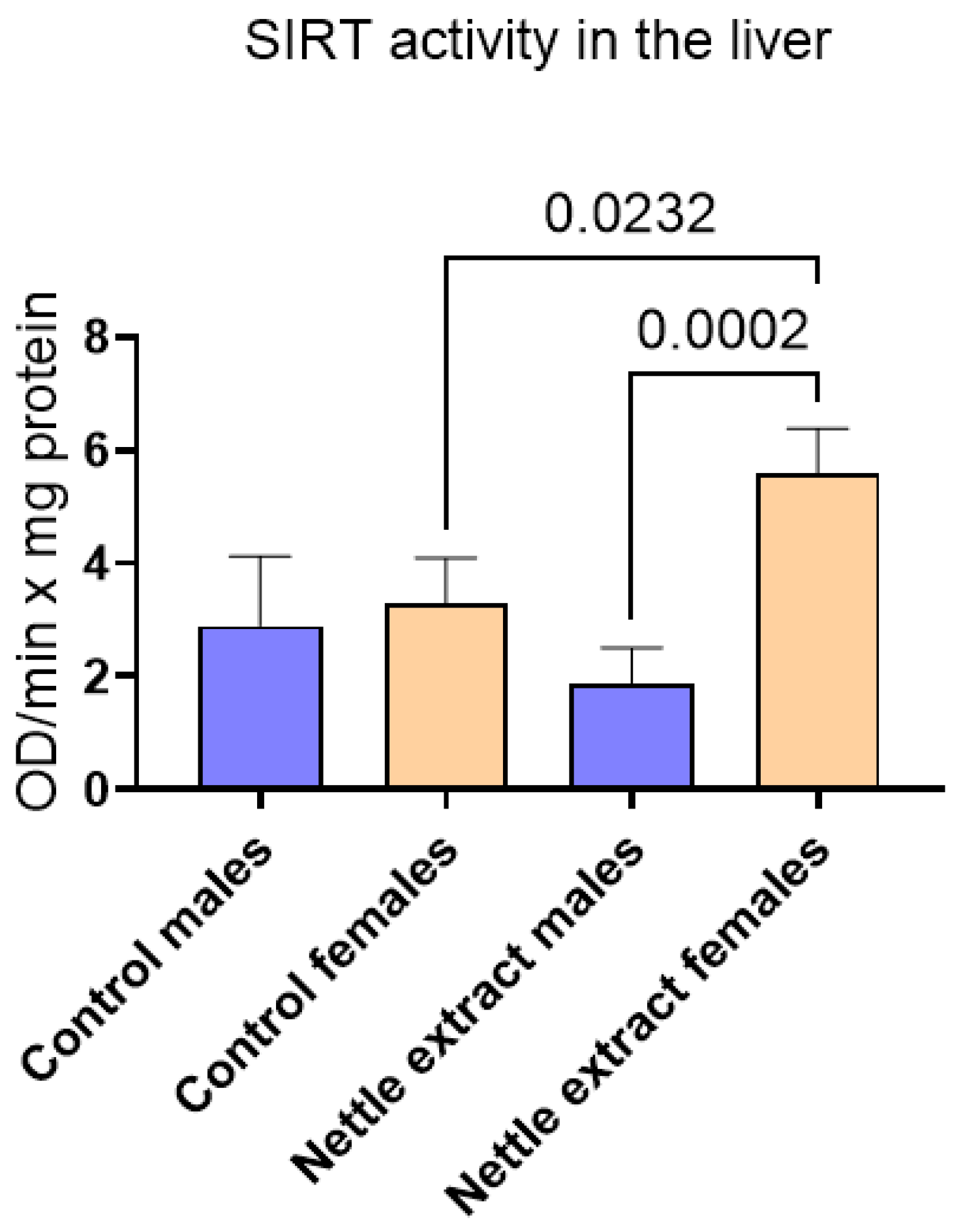
3.1. The Effects of Nettle Extract Consumption on Transcription Factors Regulators (PPARs), ACOX1 and SIRT1
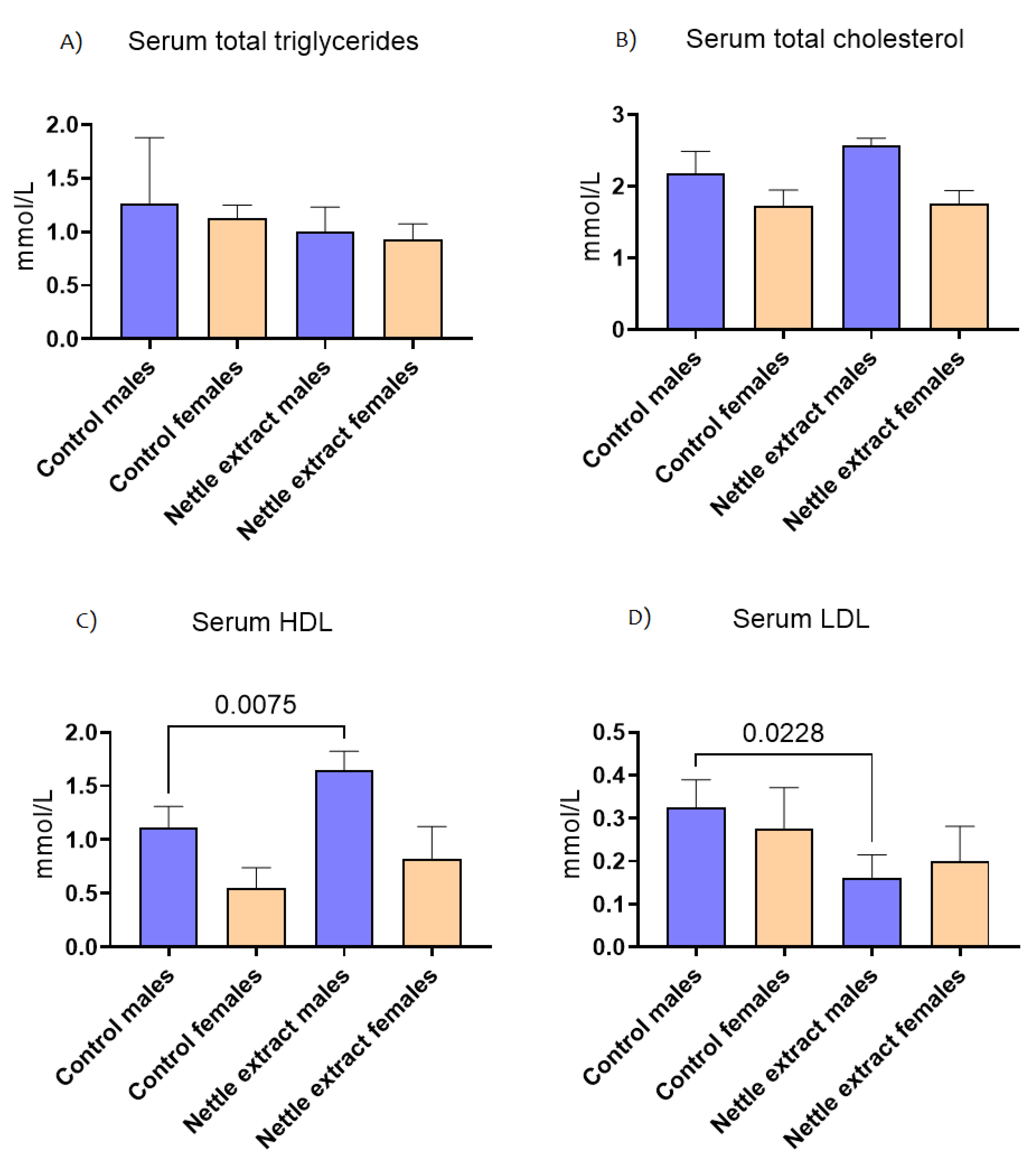
3.2. The Effects of Nettle Extract Consumption on Blood Lipid Levels (Triglycerides, Total Cholesterol, HDL and LDL) and Blood Biochemistry Parameters
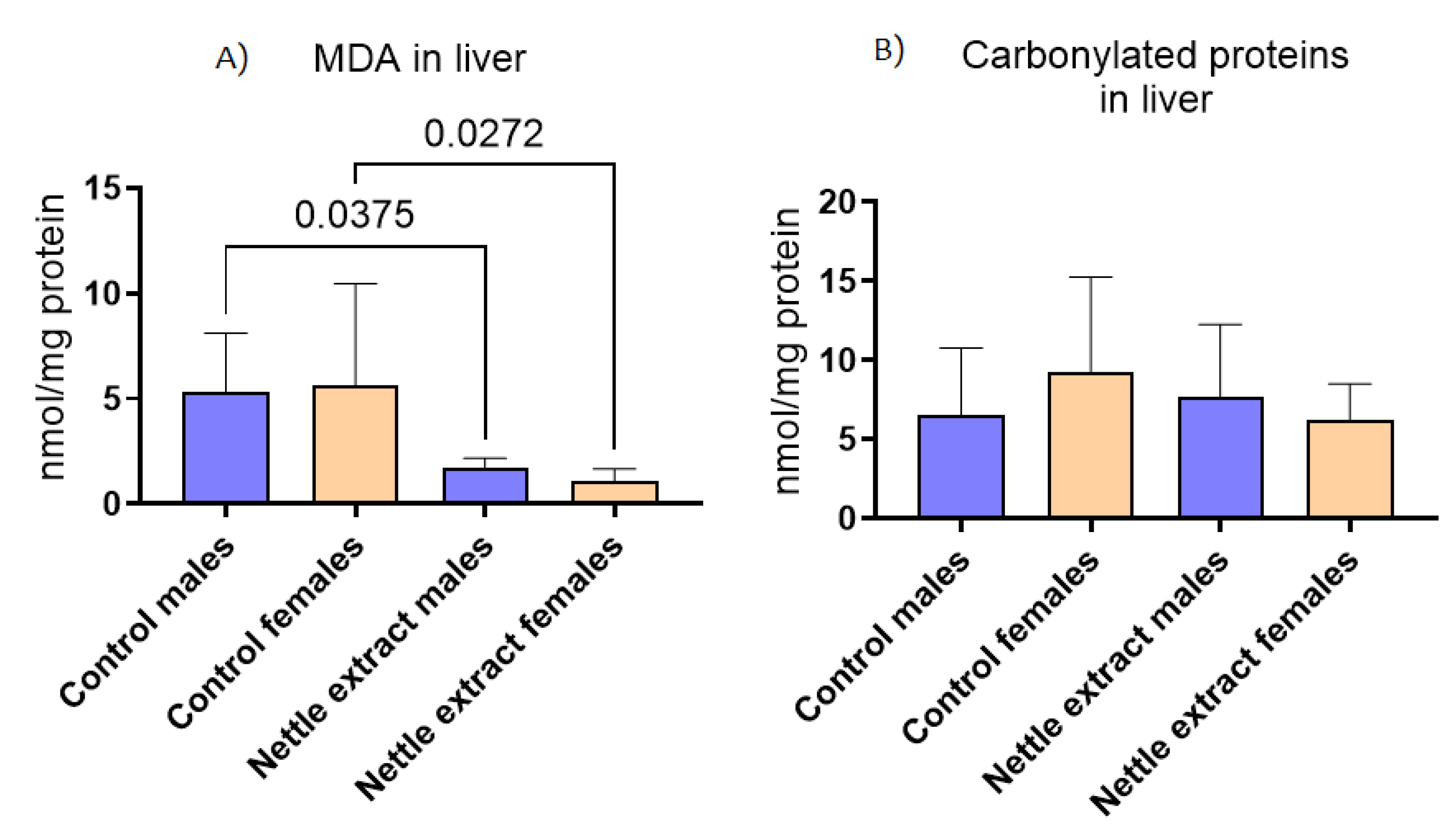
3.3. The Effects of Nettle Extract on Major Antioxidative Defense Markers in Liver Tissue
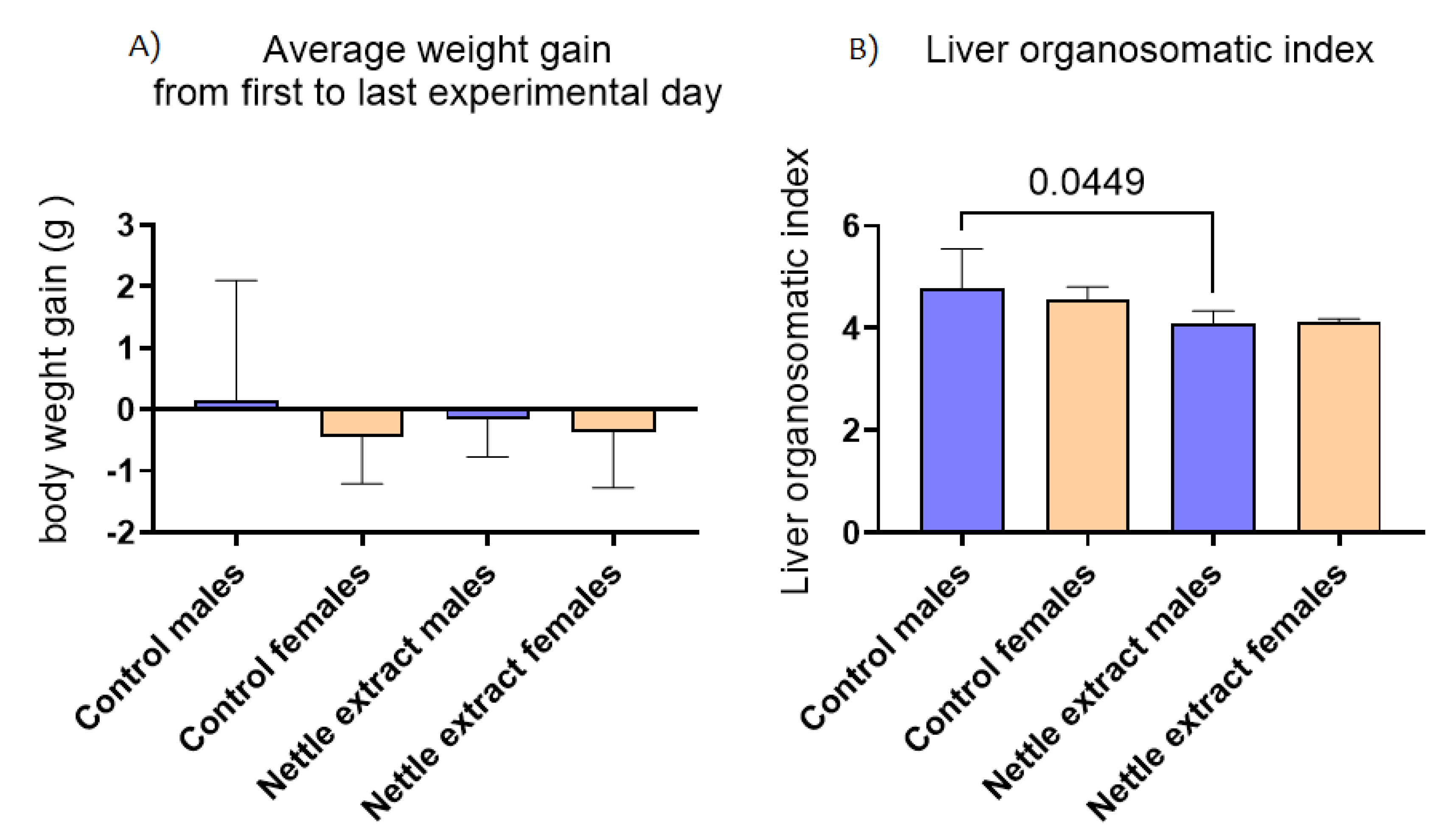
3.4. The Change in Body Mass and Orgonasomatic Index of the Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Samakar, B.; Mehri, S.; Hosseinzadeh, H. A review of the effects of Urtica dioica (nettle) in metabolic syndrome. Iran J. Basic Med. Sci. 2022, 25, 543–553. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Mahdian, D.; Hosseinzadeh, H. Medicinal plants in treatment of hypertriglyceridemia: A review based on their mechanisms and effectiveness. Phytomedicine 2019, 53, 43–52. [Google Scholar] [CrossRef]
- Payab, M.; Hasani-Ranjbar, S.; Shahbal, N.; Qorbani, M.; Aletaha, A.; Haghi-Aminjan, H.; Soltani, A.; Khatami, F.; Nikfar, S.; Hassani, S.; et al. Effect of the herbal medicines in obesity and metabolic syndrome: A systematic review and meta-analysis of clinical trials. Phytother. Res. 2020, 34, 526–545. [Google Scholar] [CrossRef]
- Ta Tahri, A.; Yamani, S.; Legssyer, A.; Aziz, M.; Mekhfi, H.; Bnouham, M.; Ziyyat, A. Acute diuretic, natriuretic and hypotensive effects of a continuous perfusion of aqueous extract of Urtica dioca in the rat. J. Ethnopharmacol. 2000, 73, 95–100. [Google Scholar] [CrossRef]
- Qayyum, R.; Qamar, H.M.U.; Khan, S.; Salma, U.; Khan, T.; Shah, A.J. Mechanisms underlying the antihypertensive properties of Urtica dioica. J. Transl. Med. 2016, 14, 254. [Google Scholar] [CrossRef]
- Ghalavand, A.; Motamedi, P.; Deleramnasab, M.; Khodadoust, M. The effect of interval training and nettle supplement on glycemic control and blood pressure in men with type 2 diabetes. Int. J. Basic Sci. Med. 2017, 2, 33–40. [Google Scholar] [CrossRef][Green Version]
- Vajic, U.J.; Grujic-Milanovic, J.; Miloradovic, Z.; Jovovic, D.; Ivanov, M.; Karanovic, D.; Savikin, K.; Bugarski, B.; Mihailovic-Stanojevic, N. Urtica dioica L. leaf extract modulates blood pressure and oxidative stress in spontaneously hypertensive rats. Phytomedicine 2018, 46, 39–45. [Google Scholar] [CrossRef]
- Petlevski, R.; Hadžija, M.; Slijepčević, M.; Juretić, D. Effect of ‘antidiabetis’ herbal preparation on serum glucose and fructosamine in NOD mice. J. Ethnopharmacol. 2001, 75, 181–184. [Google Scholar] [CrossRef]
- Golalipour, M.J.; Ghafari, S.; Kouri, V.; Kestkar, A.A. Proliferation of the β -cells of pancreas in diabetic rats treated with Urtica dioica. Int. J. Morphol. 2010, 28, 399–404. [Google Scholar] [CrossRef]
- Sahraki, M.R.; Mirshekari, H.; Sahraki, A.R.; Shafighi, E. Effect of Urtica dioica decoction on serum glucose and lipid profile in streptozotocin induced diabetic male rats. Zahedan J. Res. Med. Sci. 2013, 15, 15–18. [Google Scholar]
- Dabagh, S.; Nikbakht, M. Glycemic control by exercise and Urtica dioica Supplements in men with type 2 diabetes. Jundishapur J. Chronic Disease. Care 2016, 5, 50–56. [Google Scholar] [CrossRef]
- Khalili, N.; Fereydoonzadeh, R.; Mohtashami, R.; Mehrzadi, S.; Heydari, M.; Huseini, H.F. Silymarin, olibanum, and nettle, a mixed herbal formulation in the treatment of type II diabetes: A randomized, double-Blind, placebo-controlled, clinical trial. Evid. Based Complement. Alternat. Med. 2017, 22, 603–608. [Google Scholar] [CrossRef]
- Gohari, A.; Noorafshan, A.; Akmali, M.; Zamani-Garmsiri, F.; Seghatoleslam, A. Urtica dioica distillate regenerates pancreatic beta cells in streptozotocin-induced diabetic rats. Iran J. Med. Sci. 2018, 43, 174–183. [Google Scholar]
- Khare, V.; Kushwaha, P.; Verma, S.; Gupta, A.; Srivastava, S.; Rawat, A. Pharmacognostic evaluation and antioxidant activity of Urtica dioica L. Chin. Med. 2012, 3, 128–135. [Google Scholar] [CrossRef]
- Hanczakowska, E.; Świątkiewicz, M.; Grela, E.R. Effect of dietary inclusion of a herbal extract mixture and different oils on pig performance and meat quality. Meat Sci. 2015, 108, 61–66. [Google Scholar] [CrossRef]
- Musunuru, K. Atherogenic dyslipidemia: Cardiovascular risk and dietary intervention. Lipids 2010, 45, 907–914. [Google Scholar] [CrossRef]
- Mahjoub, S.; Davari, S.; Moazezi, Z.; Qujeq, D. Hypolipidemic effects of ethanolic and aqueous extracts of Urtica dioica in rats. World Appl. Sci. J. 2012, 17, 1345–1348. [Google Scholar]
- Vengerovsky, A.I.; Yakimova, T.V.; Nasanova, O.N. The influence of nettle and burdock extracts in combination with different diets on dyslipidemia in diabetes mellitus model. Vopr. Pitan. 2015, 84, 69–75. [Google Scholar]
- Dar, S.A.; Ganai, F.A.; Yousuf, A.R.; Balkhi, M.U.H.; Bhat, T.M.; Sharma, P. Pharmacological and toxicological evaluation of Urtica dioica. Pharm. Biol. 2013, 51, 170–180. [Google Scholar] [CrossRef]
- Raimova, K.; Abdulladjanova, N.; Kurbanova, M.; Makhmanov, D.; Kadirova Sh, O.; Tashpulatov, F. Comprehensive study of the chemical composition of Urtica dioica L. J. Crit. Rev. 2020, 7, 750–755. [Google Scholar]
- Jeong, S.M.; Kang, M.J.; Choi, H.N.; Kim, J.H.; Kim, J.I. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr. Res. Prac. 2012, 6, 201–207. [Google Scholar] [CrossRef]
- Madadi Jaberi, M.; Vahidian Rezazadeh, M.; Mogharnasi, M.; Karaji Bani, M. The effect of 8 weeks of aerobic training and consumption of hydro-alcoholic extract of nettle on apelin and hs-CRP plasma levels of overweight and obese women. Armaghane Danesh. 2016, 21, 846–859. [Google Scholar]
- Abedinzade, M.; Rostampour, M.; Mirzajani, E.; Khalesi, Z.B.; Pourmirzaee, T.; Khanaki, K. Urtica dioica and Lamium album decrease glycogen synthase kinase-3 beta and increase K-Ras in diabetic rats. J. Pharmacopunct. 2019, 22, 248–252. [Google Scholar] [CrossRef]
- Rau, O.; Wurglics, M.; Dingermann, T.; Abdel-Tawab, M.; Schubert-Zsilavecz, M. Screening of herbal extracts for activation of the human peroxisome proliferator-activated receptor. Pharmazie 2006, 61, 952–956. [Google Scholar]
- Fan, S.; Raychaudhuri, S.; Kraus, O.; Shahinozzaman, M.; Lofti, L.; Obanda, D.N. Urtica dioica Whole Vegetable as a Functional Food Targeting Fat Accumulation and Insulin Resistance-a Preliminary Study in a Mouse Pre-Diabetic Model. Nutrients 2020, 12, 1059. [Google Scholar] [CrossRef]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Elez Garofulić, I.; Malin, V.; Repajić, M.; Zorić, Z.; Pedisić, S.; Sterniša, M.; Smole Možina, S.; Dragović-Uzelac, V. Phenolic Profile, Antioxidant Capacity and Antimicrobial Activity of Nettle Leaves Extracts Obtained by Advanced Extraction Techniques. Molecules 2021, 26, 6153. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Oršolić, N.; Landeka Jurčević, I.; Đikić, D.; Rogić, D.; Odeh, D.; Balta, V.; Perak Junaković, E.; Terzić, S.; Jutrić, D. Effect of propolis on diet-induced hyperlipidemia and atherogenic indices in mice. Antioxidants 2019, 8, 156. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Landeka Jurčević, I.; Dora, M.; Guberović, I.; Petras, M.; Rimac, S.; Brnčić, S.; Đikić, D. Polyphenols from wine lees as a novel functional bioactive compound in the protection against oxidative stress and hyperlipidaemia. Food Technol. Biotechnol. 2017, 55, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Clerici, M.; Garavaglia, M.E.; Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. A step-by-step protocol for assaying protein carbonylation in biological samples. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1019, 178–190. [Google Scholar] [CrossRef] [PubMed]
- GraphPad. Software. Available online: https://www.graphpad.com/ (accessed on 5 September 2022).
- Dadvar, N.; Ghalavand, A.; Zakerkish, M.; Hojat, S.; Alijani, E.; Mahmoodkhani Kooshkaki, R. The effect of aerobic training and Urtica dioica on lipid profile and fasting blood glucose in middle age female with type II diabetes. Jundishapur. Sci. Med. J. 2016, 15, 707–716. [Google Scholar]
- Chatterji, S.; Fogel, D. Study of the effect of the herbal composition SR2004 on hemoglobin A1c, fasting blood glucose, and lipids in patients with type 2 diabetes mellitus. Integr. Med. Res. 2018, 7, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Mehran, M.M.; Norasfard, M.R.; Abedinzade, M.; Khanaki, K. Lamium album or Urtica dioica? which is more effective in decreasing serum glucose, lipid and hepatic enzymes in streptozotocin induced diabetic rats: A comparative study. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 84–88. [Google Scholar] [CrossRef]
- Das, M.; Sarma, B.; Rokeya, B.; Parial, R.; Nahar, N.; Mosihuzzaman, M.; Khan, A.; Ali, L. Antihyperglycemic and antihyperlipidemic activity of Urtica dioica on type 2 diabetic model rats. J. Diabetol. 2011, 2, 2. [Google Scholar]
- Nassiri-Asl, M.; Zamansoltani, F.; Abbasi, E.; Daneshi, M.-M.; Zangivand, A.-A. Effects of Urtica dioica extract on lipid profile in hypercholesterolemic rats. J. Chin. Integr. Med. 1986, 7, 428–433. [Google Scholar] [CrossRef]
- Kotoh, K.; Kato, M.; Kohjima, M.; Tanaka, M.; Miyazaki, M.; Nakamura, K.; Enjoji, M.; Nakamuta, M.; Takayanagi, R. Lactate dehydrogenase production in hepatocytes is increased at an early stage of acute liver failure. Exp. Ther. Med. 2011, 2, 195–199. [Google Scholar] [CrossRef]
- Pourahmadi, M.; Jashni, H.K.; Bagheri, M.; Sotoodeh Jahromi, A. The effect of hydro-alcoholic extract of Urtica dioica Root on testes in adult rats. Life Sci. J. 2014, 11, 420–424. [Google Scholar]
- Grabacka, M.; Pierzchalska, M.; Dean, M.; Reiss, K. Regulation of Ketone Body Metabolism and the Role of PPARα. Int. J. Mol. Sci. 2016, 17, 2093. [Google Scholar] [CrossRef]
- Kersten, S. Integrated physiology and systems biology of PPARα. Mol. Metab. 2014, 3, 354–371. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1-α and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef]
- McGinnis, C.D.; Jennings, E.Q.; Harris, P.S.; Galligan, J.J.; Fritz, K.S. Biochemical Mechanisms of Sirtuin-Directed Protein Acylation in Hepatic Pathologies of Mitochondrial Dysfunction. Cells 2022, 11, 2045. [Google Scholar] [CrossRef] [PubMed]
- Souza, S.A.; Held, A.; Lu, W.J.; Drouhard, B.; Avila, B.; Leyva-Montes, R.; Hu, M.; Miller, B.R.; Ng, H.L. Mechanisms of allosteric and mixed mode aromatase inhibitors. RSC Chem. Biol. 2021, 2, 892–905. [Google Scholar] [CrossRef]

| Experimental Groups | |||||
|---|---|---|---|---|---|
| Antioxidative Defence Markers in Liver | Control Males | Control Females | Nettle Extract Males | Nettle Extract Females | |
| SOD_liver (U/mg protein) | Mean | 284.61 | 163.053 | 109.998 | 79.222 |
| SD | 96.597 | 73.942 | 23.607 | 17.101 | |
| Max | 614.581 | 307.435 | 200.533 | 144.394 | |
| GSH_liver (µmol/mg protein) | Mean | 127.666 | 160.258 | 119.725 * | 96.337 * |
| SD | 27.069 | 59.972 | 20.97 | 14.227 | |
| Max | 292.21 | 274.222 | 206.334 | 194.927 | |
| CAT_liver (U/mg protein) | Mean | 0.523 | 0.600 | 0.487 | 0.725 |
| SD | 0.112 | 0.205 | 0.09 | 0.114 | |
| Max | 1.262 | 0.955 | 1.069 | 1.682 | |
| Experimental Groups | |||||
|---|---|---|---|---|---|
| Blood Chemistry Liver Markers | Control Males | Control Females | Nettle Extract Males | Nettle Extract Females | |
| AST (Ul/L) | Mean | 161.2 | 206.0 | 156.0 | 189.8 |
| SD | 35.8 | 13.9 | 8.0 | 15.0 | |
| Max | 290.0 | 245.0 | 110.0 | 223.0 | |
| ALT (U/L) | Mean | 65.0 | 88.7 | 25.5 | 137.0 |
| SD | 27.03 | 59.32 | 2.06 | 56.1 | |
| Max | 172.0 | 266.0 | 30.0 | 260.0 | |
| LDH (U/L) protein) | Mean | 706.5 | 548.0 | 1024.0 * | 1400.0 * |
| SD | 90.8 | 88.5 | 188.9 | 223.2 | |
| Max | 999.0 | 714.0 | 1570.0 | 1895.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domjanić Drozdek, S.; Odeh, D.; Đikić, D.; Gračan, R.; Oršolić, N.; Dragović-Uzelac, V.; Feher-Turković, L.; Dragičević, P.; Landeka Jurčević, I. The Effects of Nettle Extract Consumption on Liver PPARs, SIRT1, ACOX1 and Blood Lipid Levels in Male and Female C57Bl6 Mice. Nutrients 2022, 14, 4469. https://doi.org/10.3390/nu14214469
Domjanić Drozdek S, Odeh D, Đikić D, Gračan R, Oršolić N, Dragović-Uzelac V, Feher-Turković L, Dragičević P, Landeka Jurčević I. The Effects of Nettle Extract Consumption on Liver PPARs, SIRT1, ACOX1 and Blood Lipid Levels in Male and Female C57Bl6 Mice. Nutrients. 2022; 14(21):4469. https://doi.org/10.3390/nu14214469
Chicago/Turabian StyleDomjanić Drozdek, Sandra, Dyana Odeh, Domagoj Đikić, Romana Gračan, Nada Oršolić, Verica Dragović-Uzelac, Lana Feher-Turković, Petar Dragičević, and Irena Landeka Jurčević. 2022. "The Effects of Nettle Extract Consumption on Liver PPARs, SIRT1, ACOX1 and Blood Lipid Levels in Male and Female C57Bl6 Mice" Nutrients 14, no. 21: 4469. https://doi.org/10.3390/nu14214469
APA StyleDomjanić Drozdek, S., Odeh, D., Đikić, D., Gračan, R., Oršolić, N., Dragović-Uzelac, V., Feher-Turković, L., Dragičević, P., & Landeka Jurčević, I. (2022). The Effects of Nettle Extract Consumption on Liver PPARs, SIRT1, ACOX1 and Blood Lipid Levels in Male and Female C57Bl6 Mice. Nutrients, 14(21), 4469. https://doi.org/10.3390/nu14214469








