Microbiota Modulation in Patients with Metabolic Syndrome
Abstract
:1. Introduction
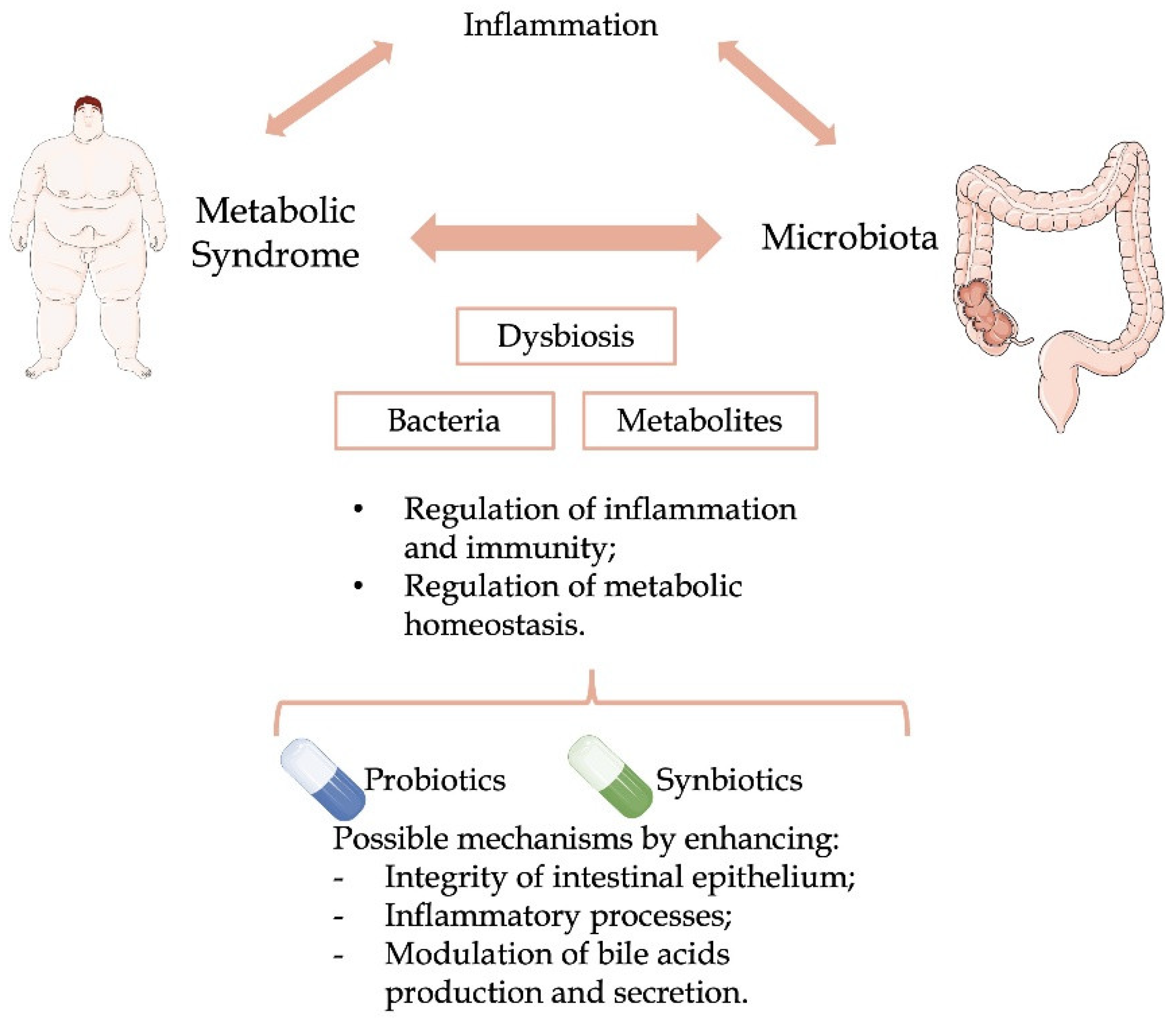
2. Metabolic Syndrome and Microbiota
3. Administration of Probiotic Supplements
3.1. Effects and Mechanisms of Action
3.2. Probiotics in MS
3.3. Synbiotics in MS
3.4. Other Foods with Probiotics in MS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo, C.; Williams, K.; Hunt, K.J.; Haffner, S.M. The National Cholesterol Education Program—Adult Treatment Panel III, International Diabetes Federation, and World Health Organization Definitions of the Metabolic Syndrome as Predictors of Incident Cardiovascular Disease and Diabetes. Diabetes Care 2007, 30, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The Metabolic Syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [Green Version]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Lee, Y.S.; Olefsky, J. Chronic tissue inflammation and metabolic disease. Genes Dev. 2021, 35, 307–328. [Google Scholar] [CrossRef]
- Ilkun, O.; Boudina, S. Cardiac Dysfunction and Oxidative Stress in the Metabolic Syndrome: An Update on Antioxidant Therapies. Curr. Pharm. Des. 2013, 19, 4806–4817. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M.; Stone, N.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic Analysis of the Human Distal Gut Microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasim, S.A.; Opulencia, M.J.C.; Ramírez-Coronel, A.A.; Abdelbasset, W.K.; Abed, M.H.; Markov, A.; Al-Awsi, G.R.L.; Shamsiev, J.A.; Hammid, A.T.; Shalaby, M.N.; et al. The emerging role of microbiota-derived short-chain fatty acids in immunometabolism. Int. Immunopharmacol. 2022, 110, 108983. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The Gut Microbiota as an Environmental Factor That Regulates Fat Storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [Green Version]
- Bäckhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.T.; Nieuwdorp, M.; Bäckhed, F. Microbial Modulation of Insulin Sensitivity. Cell Metab. 2014, 20, 753–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, T.O.; Lundgren, P.; Nath, K.; Thaiss, C.A. Metabolic control by the microbiome. Genome Med. 2022, 14, 80. [Google Scholar] [CrossRef]
- Su, X.; Gao, Y.; Yang, R. Gut Microbiota-Derived Tryptophan Metabolites Maintain Gut and Systemic Homeostasis. Cells 2022, 11, 2296. [Google Scholar] [CrossRef]
- Deschasaux, M.; Bouter, K.; Prodan, A.; Levin, E.; Groen, A.; Herrema, H.; Tremaroli, V.; Snijder, M.; Nicolaou, M.; Zwinderman, A.; et al. Differences in gut microbiota composition in metabolic syndrome and type 2 diabetes subjects in a multi-ethnic population: The HELIUS study. Proc. Nutr. Soc. 2020, 79, E183. [Google Scholar] [CrossRef]
- Pircalabioru, G.G.; Ilie, I.; Oprea, L.; Picu, A.; Petcu, L.M.; Burlibasa, L.; Chifiriuc, M.-C.; Musat, M. Microbiome, Mycobiome and Related Metabolites Alterations in Patients with Metabolic Syndrome—A Pilot Study. Metabolites 2022, 12, 218. [Google Scholar] [CrossRef]
- Qin, Q.; Yan, S.; Yang, Y.; Chen, J.; Li, T.; Gao, X.; Yan, H.; Wang, Y.; Wang, J.; Wang, S.; et al. A Metagenome-Wide Association Study of the Gut Microbiome and Metabolic Syndrome. Front. Microbiol. 2021, 12, 682721. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.L.; Vlamakis, H.; Lee, J.W.J.; Besse, L.A.; Xanthakis, V.; Vasan, R.S.; Shaw, S.Y.; Xavier, R.J. Population study of the gut microbiome: Associations with diet, lifestyle, and cardiometabolic disease. Genome Med. 2021, 13, 188. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, A.; Bastiaanssen, T.F.S.; Cryan, J.F.; Tinahones, F.J.; Vioque, J.; Corella, D.; Fito, M.; Vidal, J.; Moreno-Indias, I.; Gomez-Perez, A.M.; et al. Taxonomic and Functional Fecal Microbiota Signatures Associated With Insulin Resistance in Non-Diabetic Subjects With Overweight/Obesity Within the Frame of the PREDIMED-Plus Study. Front. Endocrinol. 2022, 13, 804455. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Qin, Q.; Chen, J.; Yan, S.; Li, T.; Gao, X.; Yang, Y.; Li, A.; Ding, S. Gut Microbiome Alterations in Patients With Visceral Obesity Based on Quantitative Computed Tomography. Front. Cell. Infect. Microbiol. 2021, 11, 823262. [Google Scholar] [CrossRef]
- Org, E.; Blum, Y.; Kasela, S.; Mehrabian, M.; Kuusisto, J.; Kangas, A.J.; Soininen, P.; Wang, Z.; Ala-Korpela, M.; Hazen, S.L.; et al. Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort. Genome Biol. 2017, 18, 70. [Google Scholar] [CrossRef] [Green Version]
- De Mello, V.D.; Paananen, J.; Lindström, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamäki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O.; et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017, 7, 46337. [Google Scholar] [CrossRef] [Green Version]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Czarnecka, K.; Czarnecka, P.; Tronina, O.; Bączkowska, T.; Durlik, M. Multidirectional facets of obesity management in the metabolic syndrome population after liver transplantation. Immun. Inflamm. Dis. 2022, 10, 3–21. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lin, T.-L.; Tsai, Y.-L.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Next generation probiotics in disease amelioration. J. Food Drug Anal. 2019, 27, 615–622. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Bose, S.; Seo, J.-G.; Chung, W.-S.; Lim, C.-Y.; Kim, H. The effects of co-administration of probiotics with herbal medicine on obesity, metabolic endotoxemia and dysbiosis: A randomized double-blind controlled clinical trial. Clin. Nutr. 2014, 33, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, V.; Leber, B.; Lemesch, S.; Trajanoski, S.; Bashir, M.; Horvath, A.; Tawdrous, M.; Stojakovic, T.; Fauler, G.; Fickert, P.; et al. Lactobacillus casei Shirota Supplementation Does Not Restore Gut Microbiota Composition and Gut Barrier in Metabolic Syndrome: A Randomized Pilot Study. PLoS ONE 2015, 10, e0141399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Festi, D.; Schiumerini, R.; Eusebi, L.H.; Marasco, G.; Taddia, M.; Colecchia, A. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014, 20, 16079–16094. [Google Scholar] [CrossRef] [PubMed]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [Green Version]
- Natividad, J.M.; Agus, A.; Planchais, J.; Lamas, B.; Jarry, A.C.; Martin, R.; Michel, M.-L.; Chong-Nguyen, C.; Roussel, R.; Straube, M.; et al. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab. 2018, 28, 737–749.e4. [Google Scholar] [CrossRef] [Green Version]
- Abenavoli, L.; Di Renzo, L.; Boccuto, L.; Alwardat, N.; Gratteri, S.; De Lorenzo, A. Health benefits of Mediterranean diet in nonalcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 873–881. [Google Scholar] [CrossRef]
- Golonka, R.M.; Yeoh, B.S.; Vijay-Kumar, M. Dietary Additives and Supplements Revisited: The Fewer, the Safer for Gut and Liver Health. Curr. Pharmacol. Rep. 2019, 5, 303–316. [Google Scholar] [CrossRef]
- Rivero-Gutiérrez, B.; Gámez-Belmonte, R.; Suárez, M.D.; Lavín, J.L.; Aransay, A.M.; Olivares, M.; Martínez-Augustin, O.; de Medina, F.S.; Zarzuelo, A. A synbiotic composed of Lactobacillus fermentum CECT5716 and FOS prevents the development of fatty acid liver and glycemic alterations in rats fed a high fructose diet associated with changes in the microbiota. Mol. Nutr. Food Res. 2017, 61, 1600622. [Google Scholar] [CrossRef]
- Park, S.; Kang, J.; Choi, S.; Park, H.; Hwang, E.; Kang, Y.; Kim, A.; Holzapfel, W.; Ji, Y. Cholesterol-lowering effect of Lactobacillus rhamnosus BFE5264 and its influence on the gut microbiome and propionate level in a murine model. PLoS ONE 2018, 13, e0203150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natividad, J.M.; Lamas, B.; Pham, H.P.; Michel, M.-L.; Rainteau, D.; Bridonneau, C.; da Costa, G.; Van Hylckama Vlieg, J.; Sovran, B.; Chamignon, C.; et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat. Commun. 2018, 9, 2802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Sung, C.Y.J.; Lee, N.; Ni, Y.; Pihlajamäki, J.; Panagiotou, G.; El-Nezami, H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306–E1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purohit, V.; Bode, J.C.; Bode, C.; Brenner, D.; Choudhry, M.A.; Hamilton, F.; Kang, Y.J.; Keshavarzian, A.; Rao, R.; Sartor, R.B.; et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: Summary of a symposium. Alcohol 2008, 42, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Khanna, S.; Walia, S.; Kondepudi, K.K.; Shukla, G. Administration of indigenous probiotics modulate high-fat diet-induced metabolic syndrome in Sprague Dawley rats. Antonie van Leeuwenhoek 2020, 113, 1345–1359. [Google Scholar] [CrossRef]
- Hibberd, A.; Yde, C.; Ziegler, M.; Honoré, A.; Saarinen, M.; Lahtinen, S.; Stahl, B.; Jensen, H.; Stenman, L. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef. Microbes 2019, 10, 121–135. [Google Scholar] [CrossRef]
- Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; Tsilingiris, D.; Magkos, F.; Stratigou, T.; Kounatidis, D.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Non-Alcoholic Fatty Liver Disease: Current Evidence and Perspectives. Biomolecules 2021, 12, 56. [Google Scholar] [CrossRef]
- Lu, X.; Jing, Y.; Zhou, X.; Zhang, N.; Tai, J.; Cao, Y. Bacillus licheniformis Zhengchangsheng® Inhibits Obesity by Regulating the AMP-Activated Protein Kinase Signaling Pathway. Probiotics Antimicrob. Proteins 2021, 13, 1658–1667. [Google Scholar] [CrossRef]
- Kang, J.-H.; Yun, S.-I.; Park, M.-H.; Park, J.-H.; Jeong, S.-Y.; Park, H.-O. Anti-Obesity Effect of Lactobacillus gasseri BNR17 in High-Sucrose Diet-Induced Obese Mice. PLoS ONE 2013, 8, e54617. [Google Scholar] [CrossRef] [Green Version]
- Fåk, F.; Bäckhed, F. Lactobacillus reuteri Prevents Diet-Induced Obesity, but not Atherosclerosis, in a Strain Dependent Fashion in Apoe−/− Mice. PLoS ONE 2012, 7, e46837. [Google Scholar] [CrossRef]
- Mularczyk, M.; Bourebaba, Y.; Kowalczuk, A.; Marycz, K.; Bourebaba, L. Probiotics-rich emulsion improves insulin signalling in Palmitate/Oleate-challenged human hepatocarcinoma cells through the modulation of Fetuin-A/TLR4-JNK-NF-κB pathway. Biomed. Pharmacother. 2021, 139, 111560. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, R.; Li, X.-F.; Wang, R.-L. Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome. Br. J. Nutr. 2011, 107, 1429–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Mercado, A.I.; Navarro-Oliveros, M.; Robles-Sánchez, C.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Muñoz-Quezada, S.; Fontana, L.; Abadía-Molina, F. Microbial Population Changes and Their Relationship with Human Health and Disease. Microorganisms 2019, 7, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haro, C.; García-Carpintero, S.; Rangel-Zuñiga, O.A.; Alcala-Diaz, J.F.; Landa, B.B.; Clemente, J.C.; Pérez-Martínez, P.; Lopez-Miranda, J.; Pérez-Jiménez, F.; Camargo, A. Consumption of Two Healthy Dietary Patterns Restored Microbiota Dysbiosis in Obese Patients with Metabolic Dysfunction. Mol. Nutr. Food Res. 2017, 61, 1700440. [Google Scholar] [CrossRef] [PubMed]
- Gøbel, R.J.; Larsen, N.; Jakobsen, M.; Mølgaard, C.; Michaelsen, K.F. Probiotics to Adolescents with Obesity. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 673–678. [Google Scholar] [CrossRef] [Green Version]
- Leber, B.; Tripolt, N.; Blattl, D.; Eder, M.; Wascher, T.C.; Pieber, T.R.; Stauber, R.; Sourij, H.; Oettl, K.; Stadlbauer, V. The influence of probiotic supplementation on gut permeability in patients with metabolic syndrome: An open label, randomized pilot study. Eur. J. Clin. Nutr. 2012, 66, 1110–1115. [Google Scholar] [CrossRef] [Green Version]
- Tripolt, N.J.; Leber, B.; Triebl, A.; Köfeler, H.; Stadlbauer, V.; Sourij, H. Effect of Lactobacillus casei Shirota supplementation on trimethylamine-N-oxide levels in patients with metabolic syndrome: An open-label, randomized study. Atherosclerosis 2015, 242, 141–144. [Google Scholar] [CrossRef]
- Tripolt, N.; Leber, B.; Blattl, D.; Eder, M.; Wonisch, W.; Scharnagl, H.; Stojakovic, T.; Obermayer-Pietsch, B.; Wascher, T.; Pieber, T.; et al. Short communication: Effect of supplementation with Lactobacillus casei Shirota on insulin sensitivity, β-cell function, and markers of endothelial function and inflammation in subjects with metabolic syndrome—A pilot study. J. Dairy Sci. 2013, 96, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Tenorio-Jiménez, C.; Martínez-Ramírez, M.J.; Del Castillo-Codes, I.; Arraiza-Irigoyen, C.; Tercero-Lozano, M.; Camacho, J.; Chueca, N.; García, F.; Olza, J.; Plaza-Díaz, J.; et al. Lactobacillus reuteri V3401 Reduces Inflammatory Biomarkers and Modifies the Gastrointestinal Microbiome in Adults with Metabolic Syndrome: The PROSIR Study. Nutrients 2019, 11, 1761. [Google Scholar] [CrossRef] [Green Version]
- Tenorio-Jiménez, C.; Martínez-Ramírez, M.J.; Tercero-Lozano, M.; Arraiza-Irigoyen, C.; Del Castillo-Codes, I.; Olza, J.; Plaza-Díaz, J.; Fontana, L.; Migueles, J.H.; Olivares, M.; et al. Evaluation of the effect of Lactobacillus reuteri V3401 on biomarkers of inflammation, cardiovascular risk and liver steatosis in obese adults with metabolic syndrome: A randomized clinical trial (PROSIR). BMC Complement. Altern. Med. 2018, 18, 306. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Maiter, D.; Thissen, J.-P.; Loumaye, A.; Hermans, M.P.; Delzenne, N.M.; de Vos, W.M.; Cani, P.D. Serum metabolite profiling yields insights into health promoting effect of A. muciniphila in human volunteers with a metabolic syndrome. Gut Microbes 2021, 13, 1994270. [Google Scholar] [CrossRef] [PubMed]
- Kassaian, N.; Feizi, A.; Aminorroaya, A.; Amini, M. Probiotic and synbiotic supplementation could improve metabolic syndrome in prediabetic adults: A randomized controlled trial. Diabetes Metab. Syndr. 2018, 13, 2991–2996. [Google Scholar] [CrossRef]
- Rahimi, F.; Pasdar, Y.; Kaviani, M.; Abbasi, S.; Fry, H.; Hekmatdoost, A.; Nikpayam, O.; Sohrab, G.; Rezaei, M.; Nachvak, S.M.; et al. Efficacy of the Synbiotic Supplementation on the Metabolic Factors in Patients with Metabolic Syndrome: A Randomized, Triple-Blind, Placebo-Controlled Trial. Int. J. Clin. Pract. 2022, 2022, 2967977. [Google Scholar] [CrossRef] [PubMed]
- Eslamparast, T.; Zamani, F.; Hekmatdoost, A.; Sharafkhah, M.; Eghtesad, S.; Malekzadeh, R.; Poustchi, H. Effects of synbiotic supplementation on insulin resistance in subjects with the metabolic syndrome: A randomised, double-blind, placebo-controlled pilot study. Br. J. Nutr. 2014, 112, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, S.; Hedayati, M.; Rashidkhani, B.; Saadat, N.; Shakerhossini, R. The Effects of Synbiotic Supplementation on Body Mass Index, Metabolic and Inflammatory Biomarkers, and Appetite in Patients with Metabolic Syndrome: A Triple-Blind Randomized Controlled Trial. J. Diet. Suppl. 2018, 16, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Impact of a short-term synbiotic supplementation on metabolic syndrome and systemic inflammation in elderly patients: A randomized placebo-controlled clinical trial. Eur. J. Nutr. 2021, 60, 655–663. [Google Scholar] [CrossRef]
- Chang, B.J.; Park, S.U.; Jang, Y.S.; Ko, S.H.; Joo, N.M.; I Kim, S.; Kim, C.-H.; Chang, D.K. Effect of functional yogurt NY-YP901 in improving the trait of metabolic syndrome. Eur. J. Clin. Nutr. 2011, 65, 1250–1255. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi-Sartang, M.; Bellissimo, N.; de Zepetnek, J.O.T.; Brett, N.; Mazloomi, S.M.; Fararouie, M.; Bedeltavana, A.; Famouri, M.; Mazloom, Z. The effect of daily fortified yogurt consumption on weight loss in adults with metabolic syndrome: A 10-week randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 565–574. [Google Scholar] [CrossRef]
- Rezazadeh, L.; Gargari, B.P.; Jafarabadi, M.A.; Alipour, B. Effects of probiotic yogurt on glycemic indexes and endothelial dysfunction markers in patients with metabolic syndrome. Nutrition 2019, 62, 162–168. [Google Scholar] [CrossRef]
- Barreto, F.M.; Simão, A.N.C.; Morimoto, H.K.; Lozovoy, M.A.B.; Dichi, I.; da Silva Miglioranza, L.H. Beneficial effects of Lactobacillus plantarum on glycemia and homocysteine levels in postmenopausal women with metabolic syndrome. Nutrition 2014, 30, 939–942. [Google Scholar] [CrossRef]
- Bernini, L.J.; Simão, A.N.C.; Alfieri, D.F.; Lozovoy, M.A.B.; Mari, N.L.; de Souza, C.H.B.; Dichi, I.; Costa, G.N. Beneficial effects of Bifidobacterium lactis on lipid profile and cytokines in patients with metabolic syndrome: A randomized trial. Effects of probiotics on metabolic syndrome. Nutrition 2016, 32, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Bellikci-Koyu, E.; Sarer-Yurekli, B.P.; Karagozlu, C.; Aydin-Kose, F.; Ozgen, A.G.; Buyuktuncer, Z. Probiotic kefir consumption improves serum apolipoprotein A1 levels in metabolic syndrome patients: A randomized controlled clinical trial. Nutr. Res. 2022, 102, 59–70. [Google Scholar] [CrossRef] [PubMed]
- da Silva Ghizi, A.; de Almeida Silva, M.; da Andrade Moraes, F.; da Silva, C.; Endringer, D.; Scherer, R.; Lenz, D.; de Lima, E.M.; Brasil, G.A.; Maia, J.F.; et al. Kefir improves blood parameters and reduces cardiovascular risks in patients with metabolic syndrome. PharmaNutrition 2021, 16, e100266. [Google Scholar] [CrossRef]
- Shapiro, H.; Suez, J.; Elinav, E. Personalized microbiome-based approaches to metabolic syndrome management and prevention. J. Diabetes 2017, 9, 226–236. [Google Scholar] [CrossRef]
- Duseja, A.; Acharya, S.K.; Mehta, M.; Chhabra, S.; Shalimar; Rana, S.; Das, A.; Dattagupta, S.; Dhiman, R.K.; Chawla, Y.K. High potency multistrain probiotic improves liver histology in non-alcoholic fatty liver disease (NAFLD): A randomised, double-blind, proof of concept study. BMJ Open Gastroenterol. 2019, 6, e000315. [Google Scholar] [CrossRef] [Green Version]
- Reinehr, T.; Roth, C.L. Fetuin-A and Its Relation to Metabolic Syndrome and Fatty Liver Disease in Obese Children Before and After Weight Loss. J. Clin. Endocrinol. Metab. 2008, 93, 4479–4485. [Google Scholar] [CrossRef] [Green Version]
- Gorenjak, M.; Gradišnik, L.; Trapečar, M.; Pistello, M.; Kozmus, C.; Škorjanc, D.; Skok, P.; Langerholc, T.; Cencič, A. Improvement of lipid profile by probiotic/protective cultures: Study in a non-carcinogenic small intestinal cell model. New Microbiol. 2014, 37, 51–64. [Google Scholar]
- Llévenes, P.; Rodrigues-Díez, R.; Cros-Brunsó, L.; Prieto, M.I.; Casaní, L.; Balfagón, G.; Blanco-Rivero, J. Beneficial Effect of a Multistrain Synbiotic Prodefen® Plus on the Systemic and Vascular Alterations Associated with Metabolic Syndrome in Rats: The Role of the Neuronal Nitric Oxide Synthase and Protein Kinase A. Nutrients 2020, 12, 117. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Liu, K.; Gao, D.; Hao, J. Protective effects of two Lactobacillus plantarum strains in hyperlipidemic mice. World J. Gastroenterol. 2013, 19, 3150–3156. [Google Scholar] [CrossRef]
- Oh, J.-H.; Schueler, K.L.; Stapleton, D.S.; Alexander, L.M.; Yen, C.-L.E.; Keller, M.P.; Attie, A.D.; van Pijkeren, J.-P. Secretion of Recombinant Interleukin-22 by Engineered Lactobacillus reuteri Reduces Fatty Liver Disease in a Mouse Model of Diet-Induced Obesity. mSphere 2020, 5, e00183-20. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araujo, R.; Borges-Canha, M.; Pimentel-Nunes, P. Microbiota Modulation in Patients with Metabolic Syndrome. Nutrients 2022, 14, 4490. https://doi.org/10.3390/nu14214490
Araujo R, Borges-Canha M, Pimentel-Nunes P. Microbiota Modulation in Patients with Metabolic Syndrome. Nutrients. 2022; 14(21):4490. https://doi.org/10.3390/nu14214490
Chicago/Turabian StyleAraujo, Ricardo, Marta Borges-Canha, and Pedro Pimentel-Nunes. 2022. "Microbiota Modulation in Patients with Metabolic Syndrome" Nutrients 14, no. 21: 4490. https://doi.org/10.3390/nu14214490
APA StyleAraujo, R., Borges-Canha, M., & Pimentel-Nunes, P. (2022). Microbiota Modulation in Patients with Metabolic Syndrome. Nutrients, 14(21), 4490. https://doi.org/10.3390/nu14214490







