Effects of Combined Inorganic Nitrate and Nitrite Supplementation on Cardiorespiratory Fitness and Skeletal Muscle Oxidative Capacity in Type 2 Diabetes: A Pilot Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Participants
2.3. Maximal Exercise Testing
2.4. Nitrate (NO3−)/Nitrite (NO2−) Supplementation
2.5. Blood Sampling and Muscle Biopsies
2.6. Histology and Fluorescence Microscopy
2.7. Skeletal Muscle Oxidative Capacity
2.8. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.1.1. Clinical Characteristics and Medications
3.1.2. Plasma NO Metabolites, VO2max and Work Rate Capacity in T2DM and Non-Diabetic Controls
3.1.3. Skeletal Muscle Fiber Types and Oxidative Capacity
3.2. Effects of Combined NO3−/NO2− Supplementation
3.2.1. Plasma NO Metabolites
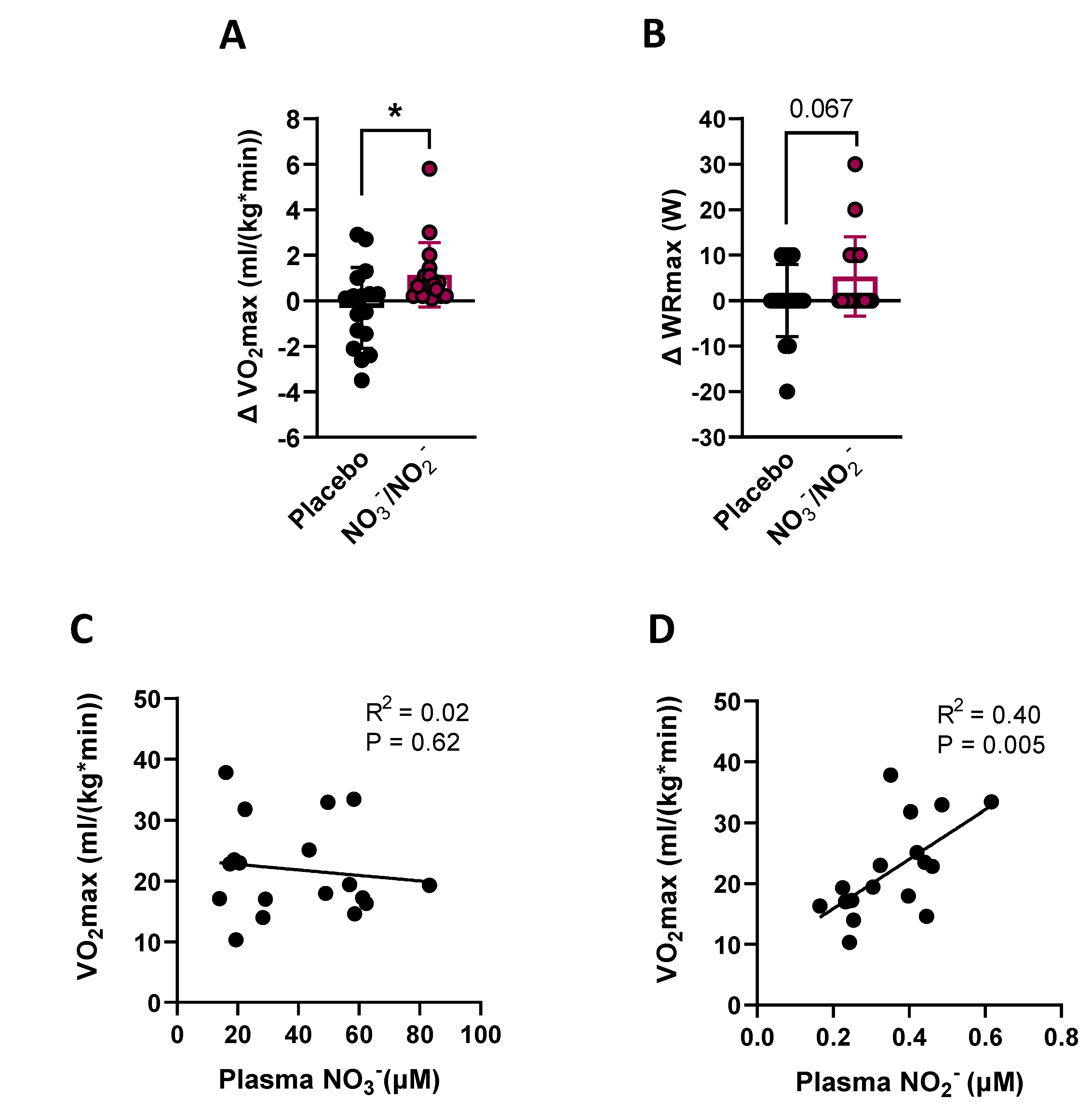
3.2.2. VO2max and WRmax
3.2.3. Skeletal Muscle Oxidative Capacity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes–Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Sattar, N.; Rawshani, A.; Franzén, S.; Rawshani, A.; Svensson, A.-M.; Rosengren, A.; McGuire, D.K.; Eliasson, B.; Gudbjörnsdottir, S. Age at Diagnosis of Type 2 Diabetes Mellitus and Associations with Cardiovascular and Mortality Risks. Circulation 2019, 139, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Salehidoost, R.; Mansouri, A.; Amini, M.; Yamini, S.A.; Aminorroaya, A. Diabetes and all-cause mortality, a 18-year follow-up study. Sci. Rep. 2020, 10, 3183. [Google Scholar] [CrossRef] [Green Version]
- Laukkanen, J.A.; Zaccardi, F.; Khan, H.; Kurl, S.; Jae, S.Y.; Rauramaa, R. Long-term Change in Cardiorespiratory Fitness and All-Cause Mortality. Mayo Clin. Proc. 2016, 91, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Regensteiner, J.G.; Sippel, J.; Mcfarling, E.T.; Wolfel, E.E.; Hiatt, W.R. Effects of non-insulin-dependent diabetes on oxygen consumption during treadmill exercise. Med. Sci. Sports Exerc. 1995, 27, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Leite, S.A.; Monk, A.M.; Upham, P.A.; Chacra, A.R.; Bergenstal, R.M. Low cardiorespiratory fitness in people at risk for type 2 diabetes: Early marker for insulin resistance. Diabetol. Metab. Syndr. 2009, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Brandenburg, S.L.; Reusch, J.E.; Bauer, T.A.; Jeffers, B.W.; Hiatt, W.R.; Regensteiner, J.G. Effects of exercise training on oxygen uptake kinetic responses in women with type 2 diabetes. Diabetes Care 1999, 22, 1640–1646. [Google Scholar] [CrossRef]
- Najafipour, F.; Mobasseri, M.; Yavari, A.; Nadrian, H.; Aliasgarzadeh, A.; Abbasi, N.M.; Niafar, M.; Gharamaleki, J.H.; Sadra, V. Effect of regular exercise training on changes in HbA1c, BMI and VO 2 max among patients with type 2 diabetes mellitus: An 8-year trial. BMJ Open Diabetes Res. Care 2017, 5, e000414. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.S.U.; Karimi, H.; Gillani, S.A.; Ahmad, S. Effects of supervised structured aerobic exercise training programme on level of Exertion, dyspnoea, VO2 max and Body Mass Index in patients with type 2 diabetes mellitus. J. Pak. Med. Assoc. 2017, 67, 1670–1673. [Google Scholar]
- Morrato, E.H.; Hill, J.O.; Wyatt, H.R.; Ghushchyan, V.; Sullivan, P.W. Physical Activity in U.S. Adults with Diabetes and At Risk for Developing Diabetes, 2003. Diabetes Care 2007, 30, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Bassett, D.R., Jr.; Howley, E.T. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 2000, 32, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Kingwell, B.A.; Formosa, M.; Muhlmann, M.; Bradley, S.J.; McConell, G.K. Type 2 Diabetic Individuals Have Impaired Leg Blood Flow Responses to Exercise: Role of Endothelium-Dependent Vasodilation. Diabetes Care 2003, 26, 899–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogensen, M.; Sahlin, K.; Fernström, M.; Glintborg, D.; Vind, B.F.; Beck-Nielsen, H.; Højlund, K. Mitochondrial Respiration Is Decreased in Skeletal Muscle of Patients with Type 2 Diabetes. Diabetes 2007, 56, 1592–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabøl, R.; Larsen, S.; Højberg, P.M.V.; Almdal, T.; Boushel, R.; Haugaard, S.B.; Andersen, J.L.; Madsbad, S.; Dela, F. Regional Anatomic Differences in Skeletal Muscle Mitochondrial Respiration in Type 2 Diabetes and Obesity. J. Clin. Endocrinol. Metab. 2010, 95, 857–863. [Google Scholar] [CrossRef] [Green Version]
- Bock, J.M.; Hughes, W.E.; Ueda, K.; Feider, A.J.; Hanada, S.; Kruse, N.T.; Iwamoto, E.; Casey, D.P. Greater α1-adrenergic-mediated vasoconstriction in contracting skeletal muscle of patients with type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H797–H807. [Google Scholar] [CrossRef]
- Bender, S.B.; Herrick, E.K.; Lott, N.D.; Klabunde, R.E. Diet-induced obesity and diabetes reduce coronary responses to nitric oxide due to reduced bioavailability in isolated mouse hearts. Diabetes Obes. Metab. 2007, 9, 688–696. [Google Scholar] [CrossRef]
- Tessari, P.; Cecchet, D.; Cosma, A.; Vettore, M.; Coracina, A.; Millioni, R.; Iori, E.; Puricelli, L.; Avogaro, A.; Vedovato, M. Nitric Oxide Synthesis Is Reduced in Subjects with Type 2 Diabetes and Nephropathy. Diabetes 2010, 59, 2152–2159. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, A.J.; Schauble, E.; Bernstein, D.; Cooke, J. Limb Blood Flow During Exercise Is Dependent on Nitric Oxide. Circulation 1998, 98, 369–374. [Google Scholar] [CrossRef]
- Joyner, M.J.; Casey, D.P. Regulation of Increased Blood Flow (Hyperemia) to Muscles During Exercise: A Hierarchy of Competing Physiological Needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef] [Green Version]
- Nisoli, E.; Falcone, S.; Tonello, C.; Cozzi, V.; Palomba, L.; Fiorani, M.; Pisconti, A.; Brunelli, S.; Cardile, A.; Francolini, M.; et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc. Natl. Acad. Sci. USA 2004, 101, 16507–16512. [Google Scholar] [CrossRef] [Green Version]
- Lira, V.A.; Brown, D.L.; Lira, A.K.; Kavazis, A.N.; Soltow, Q.A.; Zeanah, E.H.; Criswell, D.S. Nitric oxide and AMPK cooperatively regulate PGC-1α in skeletal muscle cells. J. Physiol. 2010, 588, 3551–3566. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S.; Calvert, J.W.; Elrod, J.W.; Gundewar, S.; Ji, S.Y.; Lefer, D.J. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 2007, 104, 19144–19149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundberg, J.O.; Gladwin, M.T.; Ahluwalia, A.; Benjamin, N.; Bryan, N.S.; Butler, A.; Cabrales, P.; Fago, A.; Feelisch, M.; Ford, P.C.; et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat. Chem. Biol. 2009, 5, 865–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundberg, J.O.; Weitzberg, E. NO Generation From Nitrite and Its Role in Vascular Control. Arter. Thromb. Vasc. Biol. 2005, 25, 915–922. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, J.O.; Carlstrom, M.; Larsen, F.J.; Weitzberg, E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc. Res. 2011, 89, 525–532. [Google Scholar] [CrossRef]
- Bock, J.M.; Ueda, K.; Feider, A.J.; Hanada, S.; Casey, D.P. Combined inorganic nitrate/nitrite supplementation blunts α-mediated vasoconstriction during exercise in patients with type 2 diabetes. Nitric Oxide Biol. Chem. 2022, 118, 17–25. [Google Scholar] [CrossRef]
- Bock, J.M.; Hughes, W.E.; Ueda, K.; Feider, A.J.; Hanada, S.; Casey, D.P. Glycemic management is inversely related to skeletal muscle microvascular endothelial function in patients with type 2 diabetes. Physiol. Rep. 2021, 9, e14764. [Google Scholar] [CrossRef] [PubMed]
- Bock, J.M.; Hughes, W.E.; Ueda, K.; Feider, A.J.; Hanada, S.; Casey, D.P. Dietary Inorganic Nitrate/Nitrite Supplementation Reduces Central and Peripheral Blood Pressure in Patients with Type 2 Diabetes Mellitus. Am. J. Hypertens. 2022, 35, 803–809. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Govoni, M.; Jansson, E.Å.; Weitzberg, E.; Lundberg, J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 2008, 19, 333–337. [Google Scholar] [CrossRef]
- Fuqua, J.D.; Mere, C.P.; Kronemberger, A.; Blomme, J.; Bae, D.; Turner, K.D.; Harris, M.P.; Scudese, E.; Edwards, M.; Ebert, S.M.; et al. ULK2 is essential for degradation of ubiquitinated protein aggregates and homeostasis in skeletal muscle. FASEB J. 2019, 33, 11735–12745. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.A.; Kotecki, J.A.; Jacobs, B.L.; Hornberger, T.A. Muscle Fiber Type-Dependent Differences in the Regulation of Protein Synthesis. PLoS ONE 2012, 7, e37890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W.; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Pesta, D.; Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar]
- Kenny, H.C.; Rudwill, F.; Breen, L.; Salanova, M.; Blottner, D.; Heise, T.; Heer, M.; Blanc, S.; O’Gorman, D.J. Bed rest and resistive vibration exercise unveil novel links between skeletal muscle mitochondrial function and insulin resistance. Diabetologia 2017, 60, 1491–1501. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Kunz, H.E.; Gries, K.; Hart, C.R.; Polley, E.C.; Lanza, I.R. Preserved skeletal muscle oxidative capacity in older adults despite decreased cardiorespiratory fitness with ageing. J. Physiol. 2021, 599, 3581–3592. [Google Scholar] [CrossRef]
- Jacques, M.; Kuang, J.; Bishop, D.J.; Yan, X.; Alvarez-Romero, J.; Munson, F.; Garnham, A.; Papadimitriou, I.; Voisin, S.; Eynon, N. Mitochondrial respiration variability and simulations in human skeletal muscle: The Gene SMART study. FASEB J. 2020, 34, 2978–2986. [Google Scholar] [CrossRef]
- Boushel, R.; Gnaiger, E.; Schjerling, P.; Skovbro, M.; Kraunsøe, R.; Dela, F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 2007, 50, 790–796. [Google Scholar] [CrossRef] [Green Version]
- Stuart, C.A.; McCurry, M.P.; Marino, A.; South, M.A.; Howell, M.E.A.; Layne, A.S.; Ramsey, M.W.; Stone, M.H. Slow-Twitch Fiber Proportion in Skeletal Muscle Correlates with Insulin Responsiveness. J. Clin. Endocrinol. Metab. 2013, 98, 2027–2036. [Google Scholar] [CrossRef] [Green Version]
- Kleinbongard, P.; Dejam, A.; Lauer, T.; Jax, T.; Kerber, S.; Gharini, P.; Balzer, J.; Zotz, R.B.; Scharf, R.E.; Willers, R.; et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic. Biol. Med. 2006, 40, 295–302. [Google Scholar] [CrossRef]
- Kapil, V.; Khambata, R.S.; Jones, D.A.; Rathod, K.; Primus, C.; Massimo, G.; Fukuto, J.M.; Ahluwalia, A. The Noncanonical Pathway for In Vivo Nitric Oxide Generation: The Nitrate-Nitrite-Nitric Oxide Pathway. Pharmacol. Rev. 2020, 72, 692–766. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Cai, Q.; Steinwandel, M.; Hargreaves, M.K.; Bordenstein, S.R.; Blot, W.J.; Zheng, W.; Shu, X.O. Association of oral microbiome with type 2 diabetes risk. J. Periodontal Res. 2017, 52, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Matsha, T.; Prince, Y.; Davids, S.; Chikte, U.; Erasmus, R.; Kengne, A.; Davison, G. Oral Microbiome Signatures in Diabetes Mellitus and Periodontal Disease. J. Dent. Res. 2020, 99, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Hoffmann, T.; Fischer, S.; Bornstein, S.; Graessler, J.; Noack, B. Obesity alters composition and diversity of the oral microbiota in patients with type 2 diabetes mellitus independently of glycemic control. PLoS ONE 2018, 13, e0204724. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Carlström, M.; Ghasemi, A. Inorganic nitrate: A potential prebiotic for oral microbiota dysbiosis associated with type 2 diabetes. Nitric Oxide 2021, 116, 38–46. [Google Scholar] [CrossRef]
- Hughan, K.S.; Levine, A.; Helbling, N.; Anthony, S.; DeLany, J.P.; Stefanovic-Racic, M.; Goodpaster, B.H.; Gladwin, M.T. Effects of Oral Sodium Nitrite on Blood Pressure, Insulin Sensitivity, and Intima-Media Arterial Thickening in Adults with Hypertension and Metabolic Syndrome. Hypertension 2020, 76, 866–874. [Google Scholar] [CrossRef]
- Rossman, M.J.; Gioscia-Ryan, R.A.; Santos-Parker, J.R.; Ziemba, B.P.; Lubieniecki, K.L.; Johnson, L.C.; Poliektov, N.E.; Bispham, N.Z.; Woodward, K.A.; Nagy, E.E.; et al. Inorganic Nitrite Supplementation Improves Endothelial Function with Aging: Translational Evidence for Suppression of Mitochondria-Derived Oxidative Stress. Hypertension 2021, 77, 1212–1222. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; DiMenna, F.J.; Pavey, T.G.; Wilkerson, D.P.; Benjamin, N.; Winyard, P.G.; Jones, A.M. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2010, 299, R1121–R1131. [Google Scholar] [CrossRef] [Green Version]
- Sylow, L.; Kleinert, M.; Richter, E.A.; Jensen, T.E. Exercise-stimulated glucose uptake–regulation and implications for glycaemic control. Nat. Rev. Endocrinol. 2017, 13, 133–148. [Google Scholar] [CrossRef]
- Byrkjeland, R.; Njerve, I.U.; Anderssen, S.; Arnesen, H.; Seljeflot, I.; Solheim, S. Effects of exercise training on HbA1c and VO2peak in patients with type 2 diabetes and coronary artery disease: A randomised clinical trial. Diabetes Vasc. Dis. Res. 2015, 12, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Kirwan, J.P.; Sacks, J.; Nieuwoudt, S. The essential role of exercise in the management of type 2 diabetes. Clevel. Clin. J. Med. 2017, 84, S15–S21. [Google Scholar] [CrossRef] [PubMed]
- Berends, J.E.; Berg, L.M.V.D.; Guggeis, M.A.; Henckens, N.F.; Hossein, I.J.; de Joode, M.E.; Zamani, H.; van Pelt, K.A.; Beelen, N.A.; Kuhnle, G.G.; et al. Consumption of Nitrate-Rich Beetroot Juice with or without Vitamin C Supplementation Increases the Excretion of Urinary Nitrate, Nitrite, and N-nitroso Compounds in Humans. Int. J. Mol. Sci. 2019, 20, 2277. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.R.; Hart, T.L.; Iv, J.T.H.; Woods, C.A.; Toy, A.M.; Pihera, B.C.; Aziz, M.A.; Zimmerman, G.A.; Jones, M.D.; Vantrease, W.C.; et al. Influence of Dietary Nitrate Supplementation on Physical Performance and Body Composition Following Offseason Training in Division I Athletes. J. Diet. Suppl. 2022, 19, 534–549. [Google Scholar] [CrossRef] [PubMed]
- Dutra, M.T.; Alex, S.; Silva, A.F.; Brown, L.E.; Bottaro, M. Antioxidant Supplementation Impairs Changes in Body Composition Induced by Strength Training in Young Women. Int. J. Exerc. Sci. 2019, 12, 287–296. [Google Scholar]
- Parker, B.A.; Thompson, P.D. Effect of Statins on Skeletal Muscle. Exerc. Sport Sci. Rev. 2012, 40, 188–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, B.A.; Capizzi, J.A.; Grimaldi, A.S.; Clarkson, P.M.; Cole, S.M.; Keadle, J.; Chipkin, S.; Pescatello, L.S.; Simpson, K.; White, C.M.; et al. Effect of Statins on Skeletal Muscle Function. Circulation 2012, 127, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Di Stasi, S.L.; MacLeod, T.D.; Winters, J.D.; Binder-Macleod, S.A. Effects of Statins on Skeletal Muscle: A Perspective for Physical Therapists. Phys. Ther. 2010, 90, 1530–1542. [Google Scholar] [CrossRef] [Green Version]
- Cree-Green, M.; Scalzo, R.L.; Harrall, K.; Newcomer, B.R.; Schauer, I.E.; Huebschmann, A.G.; McMillin, S.; Brown, M.S.; Orlicky, D.; Knaub, L.; et al. Supplemental Oxygen Improves In Vivo Mitochondrial Oxidative Phosphorylation Flux in Sedentary Obese Adults with Type 2 Diabetes. Diabetes 2018, 67, 1369–1379. [Google Scholar] [CrossRef] [Green Version]
- Park, S.W.; Goodpaster, B.H.; Lee, J.S.; Kuller, L.H.; Boudreau, R.; de Rekeneire, N.; Harris, T.B.; Kritchevsky, S.; Tylavsky, F.A.; Nevitt, M.; et al. Excessive Loss of Skeletal Muscle Mass in Older Adults with Type 2 Diabetes. Diabetes Care 2009, 32, 1993–1997. [Google Scholar] [CrossRef] [Green Version]
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D. Sarcopenia and type 2 diabetes mellitus: A bidirectional relationship. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1057–1072. [Google Scholar] [CrossRef]




| PRIMARY GROUP | SUB-GROUP (Muscle Biopsies) | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | T2DM | T2DM Placebo | T2DM NO3−/NO2− | Control | T2DM | T2DM Placebo | T2DM NO3−/NO2− | |
| N | 15 | 36 | 18 | 18 | 10 | 19 | 7 | 12 |
| Age (years) | 60 ± 9 | 59 ± 9 | 58 ± 9 | 59 ± 9 | 60 ± 9 | 60 ± 10 | 60 ± 12 | 59 ± 10 |
| Reported duration of T2DM | - - | 6 ± 4 | 6 ± 3 | 8 ± 5 | - - | 6 ± 4 | 5 ± 2 | 7 ± 4 † |
| Men, n (%) | 10 (67) | 26 (72) | 13 (72) | 13 (72) | 6 (60) | 13 (68) | 5 (71) | 8 (67) |
| BMI (kg/m2) | 29.5 ± 4.6 | 31.9 ± 5.0 | 32.3 ± 5.3 | 31.8 ± 5.0 | 29.4 ± 5.3 | 32.8 ± 5.6 | 32.8 ± 6.8 | 32.9 ± 5.5 |
| Glucose (mg/dL) | 95 ± 8 | 162 ± 45 *** | 159 ± 40 | 166 ± 50 | 96 ± 9 | 184 ± 47 *** | 184 ± 36 | 184 ± 55 |
| Insulin (mIU/L) | 14.9 ± 14.1 | 20.7 ± 14.3 | 21.7 ± 14.7 | 19.7 ± 14.3 | 12.2 ± 8.0 | 22.9 ± 15.7 | 24.7 ± 18.8 | 25.2 ± 18.0 |
| HbA1c (%) | 5.3 ± 0.3 | 7.4 ± 1.4 *** | 7.3 ± 1.4 | 7.5 ± 1.4 | 5.2 ± 0.3 | 7.7 ± 1.4 *** | 7.5 ± 1.3 | 7.8 ± 1.5 |
| Prescription medications, n (%) | ||||||||
| Insulin | - - | 10 (28) | 5 (28) | 5 (28) | - - | 5 (26) | 2 (29) | 3 (25) |
| Metformin | - - | 30 (83) | 15 (83) | 15 (83) | - - | 17 (89) | 6 (86) | 11 (92) |
| Sulfonylurea | - - | 13 (36) | 5 (28) | 8 (42) | - - | 11 (58) | 4 (57) | 7 (59) |
| GLP-1 | - - | 4 (11) | 2 (11) | 2 (11) | - - | 1 (5) | 1 (14) | 0 (0) |
| Thiazolidinediones (TZD) | - - | 1 (3) | 1 (6) | 0 (0) | - - | 1 (5) | 1 (14) | 0 (0) |
| Statin | 5 (33) | 26 (72) *** | 12 (67) | 14 (78) | 2 (20) | 12 (74) * | 4 (57) | 8 (67) |
| ACE Inhibitor (ACEi) | 2 (13) | 11 (31) | 5 (28) | 6 (21) | 1 (10) | 7 (37) | 3 (43) | 3 (25) |
| Angiotensin Receptor Blocker (ARB) | 0 (0) | 9 (25) * | 4 (22) | 5 (28) | 0 (0) | 5 (26) # | 2 (29) | 3 (25) |
| Beta-blocker | 2 (13) | 8 (22) | 4 (22) | 4 (22) | 1 (5) | 3 (16) | 1 (14) | 2 (17) |
| Ca2+ Channel blocker | 2 (13) | 3 (10) | 2 (11) | 1 (6) | 1 (5) | 2 (11) | 1 (14) | 1 (8) |
| Hydrochlorothiazide (HCTZ) | 0 (0) | 4 (13) | 3 (17) | 1 (6) | 0 (0) | 2 (11) | 1 (14) | 1 (8) |
| PRIMARY GROUP | SUB-GROUP (Muscle Biopsies) | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | T2DM | T2DM Placebo | T2DM NO3−/NO2− | Control | T2DM | T2DM Placebo | T2DM NO3−/NO2− | |
| N | 15 | 36 | 18 | 18 | 10 | 19 | 7 | 12 |
| Plasma Nitrate, NO3−(μM) | 12.8 ± 5.0 | 25.6 ± 13.7 *** | 27.2 ± 11.1 | 23.9 ± 16.0 | 11.4 ± 2.9 | 24.8 ± 15.8 *** | 24.4 ± 11.8 | 25.0 ± 19.0 |
| Plasma Nitrite, NO2−(μM) | 0.48 ± 087 | 0.26 ± 0.11 **** | 0.26 ± 0.13 | 0.26 ± 0.09 | 0.45 ± 0.08 | 0.26 ± 0.11 *** | 0.28 ± 0.13 | 0.25 ± 1.00 |
| VO2max (ml/(kg*min)) | 27.6 ± 8.7 | 20.3 ± 5.7 *** | 20.0 ± 4.2 | 20.7 ± 7.0 | 28.3 ± 7.1 | 20.0 ± 6.0 ** | 18.5 ± 1.1 | 20.0 ± 6.8 |
| Work Rate (Wmax) | 178 ± 46 | 136 ± 40 ** | 138 ± 32 | 135 ± 47 | 186 ± 48 | 130 ± 30 *** | 129 ± 25 | 127 ± 33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, K.D.; Kronemberger, A.; Bae, D.; Bock, J.M.; Hughes, W.E.; Ueda, K.; Feider, A.J.; Hanada, S.; de Sousa, L.G.O.; Harris, M.P.; et al. Effects of Combined Inorganic Nitrate and Nitrite Supplementation on Cardiorespiratory Fitness and Skeletal Muscle Oxidative Capacity in Type 2 Diabetes: A Pilot Randomized Controlled Trial. Nutrients 2022, 14, 4479. https://doi.org/10.3390/nu14214479
Turner KD, Kronemberger A, Bae D, Bock JM, Hughes WE, Ueda K, Feider AJ, Hanada S, de Sousa LGO, Harris MP, et al. Effects of Combined Inorganic Nitrate and Nitrite Supplementation on Cardiorespiratory Fitness and Skeletal Muscle Oxidative Capacity in Type 2 Diabetes: A Pilot Randomized Controlled Trial. Nutrients. 2022; 14(21):4479. https://doi.org/10.3390/nu14214479
Chicago/Turabian StyleTurner, Kristen D., Ana Kronemberger, Dam Bae, Joshua M. Bock, William E. Hughes, Kenichi Ueda, Andrew J. Feider, Satoshi Hanada, Luis G. O. de Sousa, Matthew P. Harris, and et al. 2022. "Effects of Combined Inorganic Nitrate and Nitrite Supplementation on Cardiorespiratory Fitness and Skeletal Muscle Oxidative Capacity in Type 2 Diabetes: A Pilot Randomized Controlled Trial" Nutrients 14, no. 21: 4479. https://doi.org/10.3390/nu14214479





