Methionine Restriction Improves Cognitive Ability by Alleviating Hippocampal Neuronal Apoptosis through H19 in Middle-Aged Insulin-Resistant Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiment
2.2. Tests of Novel Object Recognition and Morris Water Maze
2.3. TdT-Mediated dUTP Nick End Labeling (TUNEL) Fluorescent Assay and Nissl Staining Assay
2.4. Detection of Oxidative Stress Index, ROS Levels, Fasting Glucose, and Fasting Insulin
2.5. Cell Culture and Transfection
2.6. Cell Viability Assay
2.7. Measurement of ROS in HT22
2.8. Flow Cytometric Examination
2.9. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. MR Improved Cognitive Ability in HFD-Induced Insulin-Resistant Mice
3.2. MR Improved Insulin Resistance and Insulin Signaling in HFD Mice
3.3. MR Improved Hippocampal Oxidative Stress and Inhibited H19 Expression in HFD-Induced Insulin-Resistant Mice
3.4. MR Alleviated Neuronal Injury and Apoptosis in Hippocampus of HFD-Induced Insulin-Resistant Mice
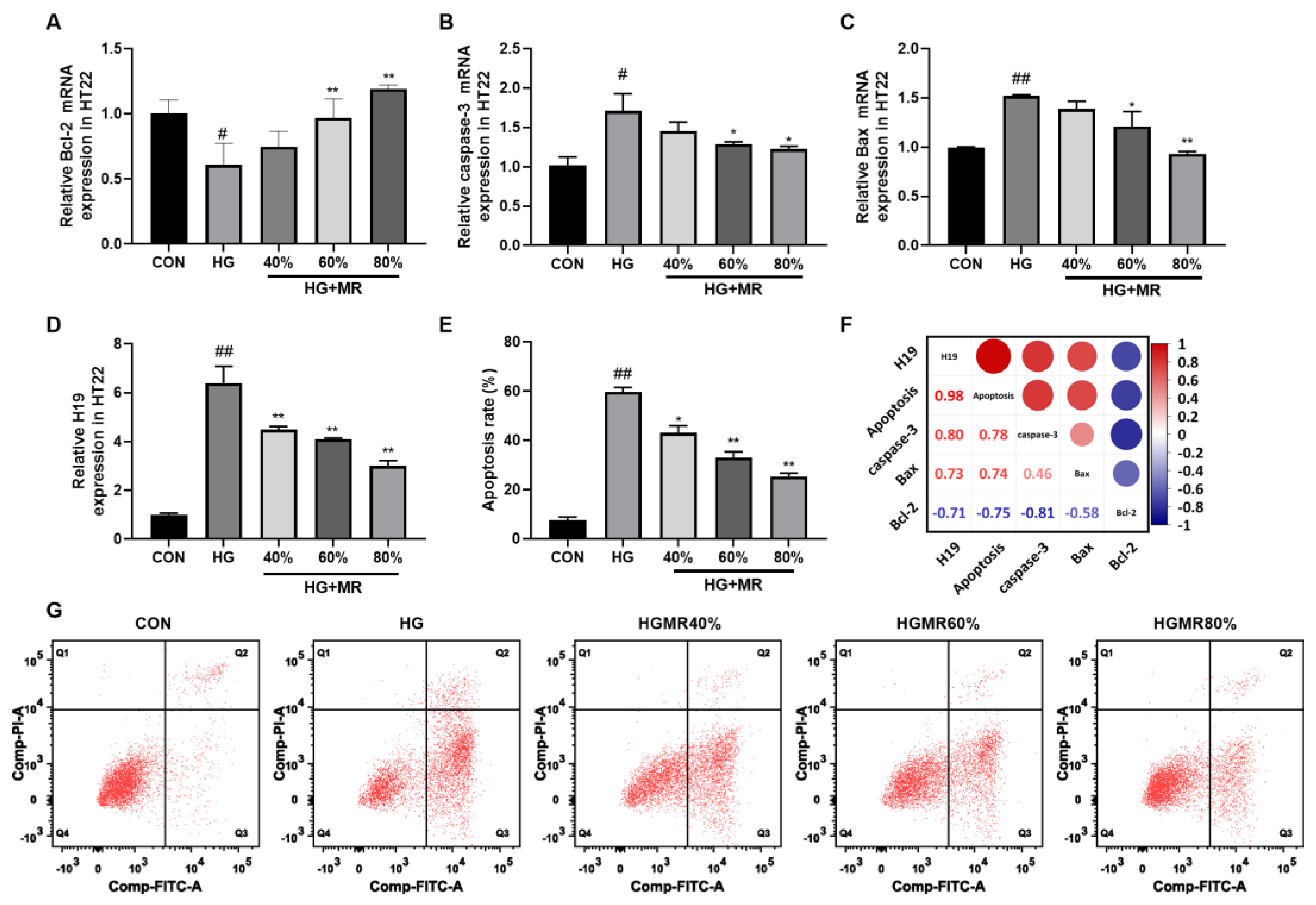
3.5. MR Inhibited the High Expression of H19 Induced by High Glucose in HT22 Cells
3.6. MR Inhibited the High Expression of H19 Induced by ROS in HT22 Cells
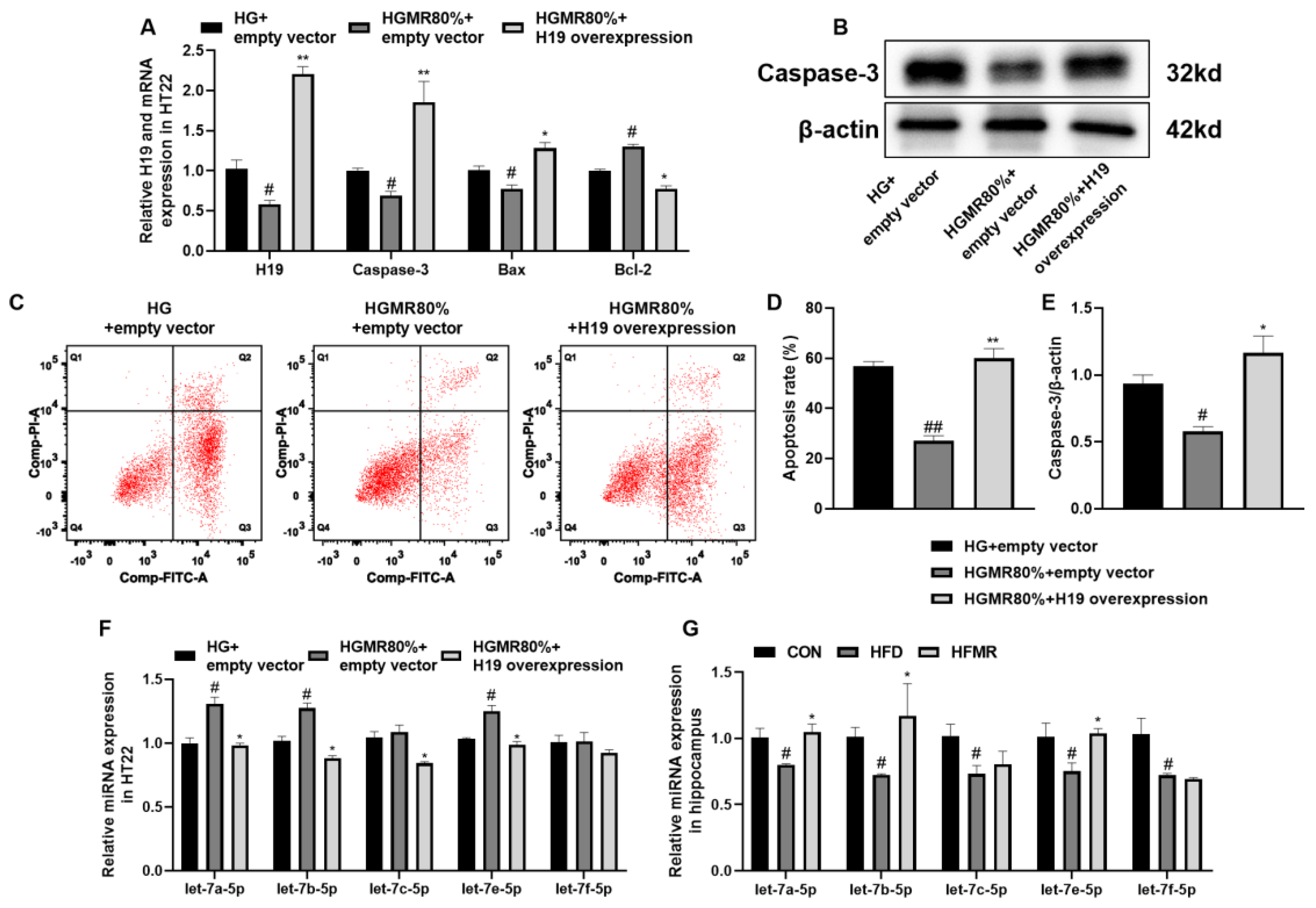
3.7. MR Inhibited HT22 Cell Apoptosis through Inhibiting H19
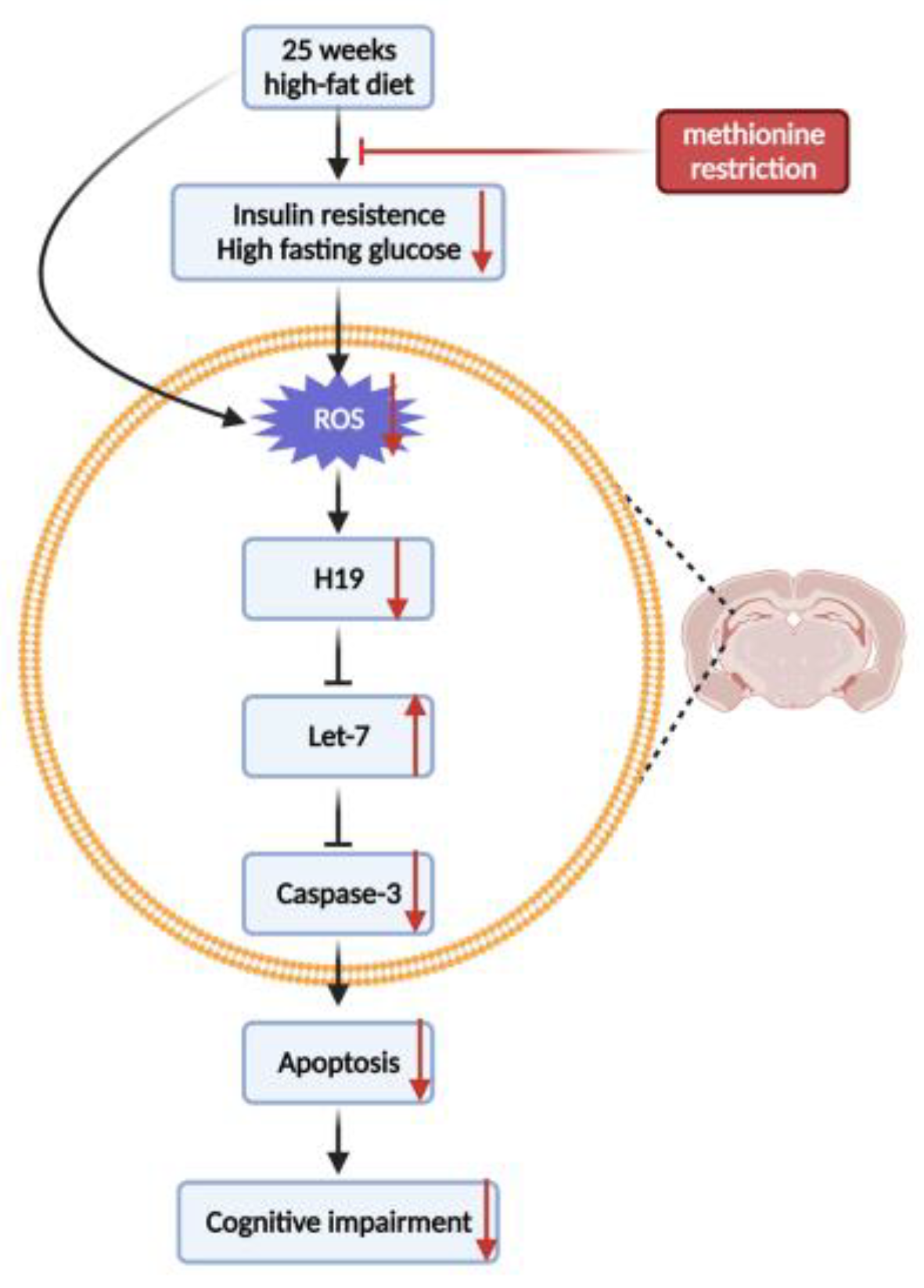
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uranga, R.M.; Bruce-Keller, A.J.; Morrison, C.D.; Fernandez-Kim, S.O.; Ebenezer, P.J.; Zhang, L.; Dasuri, K.; Keller, J.N. Intersection between metabolic dysfunction, high fat diet consumption, and brain aging. J. Neurochem. 2010, 114, 344–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sa-Nguanmoo, P.; Tanajak, P.; Kerdphoo, S.; Satjaritanun, P.; Wang, X.; Liang, G.; Li, X.; Jiang, C.; Pratchayasakul, W.; Chattipakorn, N.; et al. FGF21 improves cognition by restored synaptic plasticity, dendritic spine density, brain mitochondrial function and cell apoptosis in obese-insulin resistant male rats. Horm. Behav. 2016, 85, 86–95. [Google Scholar] [CrossRef]
- Tome-Carneiro, J.; Carmen Crespo, M.; Burgos-Ramos, E.; Tomas-Zapico, C.; Garcia-Serrano, A.; Castro-Gomez, P.; Venero, C.; Pereda-Perez, I.; Baliyan, S.; Valencia, A.; et al. Buttermilk and Krill Oil Phospholipids Improve Hippocampal Insulin Resistance and Synaptic Signaling in Aged Rats. Mol. Neurobiol. 2018, 55, 7285–7296. [Google Scholar] [CrossRef] [PubMed]
- Barcena, C.; Quiros, P.M.; Durand, S.; Mayoral, P.; Rodriguez, F.; Caravia, X.M.; Marino, G.; Garabaya, C.; Fernandez-Garcia, M.T.; Kroemer, G.; et al. Methionine Restriction Extends Lifespan in Progeroid Mice and Alters Lipid and Bile Acid Metabolism. Cell Rep. 2018, 24, 2392–2403. [Google Scholar] [CrossRef] [Green Version]
- Lees, E.K.; Krol, E.; Grant, L.; Shearer, K.; Wyse, C.; Moncur, E.; Bykowska, A.S.; Mody, N.; Gettys, T.W.; Delibegovic, M. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell 2014, 13, 817–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, K.P.; Wanders, D.; Orgeron, M.; Cortez, C.C.; Gettys, T.W. Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes 2014, 63, 3721–3733. [Google Scholar] [CrossRef] [Green Version]
- Luo, T.; Yang, Y.; Xu, Y.; Gao, Q.; Wu, G.; Jiang, Y.; Sun, J.; Shi, Y.; Le, G. Dietary methionine restriction improves glucose metabolism in the skeletal muscle of obese mice. Food Funct. 2019, 10, 2676–2690. [Google Scholar] [CrossRef]
- Bosco, D.; Fava, A.; Plastino, M.; Montalcini, T.; Pujia, A. Possible implications of insulin resistance and glucose metabolism in Alzheimer’s disease pathogenesis. J. Cell. Mol. Med. 2011, 15, 1807–1821. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Q.; Zhang, J.; Li, C.Y.; Wang, Y.; Zeng, M.J.; Cai, Z.X.; Tian, R.B.; Jia, W.; Li, X.H. Insulin resistance-induced hyperglycemia decreased the activation of Akt/CREB in hippocampus neurons: Molecular evidence for mechanism of diabetes-induced cognitive dysfunction. Neuropeptides 2015, 54, 9–15. [Google Scholar] [CrossRef]
- Suwanjang, W.; Prachayasittikul, S.; Prachayasittikul, V. Effect of 8-hydroxyquinoline and derivatives on human neuroblastoma SH-SY5Y cells under high glucose. PeerJ 2016, 4, e2389. [Google Scholar] [CrossRef]
- Lee, J.H.; Yang, D.S.; Goulbourne, C.N.; Im, E.; Stavrides, P.; Pensalfini, A.; Chan, H.; Bouchet-Marquis, C.; Bleiwas, C.; Berg, M.J.; et al. Faulty autolysosome acidification in Alzheimer’s disease mouse models induces autophagic build-up of Abeta in neurons, yielding senile plaques. Nat. Neurosci. 2022, 25, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Zilliox, L.A.; Chadrasekaran, K.; Kwan, J.Y.; Russell, J.W. Diabetes and Cognitive Impairment. Curr. Diab. Rep. 2016, 16, 87. [Google Scholar] [CrossRef] [Green Version]
- Sickmann, H.M.; Waagepetersen, H.S.; Schousboe, A.; Benie, A.J.; Bouman, S.D. Obesity and type 2 diabetes in rats are associated with altered brain glycogen and amino-acid homeostasis. J. Cereb. Blood Flow Metab. 2010, 30, 1527–1537. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Alipoor, B.; Parvar, S.N.; Sabati, Z.; Ghaedi, H.; Ghasemi, H. An updated review of the H19 lncRNA in human cancer: Molecular mechanism and diagnostic and therapeutic importance. Mol. Biol. Rep. 2020, 47, 6357–6374. [Google Scholar] [CrossRef] [PubMed]
- Pastori, C.; Wahlestedt, C. Involvement of long noncoding RNAs in diseases affecting the central nervous system. RNA Biol. 2012, 9, 860–870. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhuang, Y.; Zhao, X.; Li, X. Long Non-coding RNA in Neuronal Development and Neurological Disorders. Front. Genet. 2018, 9, 744. [Google Scholar] [CrossRef] [Green Version]
- Aliperti, V.; Skonieczna, J.; Cerase, A. Long Non-Coding RNA (lncRNA) Roles in Cell Biology, Neurodevelopment and Neurological Disorders. Noncoding RNA 2021, 7, 36. [Google Scholar] [CrossRef]
- Lim, K.H.; Yang, S.; Kim, S.H.; Chun, S.; Joo, J.Y. Discoveries for Long Non-Coding RNA Dynamics in Traumatic Brain Injury. Biology 2020, 9, 458. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Bao, H.L.; Dong, L.X.; Liu, Y.; Zhang, G.W.; An, F.M. Silenced lncRNA H19 and up-regulated microRNA-129 accelerates viability and restrains apoptosis of PC12 cells induced by Aβ25-35 in a cellular model of Alzheimer’s disease. Cell Cycle 2021, 20, 112–125. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Ji, T.F.; Luo, Q.; Yu, J.L. Long non-coding RNA H19 induces hippocampal neuronal apoptosis via Wnt signaling in a streptozotocin-induced rat model of diabetes mellitus. Oncotarget 2017, 8, 64827–64839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Tang, C.; Wang, D. LncRNA H19 promotes inflammatory response induced by cerebral ischemia-reperfusion injury through regulating the miR-138-5p-p65 axis. Biochem. Cell Biol. 2020, 98, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Han, C.L.; Ge, M.; Liu, Y.P.; Zhao, X.M.; Wang, K.L.; Chen, N.; Meng, W.J.; Hu, W.; Zhang, J.G.; Li, L.; et al. LncRNA H19 contributes to hippocampal glial cell activation via JAK/STAT signaling in a rat model of temporal lobe epilepsy. J. Neuroinflamm. 2018, 15, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Chen, X.; Guo, R.; Meng, J. Protective effects of lncRNA H19 silence against hypoxia-induced injury in PC-12 cells by regulating miR-28. Int. J. Biol. Macromol. 2019, 121, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Han, C.L.; Ge, M.; Liu, Y.P.; Zhao, X.M.; Wang, K.L.; Chen, N.; Hu, W.; Zhang, J.G.; Li, L.; Meng, F.G. Long non-coding RNA H19 contributes to apoptosis of hippocampal neurons by inhibiting let-7b in a rat model of temporal lobe epilepsy. Cell Death Dis. 2018, 9, 617. [Google Scholar] [CrossRef]
- Pu, M.; Chen, J.; Tao, Z.; Miao, L.; Qi, X.; Wang, Y.; Ren, J. Regulatory network of miRNA on its target: Coordination between transcriptional and post-transcriptional regulation of gene expression. Cell. Mol. Life Sci. 2019, 76, 441–451. [Google Scholar] [CrossRef]
- Juzwik, C.A.; Drake, S.S.; Zhang, Y.; Paradis-Isler, N.; Sylvester, A.; Amar-Zifkin, A.; Douglas, C.; Morquette, B.; Moore, C.S.; Fournier, A.E. microRNA dysregulation in neurodegenerative diseases: A systematic review. Prog. Neurobiol. 2019, 182, 101664. [Google Scholar] [CrossRef]
- Jovanovic, M.; Hengartner, M.O. miRNAs and apoptosis: RNAs to die for. Oncogene 2006, 25, 6176–6187. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Zhou, Y.; Zhang, R.; Wu, K.; Lu, Y.; Li, Y.; Duan, R.; Yao, Y.; Zhu, D.; Jia, Y. MicroRNA Let-7f-5p Promotes Bone Marrow Mesenchymal Stem Cells Survival by Targeting Caspase-3 in Alzheimer Disease Model. Front. Neurosci. 2018, 12, 333. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.; Wang, X.; Chen, S.; Liu, H.; Wang, Y.; Xu, X.; Cheng, J.; Jia, J.; Zhen, X. MicroRNA let-7c-5p protects against cerebral ischemia injury via mechanisms involving the inhibition of microglia activation. Brain. Behav. Immun. 2015, 49, 75–85. [Google Scholar] [CrossRef]
- Peng, G.; Yuan, Y.; He, Q.; Wu, W.; Luo, B.Y. MicroRNA let-7e regulates the expression of caspase-3 during apoptosis of PC12 cells following anoxia/reoxygenation injury. Brain Res. Bull. 2011, 86, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.P.; Kwok, T.T. Let-7a microRNA suppresses therapeutics-induced cancer cell death by targeting caspase-3. Apoptosis 2008, 13, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, Y.; Sun, J.; Zhang, Y.; Luo, T.; Li, B.; Jiang, Y.; Shi, Y.; Le, G. Dietary methionine restriction ameliorates the impairment of learning and memory function induced by obesity in mice. Food Funct. 2019, 10, 1411–1425. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Han, L.; Shi, Y.; Feng, C.; Yan, B.; Sun, J.; Tang, X.; Le, G. Effect of different levels of dietary methionine restriction on relieving oxidative stress and behavioral deficits in middle-aged mice fed low-, medium-, or high-fat diet. J. Funct. Foods 2020, 65, 103782. [Google Scholar] [CrossRef]
- Ren, B.; Wang, L.; Shi, L.; Jin, X.; Liu, Y.; Liu, R.H.; Yin, F.; Cadenas, E.; Dai, X.; Liu, Z.; et al. Methionine restriction alleviates age-associated cognitive decline via fibroblast growth factor 21. Redox Biol. 2021, 41, 101940. [Google Scholar] [CrossRef]
- Latimer, M.N.; Freij, K.W.; Cleveland, B.M.; Biga, P.R. Physiological and Molecular Mechanisms of Methionine Restriction. Front. Endocrinol. 2018, 9, 217. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Yang, L.; Zhong, T.; Mueller, M.; Men, Y.; Zhang, N.; Xie, J.; Giang, K.; Chung, H.; Sun, X.; et al. H19 lncRNA alters DNA methylation genome wide by regulating S-adenosylhomocysteine hydrolase. Nat. Commun. 2015, 6, 10221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitada, M.; Ogura, Y.; Monno, I.; Xu, J.; Koya, D. Effect of Methionine Restriction on Aging: Its Relationship to Oxidative Stress. Biomedicines 2021, 9, 130. [Google Scholar] [CrossRef]
- Maddineni, S.; Nichenametla, S.; Sinha, R.; Wilson, R.P.; Richie, J.P. Methionine restriction affects oxidative stress and glutathione-related redox pathways in the rat. Exp. Biol. Med. 2013, 238, 392–399. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shoorei, H.; Taheri, M. Non-coding RNAs are involved in the response to oxidative stress. Biomed. Pharmacother. 2020, 127, 110228. [Google Scholar] [CrossRef]
- Xin, L.; Lu, H.; Liu, C.; Zeng, F.; Yuan, Y.W.; Wu, Y.; Wang, J.L.; Wu, D.Z.; Zhou, L.Q. Methionine deficiency promoted mitophagy via lncRNA PVT1-mediated promoter demethylation of BNIP3 in gastric cancer. Int. J. Biochem. Cell Biol. 2021, 141, 106100. [Google Scholar] [CrossRef]
- Win-Shwe, T.T.; Fujimaki, H. Acute administration of toluene affects memory retention in novel object recognition test and memory function-related gene expression in mice. J. Appl. Toxicol. 2012, 32, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Bromley-Brits, K.; Deng, Y.; Song, W. Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J. Vis. Exp. 2011, 53, 2920. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Gil-Guzman, E.; Mahran, A.M.; Sharma, R.K.; Nelson, D.R.; Thomas, A.J.; Agarwal, A. Quality control of reactive oxygen species measurement by luminol-dependent chemiluminescence assay. J. Androl. 2001, 22, 568–574. [Google Scholar] [CrossRef]
- Xiao, F.; Yu, J.; Guo, Y.; Deng, J.; Li, K.; Du, Y.; Chen, S.; Zhu, J.; Sheng, H.; Guo, F. Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice. Metabolism 2014, 63, 841–850. [Google Scholar] [CrossRef]
- Wang, X.B.; Zou, M.X.; Li, J.; Wang, B.; Zhang, Q.S.; Liu, F.B.; Lu, G.H. LncRNA H19 targets miR-22 to modulate H2O2-induced deregulation in nucleus pulposus cell senescence, proliferation, and ECM synthesis through Wnt signaling. J. Cell. Biochem. 2018, 119, 4990–5002. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef] [Green Version]
- Mulati, A.; Ma, S.; Zhang, H.; Ren, B.; Zhao, B.; Wang, L.; Liu, X.; Zhao, T.; Kamanova, S.; Sair, A.T.; et al. Sea-Buckthorn Flavonoids Alleviate High-Fat and High-Fructose Diet-Induced Cognitive Impairment by Inhibiting Insulin Resistance and Neuroinflammation. J. Agric. Food Chem. 2020, 68, 5835–5846. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathogenesis of insulin resistance in skeletal muscle. J. Biomed. Biotechnol. 2010, 2010, 476279. [Google Scholar] [CrossRef] [Green Version]
- Soya, M.; Jesmin, S.; Shima, T.; Matsui, T.; Soya, H. Dysregulation of Glycogen Metabolism with Concomitant Spatial Memory Dysfunction in Type 2 Diabetes: Potential Beneficial Effects of Chronic Exercise. In Brain Glycogen Metabolism; DiNuzzo, M., Schousboe, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 363–383. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Zhao, L.; Han, H.; Zhang, S.; Huang, Y.; Wang, X.; Song, D.; Ma, P.; Ren, P.; et al. A neoteric dual-signal colorimetric fluorescent probe for detecting endogenous/exogenous hydrogen peroxide in cells and monitoring drug-induced hepatotoxicity. Talanta 2021, 233, 122578. [Google Scholar] [CrossRef]
- Zhuang, J.; Lu, J.; Wang, X.; Wang, X.F.; Hu, W.C.; Hong, F.S.; Zhao, X.X.; Zheng, Y.L. Purple sweet potato color protects against high-fat diet-induced cognitive deficits through AMPK-mediated autophagy in mouse hippocampus. J. Nutr. Biochem. 2019, 65, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Pugazhenthi, S.; Nesterova, A.; Jambal, P.; Audesirk, G.; Kern, M.; Cabell, L.; Eves, E.; Rosner, M.R.; Boxer, L.M.; Reusch, J.E. Oxidative stress-mediated down-regulation of bcl-2 promoter in hippocampal neurons. J. Neurochem. 2003, 84, 982–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keniry, A.; Oxley, D.; Monnier, P.; Kyba, M.; Dandolo, L.; Smits, G.; Reik, W. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat. Cell Biol. 2012, 14, 659–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, B.K.; Pfeifer, K.; Dutta, A. The H19 long noncoding RNA gives rise to microRNAs miR-675-3p and miR-675-5p to promote skeletal muscle differentiation and regeneration. Genes Dev. 2014, 28, 491–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Geng, T.; Wang, Z.; Zhang, R.; Cao, T.; Camporez, J.P.; Cai, S.Y.; Liu, Y.; Dandolo, L.; Shulman, G.I.; et al. Elevated hepatic expression of H19 long noncoding RNA contributes to diabetic hyperglycemia. JCI Insight 2018, 3, e120304. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wang, C.; Meng, F.; Xu, P. Long noncoding RNA H19 inhibition ameliorates oxygen-glucose deprivation-induced cell apoptosis and inflammatory cytokine expression by regulating the microRNA29b/SIRT1/PGC1alpha axis. Mol. Med. Rep. 2021, 23, 131. [Google Scholar] [CrossRef]
- Zhong, L.; Liu, P.; Fan, J.; Luo, Y. Long non-coding RNA H19: Physiological functions and involvements in central nervous system disorders. Neurochem. Int. 2021, 148, 105072. [Google Scholar] [CrossRef]
- Wang, J.; Cao, B.; Han, D.; Sun, M.; Feng, J. Long Non-coding RNA H19 Induces Cerebral Ischemia Reperfusion Injury via Activation of Autophagy. Aging Dis. 2017, 8, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Wang, W.T.; Ye, H.; Wei, P.P.; Han, B.W.; He, B.; Chen, Z.H.; Chen, Y.Q. LncRNAs H19 and HULC, activated by oxidative stress, promote cell migration and invasion in cholangiocarcinoma through a ceRNA manner. J. Hematol. Oncol. 2016, 9, 117. [Google Scholar] [CrossRef] [Green Version]
- Duan, S.; Li, M.; Wang, Z.; Wang, L.; Liu, Y. H19 induced by oxidative stress confers temozolomide resistance in human glioma cells via activating NF-κB signaling. Onco. Targets Ther. 2018, 11, 6395–6404. [Google Scholar] [CrossRef] [Green Version]
- Di Sanzo, M.; Chirillo, R.; Aversa, I.; Biamonte, F.; Santamaria, G.; Giovannone, E.D.; Faniello, M.C.; Cuda, G.; Costanzo, F. shRNA targeting of ferritin heavy chain activates H19/miR-675 axis in K562 cells. Gene 2018, 657, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Petri, R.; Pircs, K.; Jonsson, M.E.; Akerblom, M.; Brattas, P.L.; Klussendorf, T.; Jakobsson, J. let-7 regulates radial migration of new-born neurons through positive regulation of autophagy. EMBO J. 2017, 36, 1379–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallen, A.N.; Zhou, X.B.; Xu, J.; Qiao, C.; Ma, J.; Yan, L.; Lu, L.; Liu, C.; Yi, J.S.; Zhang, H.; et al. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol. Cell 2013, 52, 101–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Płóciennik, A.; Prendecki, M.; Zuba, E.; Siudzinski, M.; Dorszewska, J. Activated Caspase-3 and Neurodegeneration and Synaptic Plasticity in Alzheimer’s Disease. Adv. Alzheimer Dis. 2015, 4, 63–77. [Google Scholar] [CrossRef]
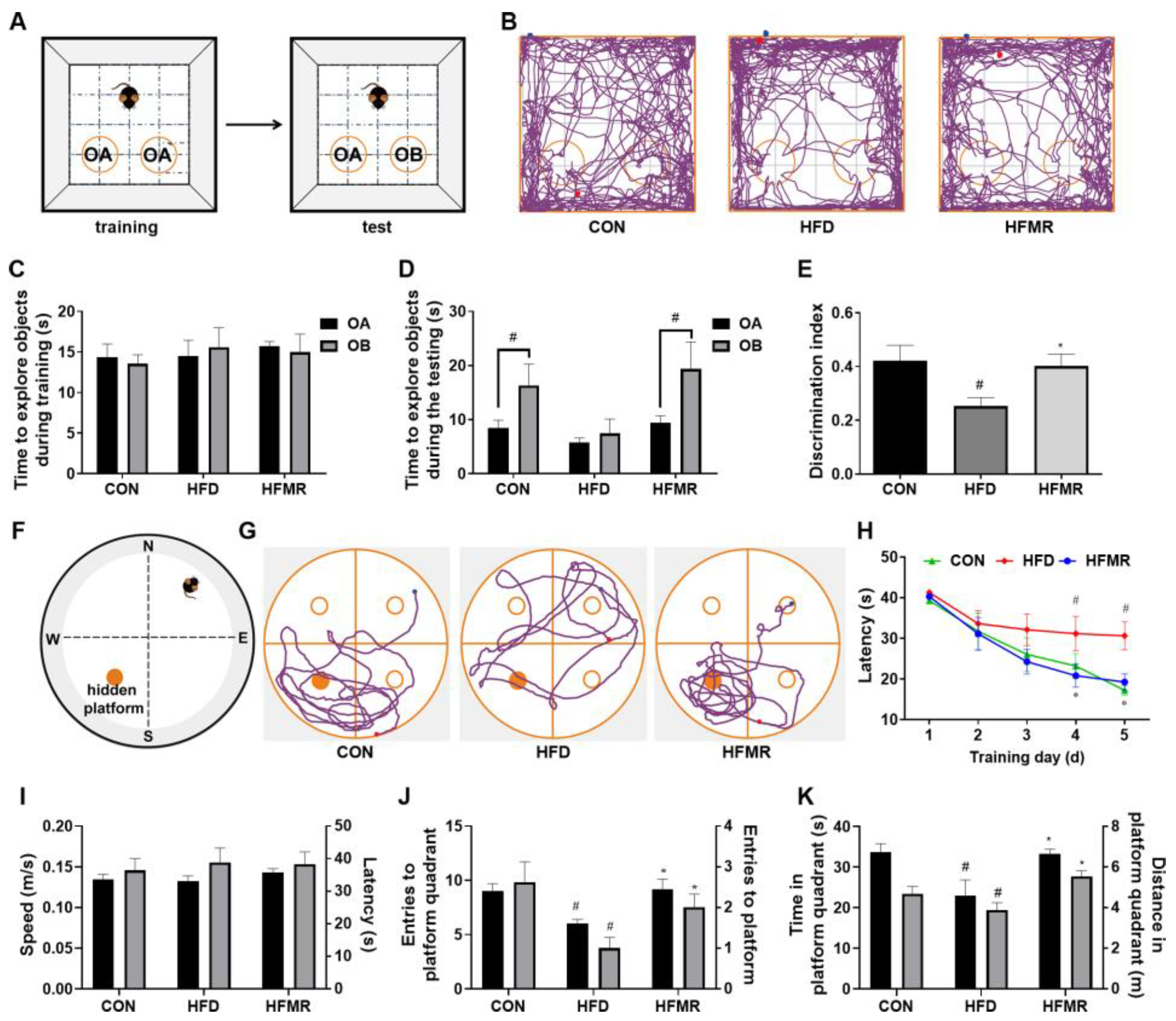


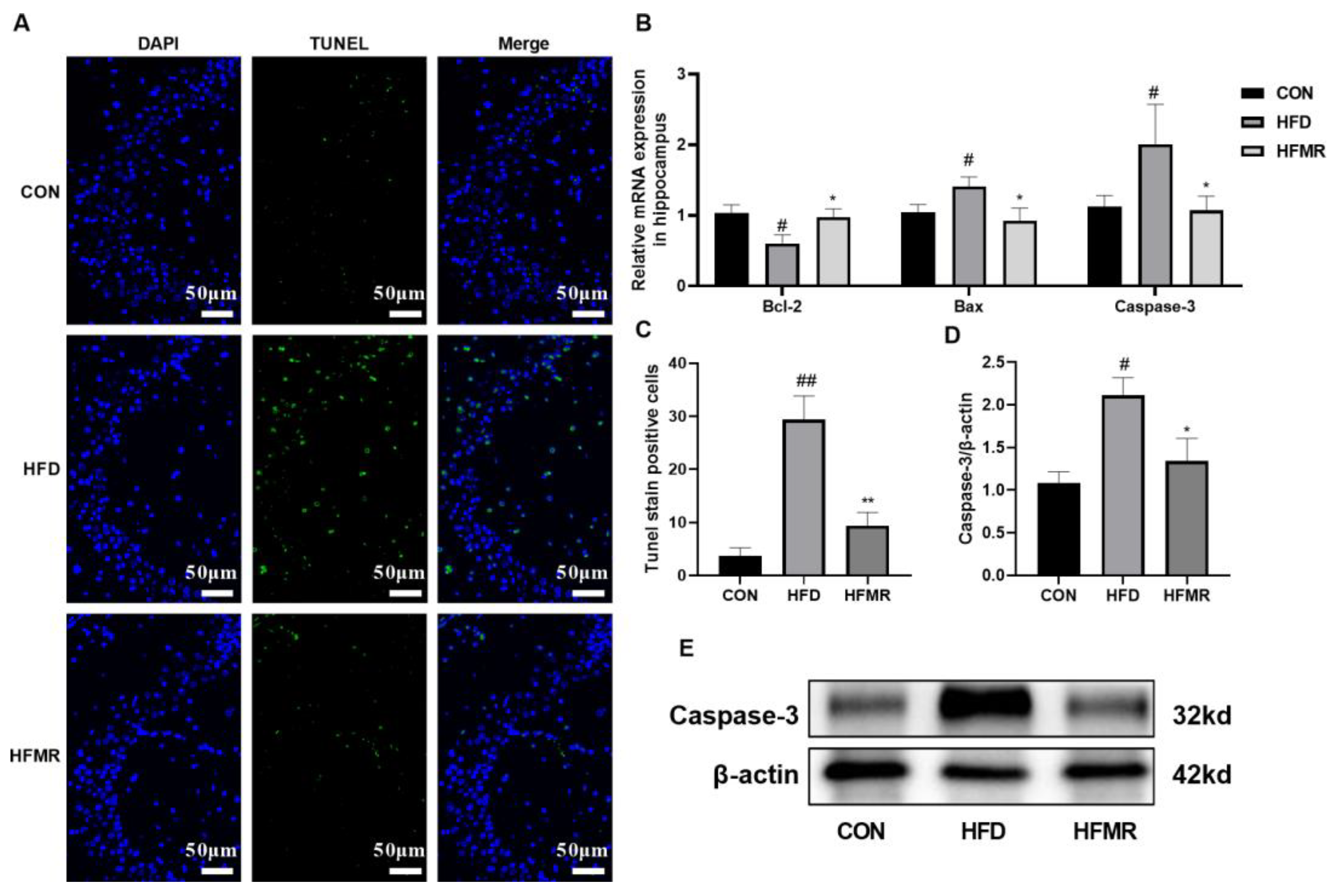
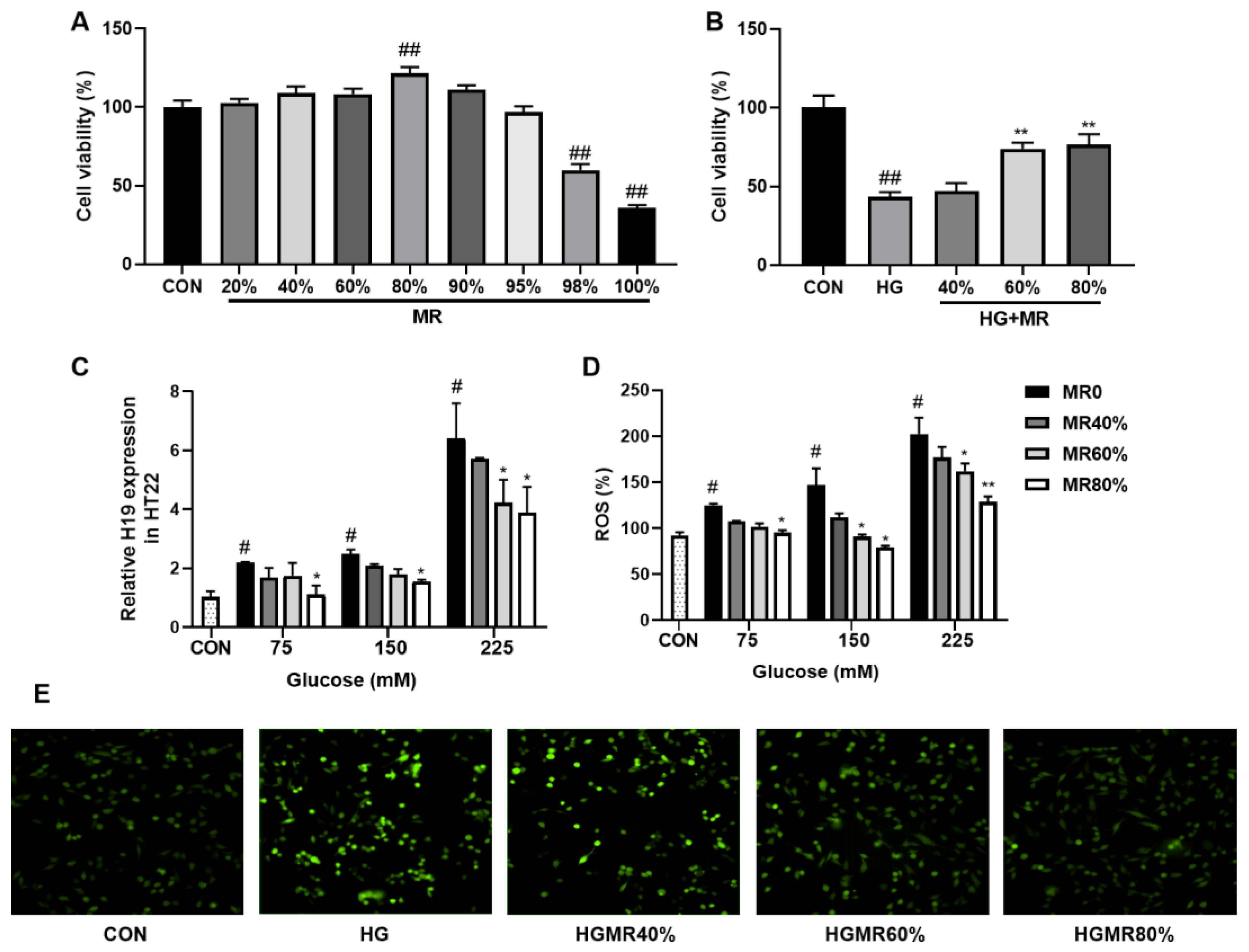
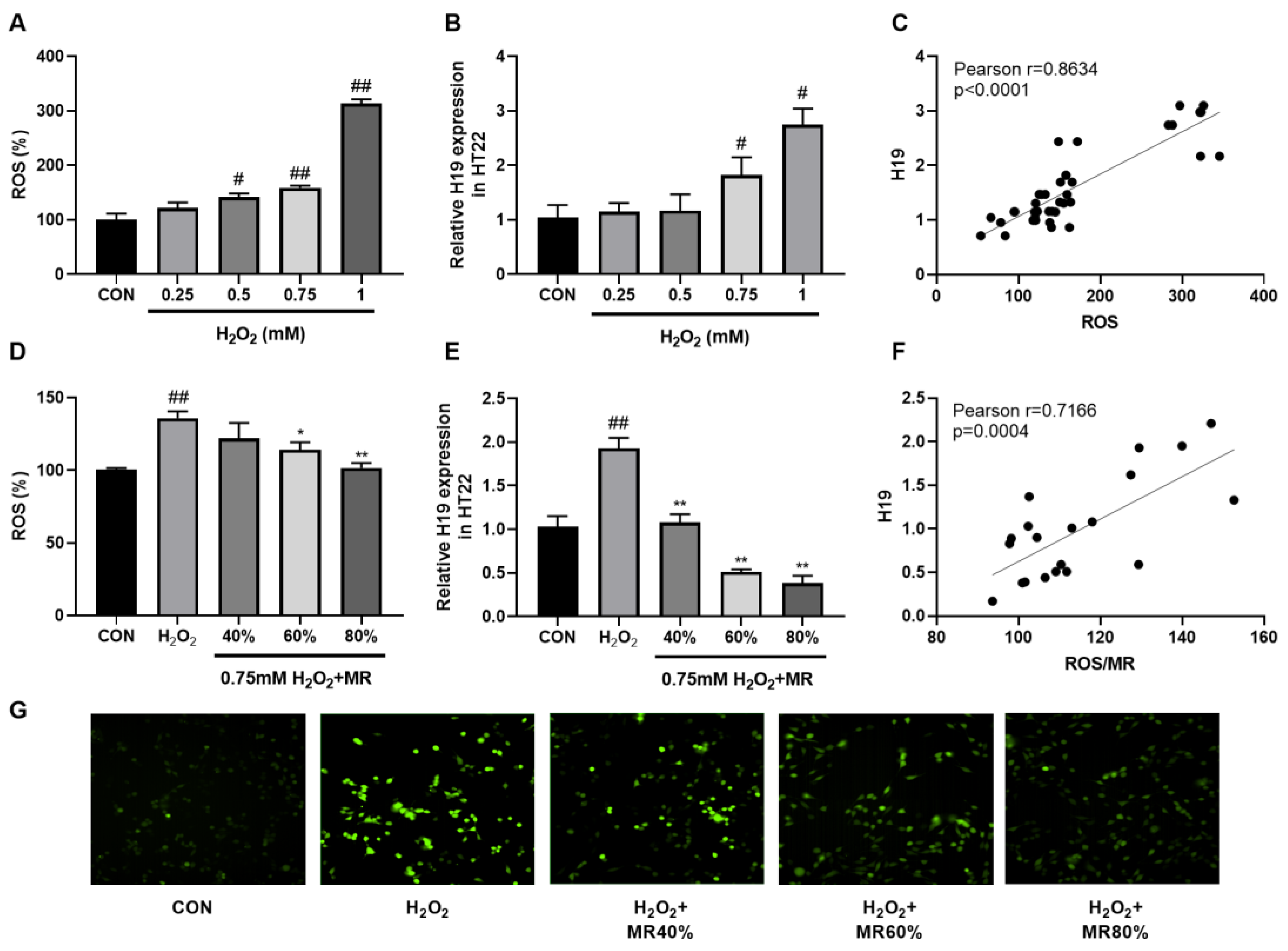
| CON | HFD | HFMR | |
|---|---|---|---|
| Body weight (g) | 41.63 ± 1.33 | 53.03 ± 0.31 ## | 41.28 ± 3.54 ** |
| Fasting glucose (mmol/L) | 9.56 ± 0.20 | 11.22 ± 0.35 ## | 9.11 ± 0.26 ** |
| Fasting insulin (mIU/L) | 7.52 ± 0.20 | 8.43 ± 0.30 # | 7.05 ± 0.24 * |
| HOMA-IR | 3.20 ± 0.26 | 4.21 ± 0.41 # | 2.86 ± 0.23 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Jiang, Y.; Li, S.; Ge, Y.; Shi, Y.; Tang, X.; Le, G. Methionine Restriction Improves Cognitive Ability by Alleviating Hippocampal Neuronal Apoptosis through H19 in Middle-Aged Insulin-Resistant Mice. Nutrients 2022, 14, 4503. https://doi.org/10.3390/nu14214503
Feng C, Jiang Y, Li S, Ge Y, Shi Y, Tang X, Le G. Methionine Restriction Improves Cognitive Ability by Alleviating Hippocampal Neuronal Apoptosis through H19 in Middle-Aged Insulin-Resistant Mice. Nutrients. 2022; 14(21):4503. https://doi.org/10.3390/nu14214503
Chicago/Turabian StyleFeng, Chuanxing, Yuge Jiang, Shiying Li, Yueting Ge, Yonghui Shi, Xue Tang, and Guowei Le. 2022. "Methionine Restriction Improves Cognitive Ability by Alleviating Hippocampal Neuronal Apoptosis through H19 in Middle-Aged Insulin-Resistant Mice" Nutrients 14, no. 21: 4503. https://doi.org/10.3390/nu14214503
APA StyleFeng, C., Jiang, Y., Li, S., Ge, Y., Shi, Y., Tang, X., & Le, G. (2022). Methionine Restriction Improves Cognitive Ability by Alleviating Hippocampal Neuronal Apoptosis through H19 in Middle-Aged Insulin-Resistant Mice. Nutrients, 14(21), 4503. https://doi.org/10.3390/nu14214503






