High Cholesterol-Induced Bone Loss Is Attenuated by Arctiin via an Action in Osteoclasts
Abstract
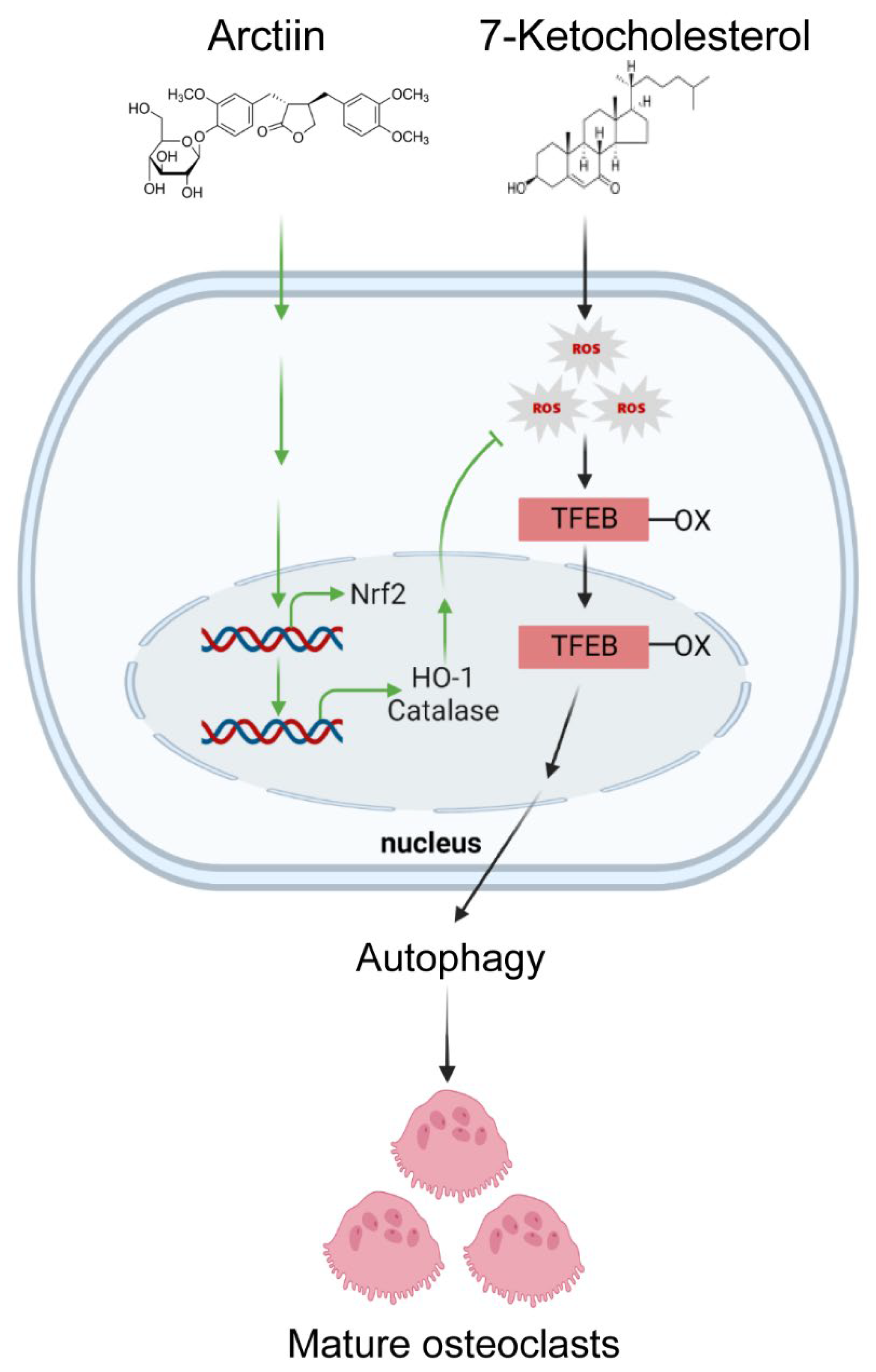
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Study Design and Animals
2.3. OC Formation
2.4. Cell Viability Assays
2.5. Bone Resorption
2.6. RNA Extraction and Quantitative Real-Time PCR (qPCR)
2.7. Western Blot Analysis
2.8. Flow Cytometry Analysis of Acidic Vesicular Organelles Stained with Acridine Orange
2.9. Detection of Intracellular Reactive Oxygen Species (ROS)
2.10. Determination of Oxidized TFEB via Carboxymethylation
2.11. SiRNA Transfection
2.12. Statistical Analysis
3. Results
3.1. Arctiin Protects Mice from AD-Induced Bone Loss
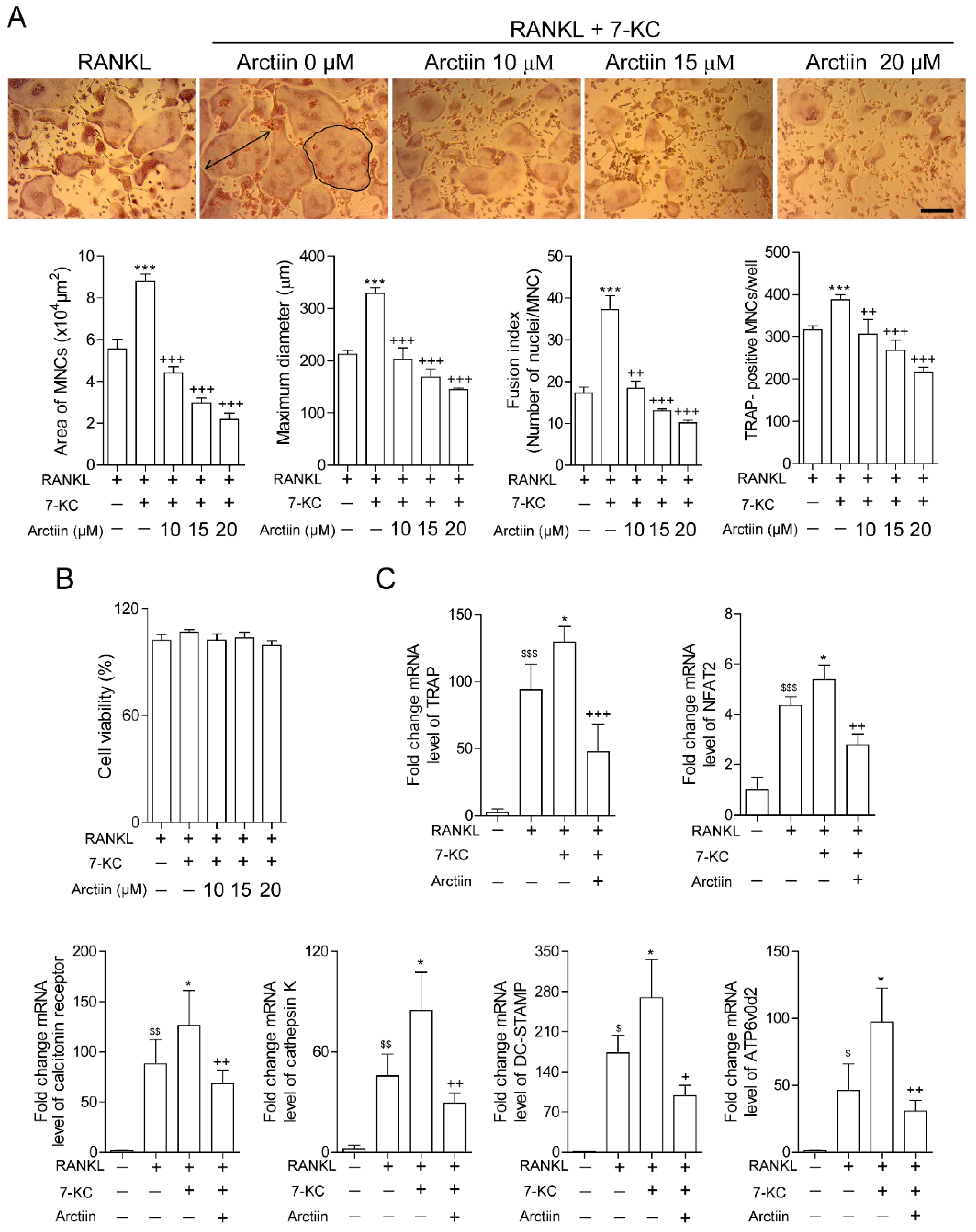
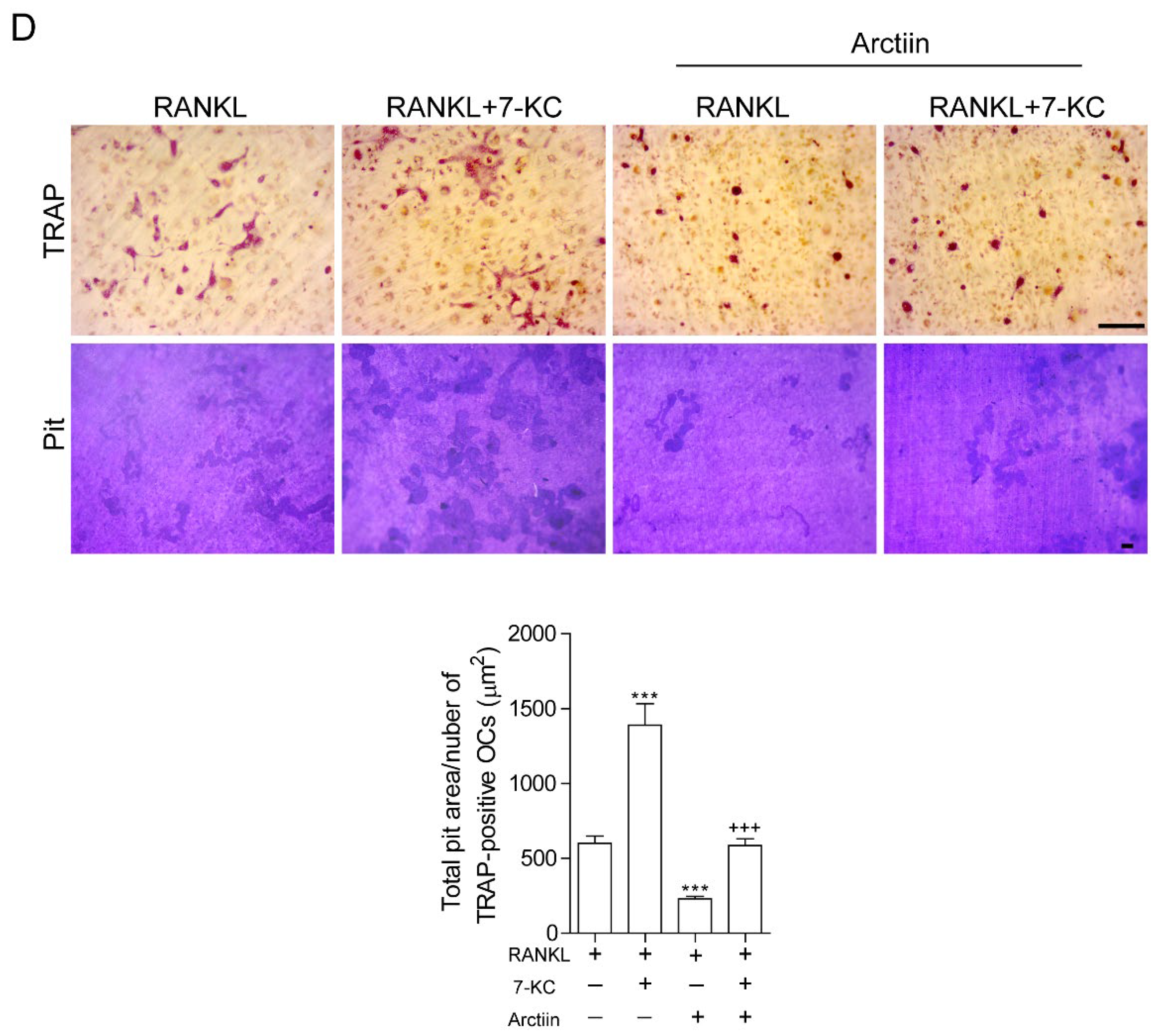
3.2. Arctiin Decreases the Number and Activity of OCs That Are Augmented by 7-KC
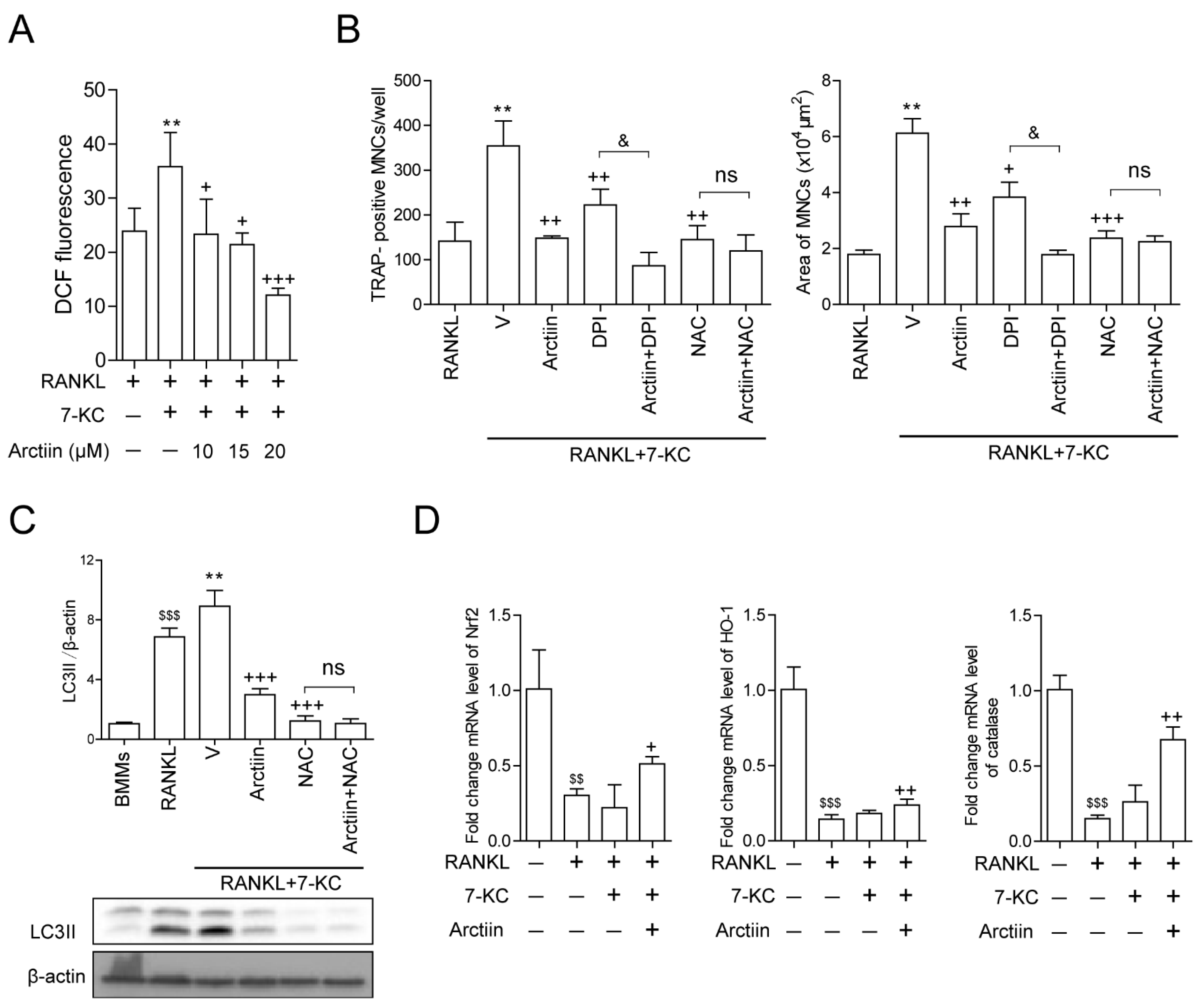
3.3. Arctiin Decreases Autophagy by Disrupting 7-KC-Stimulated TFEB Nuclear Localization
3.4. Arctiin Decreases Cytosolic ROS That Is Increased upon 7-KC Stimulation, Leading to Disrupted TFEB Nuclear Localization via Decreased Oxidation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uyama, O.; Yoshimoto, Y.; Yamamoto, Y.; Kawai, A. Bone changes and carotid atherosclerosis in postmenopausal women. Stroke 1997, 28, 1730–1732. [Google Scholar] [CrossRef] [PubMed]
- Orozco, P. Atherogenic lipid profile and elevated lipoprotein (a) are associated with lower bone mineral density in early postmenopausal overweight women. Eur. J. Epidemiol. 2004, 19, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Makovey, J.; Chen, J.S.; Hayward, C.; Williams, F.M.; Sambrook, P.N. Association between serum cholesterol and bone mineral density. Bone 2009, 44, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Sugimoto, T.; Yano, S.; Yamauchi, M.; Sowa, H.; Chen, Q.; Chihara, K. Plasma lipids and osteoporosis in postmenopausal women. Endocr. J. 2002, 49, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Oxlund, H.; Andreassen, T.T. Simvastatin treatment partially prevents ovariectomy-induced bone loss while increasing cortical bone formation. Bone 2004, 34, 609–618. [Google Scholar] [CrossRef]
- Parhami, F.; Tintut, Y.; Beamer, W.G.; Gharavi, N.; Goodman, W.; Demer, L.L. Atherogenic high-fat diet reduces bone mineralization in mice. J. Bone Miner. Res. 2001, 16, 182–188. [Google Scholar] [CrossRef]
- Stocker, R.; Keaney Jr, J.F. Role of oxidative modifications in atherosclerosis. J. Physiol. 2004, 84, 1381–1478. [Google Scholar] [CrossRef]
- Gargiulo, S.; Gamba, P.; Testa, G.; Leonarduzzi, G.; Poli, G. The role of oxysterols in vascular ageing. J Physiol. 2016, 594, 2095–2113. [Google Scholar] [CrossRef]
- Brown, A.J.; Jessup, W. Oxysterols and atherosclerosis. Atherosclerosis 1999, 142, 1–28. [Google Scholar] [CrossRef]
- Larsson, H.; Böttiger, Y.; Iuliano, L.; Diczfalusy, U. In vivo interconversion of 7-beta-hydroxycholesterol and 7-ketocholesterol, potential surrogate markers for oxidative stress. Free. Radic. Biol. Med. 2007, 43, 695–701. [Google Scholar] [CrossRef]
- Hitsumoto, T.; Takahashi, M.; Iizuka, T.; Shirai, K. Clinical significance of serum 7-ketocholesterol concentrations in the progression of coronary atherosclerosis. J. Atheroscler. Thromb. 2009, 16, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Sul, O.J.; Kim, J.E.; Ke, K.; Suh, J.H.; Choi, H.S. Atherogenic diet-induced bone loss is primarily due to increased osteoclastogenesis in mice. J. Nutr. Biochem. 2020, 79, 108337. [Google Scholar] [CrossRef] [PubMed]
- Sul, O.J.; Li, G.; Kim, J.E.; Kim, E.S.; Choi, H.S. 7-ketocholesterol enhances autophagy via the ROS-TFEB signaling pathway in osteoclasts. J. Nutr. Biochem. 2021, 96, 108783. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, M.; Zuo, Z. Overview of the anti-inflammatory effects, pharmacokinetic properties and clinical efficacies of arctigenin and arctiin from Arctium lappa L. Acta Pharmacol. Sin. 2018, 39, 787–801. [Google Scholar] [CrossRef]
- Min, B.; Lee, H.; Song, J.H.; Han, M.J.; Chung, J. Arctiin inhibits adipogenesis in 3T3-L1 cells and decreases adiposity and body weight in mice fed a high-fat diet. Nutr. Res. Pract. 2014, 8, 655–661. [Google Scholar] [CrossRef]
- Xu, X.; Zeng, X.Y.; Cui, Y.X.; Li, Y.B.; Cheng, J.H.; Zhao, X.D.; Xu, G.H.; Ma, J.; Piao, H.N.; Jin, X.; et al. Antidepressive effect of arctiin by attenuating neuroinflammation via HMGB1/TLR4- and TNF-α/TNFR1-mediated NF-κB activation. ACS Chem. Neurosci. 2020, 11, 2214–2230. [Google Scholar] [CrossRef]
- Zhou, B.; Weng, G.; Huang, Z.; Liu, T.; Dai, F. Arctiin prevents LPS-induced acute lung injury via inhibition of PI3K/AKT signaling pathway in mice. Inflammation 2018, 41, 2129–2135. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Z.T.; Li, M.; Yuan, Y.L.L.; Cui, S.S.; Wang, G.H.; Li, R.M. An integrated strategy for revealing the pharmacological changes based on metabolites profiling and network pharmacology: Arctiin as an example. J. Chromatography. B Anal. Technol. Biomed. Life Sci. 2020, 10, 1157–122270. [Google Scholar] [CrossRef]
- Chen, D.; Ye, Z.; Wang, C.; Wang, Q.; Wang, H.; Kuek, V.; Wang, Z.; Qiu, H.; Yuan, J.; Kenny, J.; et al. Arctiin abrogates osteoclastogenesis and bone resorption via suppressing RANKL-induced ROS and NFATc1 activation. Pharmacol. Res. 2020, 159, 104944. [Google Scholar] [CrossRef]
- Ke, K.; Safder, M.A.; Sul, O.J.; Kim, W.K.; Suh, J.H.; Joe, Y.; Chung, H.T.; Choi, H.S. Hemeoxygenase-1 maintains bone mass via attenuating a redox imbalance in osteoclast. Mol. Cell. Endocrinol. 2015, 409, 11–20. [Google Scholar] [CrossRef]
- Okayasu, M.; Nakayachi, M.; Hayashida, C.; Ito, J.; Kaneda, T.; Masuhara, M.; Suda, N.; Sato, T.; Hakeda, Y. Low-density lipoprotein receptor deficiency causes impaired osteoclastogenesis and increased bone mass in mice because of defect in osteoclastic cell-cell fusion. J. Biol. Chem. 2012, 287, 19229–19241. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Son, H.J.; Sul, O.J.; Suh, J.H.; Choi, H.S. 4-Phenylbutyric acid protects against lipopolysaccharide-induced bone loss by modulating autophagy in osteoclasts. Biochem. Pharmacol. 2018, 151, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Bil, J.; Winiarska, M.; Nowis, D.; Bojarczuk, K.; Dabrowska-Iwanicka, A.; Basak, G.W.; Sułek, K.; Jakobisiak, M.; Golab, J. Bortezomib modulates surface CD20 in B-cell malignancies and affects rituximab-mediated complement-dependent cytotoxicity. Blood 2010, 115, 3745–3755. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, E.; Buricchi, F.; Raugei, G.; Ramponi, G.; Chiarugi, P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol. Cell. Biol. 2005, 25, 6391–6403. [Google Scholar] [CrossRef]
- Farnaghi, S.; Crawford, R.; Xiao, Y.; Prasadam, I. Cholesterol metabolism in pathogenesis of osteoarthritis disease. Int. J. Rheum. Dis. 2017, 20, 131–140. [Google Scholar] [CrossRef]
- Luo, H.; Li, J.; Cao, H.; Tan, Y.; Magdalou, J.; Chen, L.; Wang, H. Prenatal caffeine exposure induces a poor quality of articular cartilage in male adult offspring rats via cholesterol accumulation in cartilage. Sci. Rep. 2015, 5, 17746. [Google Scholar] [CrossRef]
- Arai, A.; Kim, S.; Goldshteyn, V.; Kim, T.; Park, N.H.; Wang, C.Y.; Kim, R.H. Beclin1 modulates bone homeostasis by regulating osteoclast and chondrocyte differentiation. J. Bone Miner. Res. 2019, 34, 1753–1766. [Google Scholar] [CrossRef]
- Settembre, C.; Malta, C.D.; Polito, V.A.; Garcia Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef]
- Di Malta, C.; Cinque, L.; Settembre, C. Transcriptional regulation of autophagy: Mechanisms and diseases. Front. Cell Dev. Biol. 2019, 7, 114. [Google Scholar] [CrossRef]
- Settembre, C.; Zoncu, R.; Medina, D.L.; Vetrini, F.; Erdin, S.; Erdin, S.; Huynh, T.; Ferron, M.; Karsenty, G.; Vellard, M.; et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012, 31, 1095–1108. [Google Scholar] [CrossRef]
- Wang, H.F.; Wang, N.N.; Xu, D.L.; Ma, Q.L.; Chen, Y.; Xu, S.Q.; Xia, Q.; Zhang, Y.; Prehn, J.; Wang, G.; et al. Oxidation of multiple MiT/TFE transcription factors links oxidative stress to transcriptional control of autophagy and lysosome biogenesis. Autophagy 2020, 16, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Kang, S.W.; Jeong, W.; Chang, T.S.; Yang, K.S.; Woo, H.A. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr. Opin. Cell Biol. 2005, 17, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Yabaji, S.M.; Mishra, A.K.; Chatterjee, A.; Dubey, R.K.; Srivastava, K.; Srivastava, K.K. Peroxiredoxin-1 of macrophage is critical for mycobacterial infection and is controlled by early secretory antigenic target protein through the activation of p38 MAPK. Biochem. Biophys. Res. Commun. 2017, 17, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Kim, S.; Park, J.G.; Jung, I.H.; Lee, M.N.; Jeon, S.J.; Kweon, H.Y.; Yu, D.Y.; Lee, S.H.; Jang, Y.; et al. Prdx1 (peroxiredoxin 1) deficiency reduces cholesterol efflux via impaired macrophage lipophagic flux. Autophagy 2018, 14, 120–133. [Google Scholar] [CrossRef]
- Min, Y.; Kim, M.J.; Lee, S.; Chun, E.; Lee, K.Y. Inhibition of TRAF6 ubiquitin-ligase activity by PRDX1 leads to inhibition of NFKB activation and autophagy activation. Autophagy 2018, 14, 1347–1358. [Google Scholar] [CrossRef]
- Vasconcelos, A.R.; dos Santos, N.B.; Scavone, C.; Munhoz, C.D. Nrf2/ARE Pathway modulation by dietary energy regulation in neurological disorders. Front. Pharmacol. 2019, 10, 33. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, L.; Liang, Y.; Li, J.; Pan, X. Arctiin suppresses H9N2 avian influenza virus-mediated inflammation via activation of Nrf2/HO-1 signaling. BMC Complement. Med. Ther. 2021, 21, 289. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, L.; Yao, W.; Han, J.; Wang, G. Arctiin antagonizes triptolide-induced hepatotoxicity via activation of Nrf2 pathway. BioMed Res. Int. 2020, 2020, 2508952. [Google Scholar] [CrossRef]
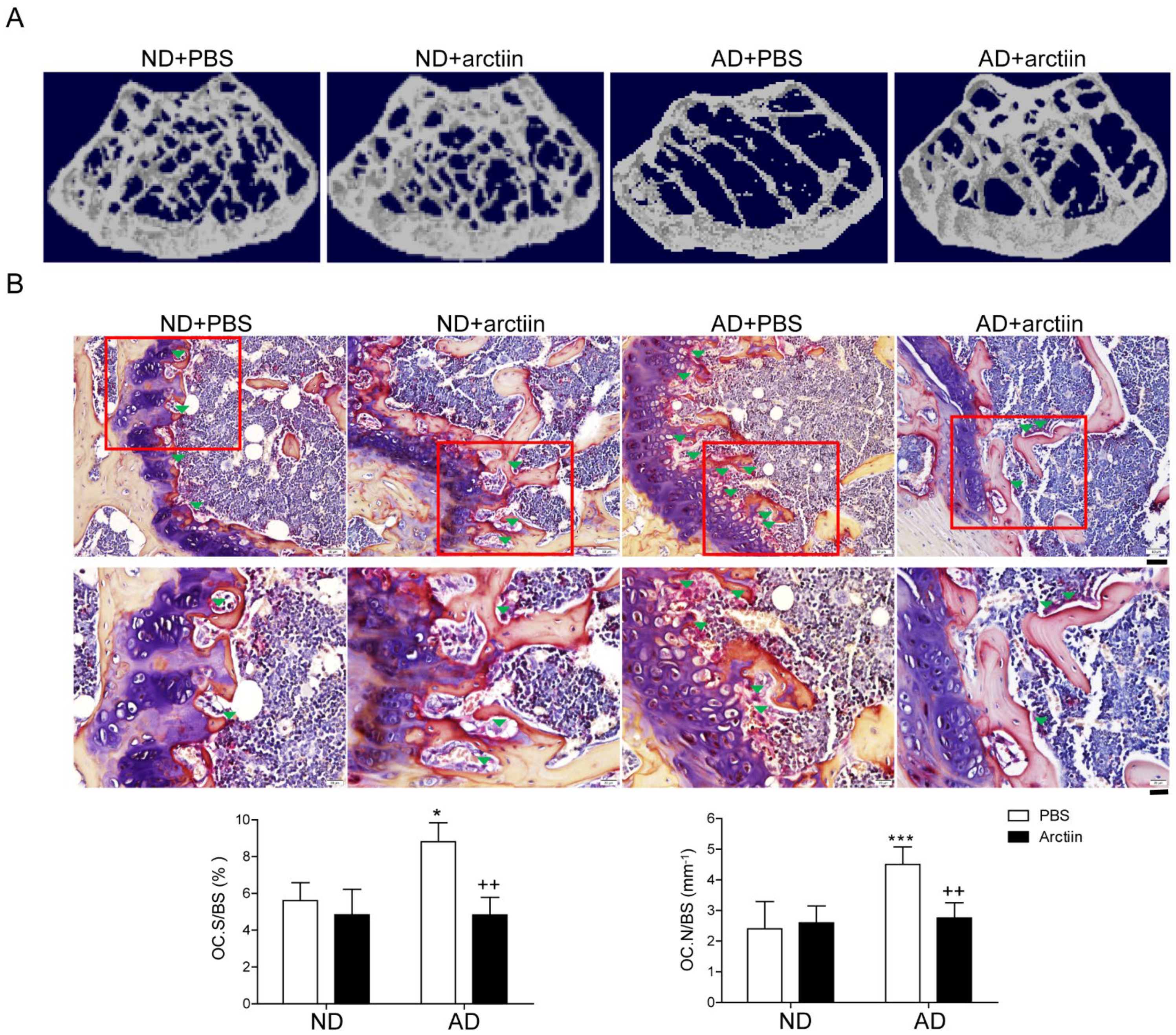


| Parameter | ND | AD | ||
|---|---|---|---|---|
| PBS | Arctiin | PBS | Arctiin | |
| BMD [mg/cm3] | 270.7 ± 16.23 | 266 ± 4.00 | 192.8 ± 7.11 *** | 232.3 ± 9.10 ++ |
| BV/TV [%] | 23.00 ± 1.44 | 23.03 ± 0.42 | 16.43 ± 0.51 *** | 20.25 ± 1.01 ++ |
| Tb.Th [μm] | 75.58 ± 2.47 | 73.19 ± 2.05 | 66.10 ± 1.04 ** | 74.92 ± 1.34 +++ |
| Tb.N [mm−1] | 3.04 ± 0.12 | 3.15 ± 0.04 | 2.441 ± 0.06 *** | 2.71 ± 0.09 + |
| Tb.Sp [μm] | 253.1 ± 10.50 | 250.2 ± 3.71 | 388.2 ± 17.45 ** | 326.4 ± 18.30 + |
| ALP [U/L] | 30.01 ± 0.36 | 30.43 ± 3.59 | 53.75 ± 3.25 *** | 55.22 ± 1.89 |
| OCN [ng/mL] | 18.60 ± 0.86 | 20.95 ± 2.76 | 27.37 ± 2.16 * | 23.33 ± 2.48 |
| CTX-1 [ng/mL] | 17.25 ± 1.94 | 16.03 ± 2.70 | 26.81 ± 1.30 ** | 19.27 ± 1.23 + |
| H2O2 [μM] | 60.86 ± 2.41 | 59.08 ± 1.15 | 69.29 ± 0.69 ** | 61.72 ± 1.83 ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Park, J.-N.; Park, H.-J.; Suh, J.-H.; Choi, H.-S. High Cholesterol-Induced Bone Loss Is Attenuated by Arctiin via an Action in Osteoclasts. Nutrients 2022, 14, 4483. https://doi.org/10.3390/nu14214483
Li G, Park J-N, Park H-J, Suh J-H, Choi H-S. High Cholesterol-Induced Bone Loss Is Attenuated by Arctiin via an Action in Osteoclasts. Nutrients. 2022; 14(21):4483. https://doi.org/10.3390/nu14214483
Chicago/Turabian StyleLi, Guoen, Jung-Nam Park, Hyun-Jung Park, Jae-Hee Suh, and Hye-Seon Choi. 2022. "High Cholesterol-Induced Bone Loss Is Attenuated by Arctiin via an Action in Osteoclasts" Nutrients 14, no. 21: 4483. https://doi.org/10.3390/nu14214483
APA StyleLi, G., Park, J.-N., Park, H.-J., Suh, J.-H., & Choi, H.-S. (2022). High Cholesterol-Induced Bone Loss Is Attenuated by Arctiin via an Action in Osteoclasts. Nutrients, 14(21), 4483. https://doi.org/10.3390/nu14214483





